 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability
Paul J. A.
Withers
*a,
James J.
Elser
b,
Julian
Hilton
c,
Hisao
Ohtake
d,
Willem J.
Schipper
e and
Kimo C.
van Dijk
f
aSchool of Environment, Natural Resources and Geography, Bangor University, Bangor, Gwynedd LL57 2UW, UK. E-mail: p.withers@bangor.ac.uk
bSchool of Life Sciences, Arizona State University, Tempe, Arizona 85287-4501, USA
cAleff Group, Cross Keys Centre, 36 Erith High Street, Erith, London, DA8 1QY, UK
dDepartment of Biotechnology, Osaka University, Yamada-oka 2-1, Suita-shi, Osaka, Japan
eWillem Schipper Consulting, Oude Vlissingseweg 4, 4336 AD Middelburg, The Netherlands
fDepartment of Soil Quality, Wageningen University, P.O. Box 47, 6700 AA Wageningen, The Netherlands
First published on 9th March 2015
Abstract
The sustainability of global phosphorus (P) use is emerging as a major societal goal to secure future food, energy, and water security for a growing population. Phosphate rock (PR) is a critical raw material whose inefficiency of use is leading to widespread eutrophication and uncertainties about supplies of affordable fertilizers. Green chemistry and green engineering can be applied to help close the global P cycle by addressing three sustainability challenges: (1) consume less PR and with greater efficiency, (2) minimise P losses and generation of waste P that can no longer be re-used, and (3) set economically, socially and environmentally acceptable P sustainability targets to lower P demand. Greater precision in P use by the agriculture sector (the main P flow) supported by smarter PR mining and processing technology could greatly improve global P use efficiency. Emerging bio-based and green chemical technologies could be more widely applied to enhance first- and second-generation valorization of low-grade PR ores, manures, by-products and residues to provide renewable secondary sources of P and other essential elements and compounds. All sectors of society have the potential to lower their P demands, and all production systems could be redesigned to facilitate recovery and recycling of P. Collectively these ‘green engineering’ actions at sector and regional level can help achieve planetary P sustainability.
1. Introduction
“This is no way to run a biogeochemical cycle.”(J. Elser quoted in Lougheed (2011)).1
Phosphorus (P) is a critical element for our food production systems, manufacturing industries, and general economic growth whose long-term security of supply is of major concern for regional and national economies. Phosphate rock (PR) is mined for processing into P derivates, such as phosphoric acid (PA, H3PO4) and white phosphorus (P4), for final use in various products that society uses ranging from fertilizers to toothpaste to car batteries. The mineable reserves of PR are essentially finite for the human era because of the geological timescales over which the natural cycling and therefore renewability of P occurs. Current high rates of P consumption (over 20 Tg P year−1)2 driven largely by fertilizer use are putting increasing pressure on the global supplies of this vital resource, leading to rising and volatile prices.3 Estimates of how long global PR reserves will last are uncertain and currently vary from <100 up to 400 years, but their accessibility and cost are a major concern for many countries with no PR reserves, such as Europe.4,5 Future demand for P in emerging economies, notable in Africa and Asia, is anticipated to be very high, while rising demands are also imposed by expanding biofuel production.6 Ironically, a more immediate environmental problem is the widespread leakage of P to waterbodies where it causes nuisance algal blooms, loss of aquatic biodiversity, and increased risk to human health.7 These pressing problems have been evidenced quite tangibly in the bloom-induced drinking water crises that have emerged in recent years in Lake Taihu (China)8 and Lake Erie (USA/Canada).9 Phosphorus is therefore both a critical element and a pollutant and must be used more efficiently and sustainably in the future to help safeguard future food, energy and water security.5,10–12
Phosphorus has no substitute, but can be continually re-used, and is thus a prime example of a critical resource that could be utilized more efficiently in a circular economy to support sustainable growth with less pollution.13,14 The current P cycle is inherently inefficient because the vast majority of the P that is mined each year becomes dissipated in low-grade PR ores, manures, by-products and residues that are not fully re-used or re-used uniformly, and in surface waters and seas. Actual rates of P re-use will clearly vary considerably between sectors and countries, but a recent review of P budgets across different geographical scales suggests it is <20% of total P inflows.15,16 This is a low percentage and there continues to be large scale disposal of P to landfill in developed countries. A large proportion of dissipated P (wrongly termed waste) could potentially be re-used as secondary P resources subject to technological and financial constraints.17–19 A distinction has been made between strategies that enhance P re-use through more uniform recycling directly back to land, and those that first require recovery through innovation in bio- and chemical engineering.5 True wastage occurs when P is dissipated to the oceans. These permanent P losses have quadrupled due to anthropogenic activity in the 20th century and must also be reduced.20
Achieving long-term sustainability of P use in society will require insights from a variety of emerging approaches, including “green chemistry” and “green engineering”.21,22 In the context of P, these two concepts adopt the same core principles of (a) the development of benign products and processes, (b) the elimination of waste, (c) the use of renewable (secondary) resources, and (d) the design of output-driven production systems with minimum requirements and maximum efficiency.23 These green principles will be critical in developing new strategies for how we use P in the technosphere and how products can be designed in the future with a focus on recycling. For example, an immediate green chemistry challenge is to remove and re-use the potentially harmful elements naturally present in PR, such Cadmium (Cd), Uranium (U) and Lead (Pb), that might persist in the environment or through the food chain.24 The cradle-to-cradle philosophy implicit in green chemistry has yet to be fully adopted by sectors in the P cycle, although the conceptual basis for achieving this transition exists through P accounting and recent drivers towards industry sustainability and a circular economy.13,25–27 This probably relates to the relatively recent recognition of P as a critical resource by government and industry, and P sustainability as a serious societal problem. For example, PR has only just been recognized in Europe as a critical raw material.28 Considering the environmental impact of the whole life cycle, and afterlife, of P products and not just the impact of their initial manufacture will also require a large paradigm shift in attitude by all stakeholders and actors in defining societal P needs and how systems can be best designed to meet them.29–31
This paper outlines a variety of avenues for the application of green chemistry principles and practice to transform P use in society. We describe a variety of existing and emerging technologies and strategies for elemental re-use in the P cycle. If not constrained by cost, their adoption will help deliver a sustainable P system that can sustain future generations with abundant food, feed, fibre, fuel, clean water and other essential P products society relies on. After a synopsis of global P use, we consider sustainability and green chemistry challenges in recycling and recovery technologies within different sectors and then system design with some regional examples of progress. Our ultimate objective is to inspire green chemists and engineers to take a major role in developing and implementing a more efficient and sustainable P cycle.
2. Phosphorus distribution in the technosphere
Information on how mined PR is dispersed in the technosphere is needed to identify where opportunities for green chemistry and engineering are most likely to occur in the P cycle (Fig. 1). Numerous attempts at global P budgets have been made, but they are all simplified to some degree or another, and contain large errors because accurate data for all the stores and flows are very difficult to obtain or estimate. However some important general trends emerge. Anthropogenic flows of P are much greater than in natural systems, but are still relatively small (<50 Tg year−1) compared to the very large global stores of P present in the pedosphere, (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–200
000–200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Tg), biosphere (550–3050 Tg) and hydrosphere (8300–123
000 Tg), biosphere (550–3050 Tg) and hydrosphere (8300–123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Tg).20,33 They are also very widely dispersed because PR derivates have a very wide range of uses in industrialized society and are therefore traded globally: for example in fertilizers, feeds, foods, pesticides, plastics, computer chips, lubricants, chemical extractants, detergents, toothpaste, car batteries and flame retardants. This P becomes further dispersed in industrial and municipal wastewater, solid waste, livestock manures and crop residues after its first use. Variation in demography, agricultural intensity and industrial activity in different regions, countries and catchments will therefore lead to different priorities for improving P resource use efficiency, choosing the most economic recycling and recovery options and reducing environmental impacts.15,34 This regional variation is an important aspect regarding achieving progress in global P sustainability.
000 Tg).20,33 They are also very widely dispersed because PR derivates have a very wide range of uses in industrialized society and are therefore traded globally: for example in fertilizers, feeds, foods, pesticides, plastics, computer chips, lubricants, chemical extractants, detergents, toothpaste, car batteries and flame retardants. This P becomes further dispersed in industrial and municipal wastewater, solid waste, livestock manures and crop residues after its first use. Variation in demography, agricultural intensity and industrial activity in different regions, countries and catchments will therefore lead to different priorities for improving P resource use efficiency, choosing the most economic recycling and recovery options and reducing environmental impacts.15,34 This regional variation is an important aspect regarding achieving progress in global P sustainability.
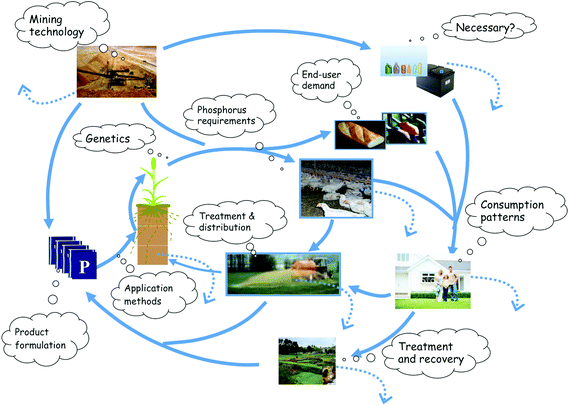 | ||
| Fig. 1 Phosphorus cycling in the technosphere showing thought bubbles suggesting where green technologies and innovations could improve P sustainability by reducing P fluxes, losses and wastage. Points of P loss are shown by dotted lines. Adapted from Sylvester-Bradley and Withers.32 | ||
Two major issues emerge from global P budgeting. The first is that food consumption patterns and global trade in food, feed and livestock products have a major impact on P flows and their global distribution.35 In particular, increasing demand for meat is a major driver of fertilizer and feed P consumption and a major cause of global inefficiency of P use.36 This is because large areas of agricultural land are needed to grow the fodder and grain to feed animals, and 70–80% of the P consumed by animals is excreted and must be handled. The second issue is that the amount of new PR mined each year is currently about 30 Tg of P, whilst the actual incremental demand for new P by a growing world population is estimated at only 1 Tg P year−1.2,37 This highlights the gross inefficiency of the global P cycle and the necessary infrastructure and societal costs of handling dissipated P. Sheldon introduced the E Factor (or Environmental Factor) to describe the ratio of the amounts of waste generated per unit of product processed.23 Although not strictly comparable, PR production has an E factor of at least 30 making it similar to the fine chemical industry in terms of waste generation.
The first stage of waste generation is in the mining operation and subsequent processing to PA, with global estimates of unrecovered P amounting to 7–11 Tg P year−1.2 Over 80% of mined PR is used to manufacture fertilizers (chiefly through PA), with the remainder used in feed and food additives mainly as calcium and sodium phosphates (through PA), and in the chemical industry as white phosphorus (from PR).38 The majority of fertilizer P accumulates in the soil from where crops take up their P requirements to be harvested or grazed into food for animals and humans. Excreted P is either recycled back to the land, incinerated, taken to landfill after treatment or discharged to water (Fig. 1). Fluxes of P through animals are similar in size to those through crops (arable crops and managed grass, ca. 20–25 Tg year−1, and dominate the technosphere P budget.33,39 Globally there is more P in animal excreta than in manufactured fertilizers, but both grazing returns and collected manure are not recycled uniformly. Animal slaughter also leads to significant amounts of slaughter waste which is often co-incinerated for its fuel value in cement works and power stations. Global estimates of P in slaughterhouse waste suggest it could be 10–15% of total P imports and therefore significant.40 For example, for Europe it represents about 70% of the P imported into the livestock sector.15
High animal stocking rates in some areas lead to an oversupply of collected manure P for the available land area. This misplacement of P, together with its intrinsically high P content, is both a source of localized P surplus, P accumulation in soil and P loss in land runoff causing a high eutrophication risk.41,42 Surpluses of P in soil also arise due to excessive fertilizer use.43,44 Global surpluses of P accumulating in agricultural soils are estimated at 11–16 Tg P year−1.45,46 The legacy soil P associated with cumulative past P surpluses therefore represents a considerable P resource base to reduce P fertilizer use.5,47 For example, Ringeval et al. recently estimated that over 80% of the total P in French soils (0–35 cm) was of anthropogenic origin.48 Estimates of global P losses in land runoff to water associated with erosion and direct losses from the fertilizers and manures applied are typically quoted at 13–24 Tg year−1.2,33,46 Although there is large uncertainty around these runoff losses, there is clearly potential to reduce them.
The flux of P associated with consumed food, with smaller contributions from detergents and other materials is ca. 5 Tg year−1.45,49 The majority of this P input ends up in wastewater, or in solid (largely food) waste. Wastewater P is either discharged directly to watercourses, or treated in a septic tank or collectively at a treatment centre. Wastewater treatment plants (WWTPs) are estimated to receive about 4 Tg P year−1, of which up to 90% is removed in the form of sewage sludge depending on local effluent regulations. Of this P rich sludge, ca. 50% is returned to the land as biosolids, but this figure can vary considerably; for example in Europe between 0 and 80%.15 The remainder of sewage P, i.e. that is not captured in the sludge, is discharged to water as treated sewage effluent amounting to ca. 1.5 Tg P year−1.50 Globally consumer waste is increasingly being recycled, but the majority is still disposed of in landfill or incinerated.51 Total amounts of household and industrial P that are disposed of to landfill, or incinerated are estimated at 2–3 Tg P year−1.2 The total amounts of P potentially recoverable from wastewater effluent, landfill and incineration are therefore only ca. 5 Tg P year−1, and much lower than the larger global fluxes of P retrievable from agricultural systems.
Global usage of P in the chemical industry is estimated at only 1–3 Tg year−1, but there are still opportunities to recycle industrial residues containing P.18,49,52 The use of P in applications that might be considered non-essential are also relatively minor (1 Tg P year−1), but still contribute to the P cycle.2 This evidently raises debate on which applications of P are considered unnecessary and what the consequences of replacement would be. Detergent P is one example of unnecessary P that is now being phased out in the USA and Europe.2,5
3. Greening the phosphorus cycle
The wide dispersal of P in the technosphere and the large variability in P flows across sectors in the P cycle suggests that P sustainability targets are best set and met at the sector and regional level.15,34,53 A large proportion of dissipated P is contained in mined ores that do not pass the bone phosphate of lime standard for processing (BPL <68), and in sector wastes, by-products, residues and wastewaters. These secondary materials provide a range of accessible resources from which P could be recovered by green chemical and bio-technologies into safe, renewable P sources for re-use, and for substitution of primary PR, with minimum waste (Fig. 2). Many technologies are still in their infancy (i.e. pilot stage), whilst others have been already implemented.18,55,56 These secondary sources are also increasingly being considered as sources for other essential elements and compounds that society uses. For example, Westerhoff et al.57 recently identified a range of valuable metals in municipal sludge and suggested that the 13 most lucrative (Ag, Cu, Au, P, Fe, Pd, Mn, Zn, Ir, Al, Cd, Ti, Ga, and Cr) had a value of US $280 per tonne of sludge. Wastes and wastewaters generated in the food chain are also renewable sources of energy (electricity and fuel), and a number of essential ‘functionalized’ compounds that society needs including flavonoids, waxes, fatty acids and biopolymers (Fig. 3).58–60 This raises the question over whether first generation valorization of secondary bioresources from the food chain through recycling P to land is the most sustainable route.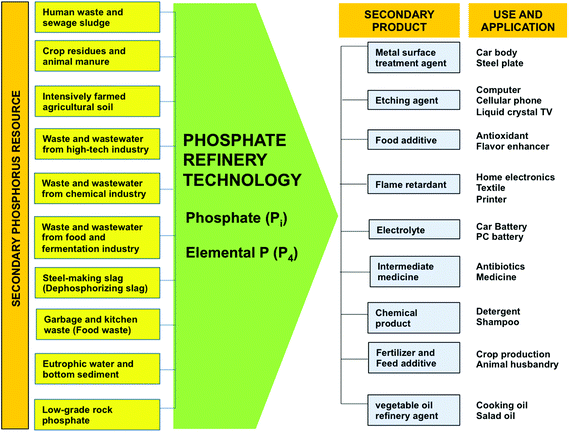 | ||
| Fig. 2 The Phosphate Refinery for production of renewable secondary P sources in agriculture and industry. After Ohtake.18,54 | ||
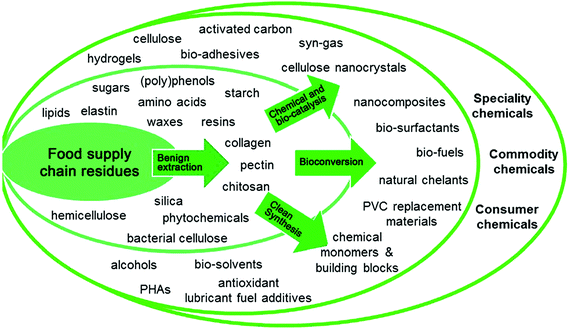 | ||
| Fig. 3 Components present in food chain waste and their uses in common consumer applications, highlighting sectors of the chemical industry that could benefit from the use of such a renewable resource. After Clark et al.58 | ||
3.1 The mining sector
The first stage in the P cycle for progress in sustainability is in the PR mining operation. A recent green chemistry development by the fertilizer industry has been a renewed interest in comprehensive extraction (CX), Table 1. CX sees PR not just as a source of P, but as an ore “which can contain the entire periodic table”. Since PR contains U and a number of rare earths essential to society, the co-extraction U and rare earths from PR and PA is a prime example of how the mining industry can gain added value from PR processing.62 Technologies for co-extraction of P and U from lower grade ores by adding solvent extraction circuits to existing wet process technology for PA manufacture, or though ion exchange, are now being revisited with commercial-scale projects starting in Brazil and India in 2017.63 Simpler, gravity separation techniques (shaking table) have been shown to work for extracting both heavy and light rare earths from flotation tailings (heavy) and from phosphogypsum (PG) (light). Thorium (Th) is another metal in PR ores and PA that could also be potentially recovered to provide a long-term supply of safe nuclear energy, with pioneering plants in China, India and Brazil.63 Co-extraction technologies also help to remove the environmental hazards associated with PR-derived products making them more benign for future P re-use; a clear green chemistry goal. A future challenge for these technologies is to remain economic.| • address all available resources from a given site/deposit in an integrated resource management strategy |
| • disturb the ground once |
| • construct regulation of naturally occurring radioactive materials (NORM) industries based on shared values between operators and regulators |
| • obtain and keep a social licence to operate, focused on equitable distribution of benefits between stakeholders and stockholders |
| • sequence extraction procedures and select extraction technologies to optimise deposit returns, e.g. by classifying and progressing resources on a whole “energetic basin” project management basis |
| • extract and store resources that would otherwise be wasted or dispersed for future use |
| • manage resources across the whole life-cycle seeking to conserve primary resources and substitute secondary resources for primary where feasible |
| • align to the waste hierarchy seeking all opportunities for re-use and recycling of by-products, residues and “wastes”, resulting in a zero waste outcome |
| • promote new product development as strategic alternative to waste disposal (e.g. from recycling tailings or residues) |
| • ensure a net positive contribution to food, energy and water security as part of a wider commitment to sustainable development |
The challenge of recovery of P from low-grade PR ores (BPL values of 50 or above) is stimulating innovation in an industry that has been very change averse. Ecophos and the Improved Hard process (IHP) are typical examples of such innovation. The Ecophos process expands on the well-known hydrochloric acid route to dissolve phosphate from rock (http://www.ecophos.com). This serves as an alternative to the standard sulphuric acid method (wet process), which is applied worldwide to produce PA. Unlike the latter, the hydrochloric acid route does not create an insoluble stream of PG to separate the calcium oxide from the PA, but instead produces soluble calcium chloride which remains mixed with the PA product. This poses a separation challenge which can be surmounted by solvent extraction of the PA, or precipitation with lime. The latter chemistry yields feed grade phosphates in the form of dicalcium phosphate. This process has a marked operational cost advantage if hydrochloric acid waste is locally available and is more sustainable than the market procurement of sulphur (S), which is needed to produce sulphuric acid for the conventional wet process of PA manufacture. The IHP uses the local formation of P4 in a heated mixture of low-grade rock and a low-cost reduction agent such as petcoke. The off gases are oxidized immediately, giving off sufficient heat to keep the process going without significant external energy input. The now-oxidized P is hydrolysed to PA which is used in fertilizer and industrial phosphates manufacture. The process is eminently suited to use low-grade rocks, being competitive in variable cost for these, and might use other locally available P-rich waste also as input. This process has seen a very long development phase and is now at pilot stage near Fort Meade, Florida. The operator anticipates this will become commercially viable sometime in the coming years based on a 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t PA yr−1 production plant.64
000 t PA yr−1 production plant.64
Another sustainable development within the mining industry has been the focus on registering mine and PA processing wastes, reducing their volume and re-using them. The efficiency of P extraction from PR ore is very variable, with estimates of P losses and wastage during mining, beneficiation, chemical processing and handling of between 15 and 50% (average 30%) depending on PR quality and the methods deployed.38,49,63 This wastage could be substantially reduced by optimising the PR extraction process and recovering P from mine tailings and the by-product PG produced during PA manufacture.19 About 20 Tg (dry weight) of mine tailings equivalent to 1 Tg P year−1, and about 160–170 Tg of PG (dry weight) equivalent to 0.5 Tg P year−1 are produced each year.63 Reducing ore waste at the mine face is being explored through laser technology to improve ore quality detection, and mine tailings can be flocculated and thickened using polymers and re-used to strengthen concrete.63 As a result of an evidence-based review by the International Atomic Energy Agency,65 PG is also no longer classed as a hazardous waste (it contains the radionuclide Radium, Ra), but as a co-product, encouraging its re-use rather than indefinite disposal to stacks. By 2015, annualised re-use of PG as a soil amendment and fertilizer in agriculture, as a building and road base material in the construction industry and as a source of ammonium sulphate (through the Merseburg process) will be at least 30 Tg year−1 from a near zero base in 2008. However, although their re-use has increased, the recovery of P from mine tailings and PG remains a challenge and one potentially for green chemistry to tackle.
3.2 The agricultural sector
The agricultural sector generates the largest amounts of potentially recoverable and recyclable P. Most of the dissipated P from past fertilizer and recycled manure inputs has accumulated in farmed topsoil (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Tg),53 and in some cases subsoil.66 Progress in the recovery of this legacy soil P in situ is largely dependent on improving its accessibility to plants through bio-engineering based on the inherent traits present in plants and microorganisms for soil P acquisition.67,68 A more accessible regional source of secondary P in agriculture is livestock manure. Although livestock manure is a multifunctional fertilizer, containing organic matter and essential major nutrients and trace elements, its nutrient composition is not ideally balanced for many crops. In areas with intensive livestock farming and limited land area (e.g. large pig and poultry units), uniform spreading of manure on fields is limited by its P content and costly transport over longer distances, or treatment, is needed.69 Due to the necessity for local disposal, spreading allowances quickly exceed crop P requirement due to their low N
000 Tg),53 and in some cases subsoil.66 Progress in the recovery of this legacy soil P in situ is largely dependent on improving its accessibility to plants through bio-engineering based on the inherent traits present in plants and microorganisms for soil P acquisition.67,68 A more accessible regional source of secondary P in agriculture is livestock manure. Although livestock manure is a multifunctional fertilizer, containing organic matter and essential major nutrients and trace elements, its nutrient composition is not ideally balanced for many crops. In areas with intensive livestock farming and limited land area (e.g. large pig and poultry units), uniform spreading of manure on fields is limited by its P content and costly transport over longer distances, or treatment, is needed.69 Due to the necessity for local disposal, spreading allowances quickly exceed crop P requirement due to their low N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio. In such cases, manure application becomes diffuse landfilling (push model) rather than a demand-driven application. A selective technology for partial chemical removal of P from manure through acidulation and solid/liquid separation may remedy this and enable more demand-driven application of P-poor solid fraction of manure as organic fertilizer, and the separate re-use of a precipitated P-rich concentrate (e.g. struvite or calcium phosphates) elsewhere as an inorganic P fertilizer (Fig. 4). The recoveries of P from manure and liquid manure fractions by struvite and calcium phosphate precipitation can be high (50–90%).55 For example, Suzuki recovered 171 g of 95% pure struvite from 1 m3 of pig slurry in a crystallization plant.70 Potential alternative technologies for partial removal of P from manures to decrease their N
P ratio. In such cases, manure application becomes diffuse landfilling (push model) rather than a demand-driven application. A selective technology for partial chemical removal of P from manure through acidulation and solid/liquid separation may remedy this and enable more demand-driven application of P-poor solid fraction of manure as organic fertilizer, and the separate re-use of a precipitated P-rich concentrate (e.g. struvite or calcium phosphates) elsewhere as an inorganic P fertilizer (Fig. 4). The recoveries of P from manure and liquid manure fractions by struvite and calcium phosphate precipitation can be high (50–90%).55 For example, Suzuki recovered 171 g of 95% pure struvite from 1 m3 of pig slurry in a crystallization plant.70 Potential alternative technologies for partial removal of P from manures to decrease their N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio for land application and reduce the need for costly manure export pose a challenge for green chemistry. One green engineering option might be to use simple water extraction and micro-filtration of animal slurries to manipulate their N
P ratio for land application and reduce the need for costly manure export pose a challenge for green chemistry. One green engineering option might be to use simple water extraction and micro-filtration of animal slurries to manipulate their N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios since a significant proportion P in manures and slurries is water-extractable.71,72
P ratios since a significant proportion P in manures and slurries is water-extractable.71,72
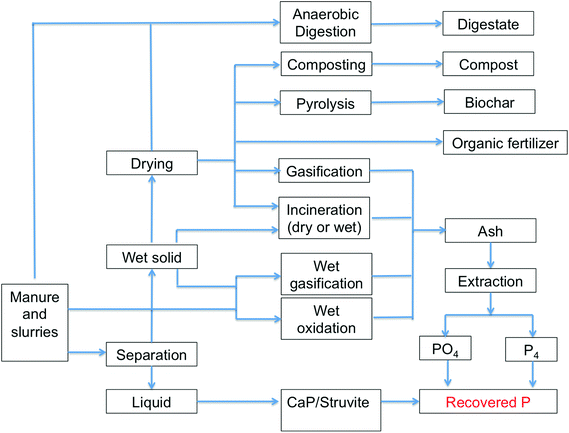 | ||
| Fig. 4 Schematic overview of the main options to recover P from manures and biosolids. Adapted from Oenema et al.53 and Schoumans et al.55 | ||
Drying, or prior separation before drying and pelletizing may pose an environmentally acceptable solution, allowing the dried manure (nutrient concentrate) to be transported over longer distances to crop producing areas with P demand. Separation of the P-poor liquid fraction to allow more efficient drying of the P-rich solid fraction requires separate treatment and/or disposal of the liquid which increases cost. Green chemistry techniques to combust whole (wet) manures without separation by supercritical and subcritical oxidation or and water gasification for energy production could provide a solution, but are still in their infancy.55 Alternatively, bulky manures or separated solids can be anaerobically digested, incinerated or pyrolysed to provide energy (Fig. 4). Pyrolysis can promote the conversion of animal manure into charcoal (i.e. biochar) by heating to 300–550 °C in the absence of oxygen, thereby reducing the solid volume. Biochar is proving a useful soil amendment and fertilizer with low greenhouse gas emissions.73 Pyrolysis has also been used as a pre-treatment for raw biomass which is not suitable for direct combustion because of a low energy density and high moisture content.74 Gasification heats manure at higher temperatures (800–1000 °C) but loses more of the carbon as CO2.55 Incineration of animal manure is an attractive option in regions with a high animal density, particularly for handling chicken manure, and the incineration ash, contains a considerable amount of P and can be processed into fertilizers (see wastewater sector below).75
3.3 The food processing sector
After leaving the farm significant amounts of P are dissipated in slaughter wastes, food processing waste and wastewater. After various steps aiming at recovery of valuable by-products such as animal fats, gelatine and proteins, livestock slaughter waste becomes Meat and Bone Meal (MBM). This is a mixture of calcium phosphate (bones) and proteins (meat) potentially usable as a fertilizer or feed. Various streams of MBM occur worldwide with different protein/bone phosphate ratios, and generally have the highest P concentrations of waste flows suitable to substitute PR. After the Bovine Spongiform Encephalopathy (BSE) crisis, Europe required a BSE-sensitive fraction MBM, Category I, to be thermally destroyed instead of being used as feed ingredients for proteins and minerals.40,76 Currently, these materials with high calorific value are often used as bio-co-fuel in power plants, to give carbon dioxide (CO2) credits. This unfortunately dilutes their considerable P content beyond recovery. MBM mono-incineration and smart development to recover P from the ashes (as for other incinerated wastes) needs to be implemented to avoid this P wastage. In one process, MBM is incinerated with alkaline earth compounds at 1000 °C in a rotary kiln to improve P availability in the ash and marketed for use in agriculture (ULOPHOS®).77Wastewater from the vegetable industry has been conventionally treated with iron salts to remove P to allow discharge to rivers, but alternative green chemistry approaches are now being explored. In the potato industry after anaerobic digestion of the organic matter to produce biogas, P is recovered from the P-rich wastewater as struvite by adjusting pH and adding magnesium chloride in the NuReSyS-P process (http://www.nuresys.com).56 The process is suitable for any anaerobic digestate or P-rich wastewater (>55 mg PO4-P L−1) with up to 80% P recovery and produces a crystalline struvite product suitable for re-use as fertilizer. A similar process used in the potato industry uses a fluid-bed crystallizer partially filled with sand or mineral to seed the crystallization process to struvite or calcium phosphate (Crystalactor®).78 The process is accurately controlled to limit the growth of the crystals to approximately 1 mm which then move to the bottom of the bed for removal and drying. The advantage of this process is that there is no residual waste because the nearly dry phosphate pellets are fully recovered and useable. However, the pH adjustments to the inflow necessary before entry to the bed are quite demanding in terms of energy (CO2 stripping) and chemicals (acids/bases).56 A comparable approach currently under investigation is recovering P from vegetable wastewater as calcium phosphate for subsequent conversion to PA for use in the chemical industry (http://www.biorefine.eu).
Food waste contains non-negligible amounts of P, not least because society wastes at least a third of its food, whilst in rich countries it can be up to 40%.79,80 As food waste has by definition been safe for consumption before its expiry, it is suited for composting (provided packaging can be separated) and the small amount of P in the ensuing compost contributes to its wider agricultural value. As such, it may not be necessary to selectively extract P from food waste, but rather see it as an essential if minor part of its wider agricultural value as a soil amendment, or as a source of other second generation critical compounds.81
3.4 The wastewater sector
Phosphorus recovery from human sewage (e.g. excreta, detergents and food washings) has generated most P recovery research not least because accessibility is already guaranteed. WWTP need to remove P to reduce effluent P concentrations discharged to water, most commonly by conventional anaerobic digestion and Fe/Al dosing. The remaining P-rich sludge (biosolid) can be directly recycled to land as another multifunctional fertilizer, but the presence of pathogens, pharmaceuticals and hormone residues and heavy metals are a continual cause for concern.82 The plant availability of recycled Fe-bound P to agricultural crops can also be low.83 Urine diversion from human excreta via a specially designed toilet is an option for improving the direct re-cycling of human waste to agriculture, especially in developing countries where the majority of households are not already directly connected to WWTP.84,85 Mihelcic et al.86 suggested there is 1.7 Tg P in global urine, and recycling urine as a fertilizer seems to be socially acceptable.87To avoid potential environmental and human health risks, there is an increasing global trend towards sludge incineration with the ashes offering an entry point for P recovery and metal removal to produce fertilizers that are safer to store, handle and apply.88,89 The green chemistry advantage of this route is the near-complete collection of sewage P provided WWTPs are well-designed and meet operational criteria. There is also potential to recover other non-renewable elements of value to society (e.g. K, Zn, Cu, Se).57 While P recovery from liquid phases at WWTP is only 40–50% at most, recovery of P from ashes is up to 90%.56 Thermo-chemical and wet chemistry technologies using acid or caustic digestion of these ashes can produce P products with high P-availability suitable for animal feed or fertilizers, or as elemental P, whilst also removing any heavy metal contamination that might otherwise reduce the recycling value.52,55 For example an innovative refinement of the hydrochloric acid technology used in the Ecophos process to produce feed phosphate from sewage sludge ashes is currently being investigated and may achieve full scale in 2017 (http://www.ecophos.com). Two full-scale plants have been implemented in Japan to recover P from incinerated sludge ash using alkaline (NaOH) leaching technology to minimize the leaching of heavy metals from the ash that would otherwise contaminate the recovered product.18 The relatively low level of Ca (typically less than 10% CaO by weight) in the ash makes alkaline leaching sufficiently efficient. Such routes are economical wherever a local source of attractively priced NaOH (e.g. as by-product) is available.
An alternative green engineering approach to P removal in WWTPs is by biological treatment, a setup where little or no further chemical P removal is needed.54 In enhanced biological P removal (EBPR), alternate anaerobic and aerobic cycles facilitate polyphosphate accumulation in microorganisms.90 Inorganic P (Pi) can be released from EBPR sludge in a more concentrated form by various technologies, including heat treatment,91 anaerobiosis,92 anaerobic digestion,93 and incineration followed by chemical leaching.94,95 P can also be recovered from Pi-rich solution using precipitation technologies with inorganic cations such as Ca2+ or Mg2+, producing either calcium hydroxyapatite (Ca10(PO4)6(OH)2), or struvite (MgNH4PO4·6H2O).96,97 Struvite poses a scaling issue in EBPR plants, and its targeted precipitation therefore offers considerable operational cost savings. The recovered product has so far proved a useful slow-release fertilizer, but recovery of struvite from liquid phases is only applicable in WWTP with EBPR.55 Recovery of struvite directly from the digested sludge is also in operation (e.g. Airprex, and Seaborne processes), but not on a large scale due to economic feasibility and national legislation.56 In the Seaborne process, P and metals are recovered separately. Digested sludge is first acidified with sulphuric acid to mobilise P and heavy metals, the metals are removed with sulphur-rich digester gas and the P is precipitated as struvite by addition of sodium hydroxide. Both struvite and ammonium sulphate produced from the process can be re-used in agriculture. A more green chemistry approach at the pilot stage is the Budenheim process which uses carbon dioxide rather than acid to dissolve the P in the sludge before precipitating the P as calcium phosphate.77 The CO2 extractions are very efficient at mobilising the P with 60–70% recovery and the used CO2 is recycled in the process (http://www.budenheim.com).
A number of potential green and bio-engineering solutions to recover P from dilute wastewaters are at the pilot scale. Amorphous calcium silicate hydrates (A-CSHs) synthesized using natural and low cost materials, such as siliceous shale and calcium hydroxide (Ca(OH)2) have proved an effective option to recover P from aqueous solutions by simple adsorption.98 Similar suitable materials can even be obtained from construction material production waste. The lack of any need for pH adjustment and the high settling rates, filterability, and dewaterability of recovered P are the advantages of A-CSHs over conventional CaCl2 and Ca(OH)2 chemistry.99 No chemical coagulants are required for P recovery by A-CSHs, and, unlike Ca(OH)2, no significant carbonate inhibition occurs with P recovery with A-CSHs. Other novel phosphate-binding materials for treating wastewater have included polymeric hydrogels synthesized by chemically crosslinking linear poly(allylamine) PAA⋯HCl chains with epichlorohydrin, and ion-exchangeable ceramic beads (0.55 mm diameter and 85% porosity) which exhibit a high specificity for fast phosphate adsorption over a wide pH range (pH 2 to 14), and can be used more than 100 times.100,101 With an increasing need to further lower WWTP effluent P concentrations for eutrophication control, sorption and ion exchange may become more attractive green engineering approaches for enhanced wastewater P recovery in the future.102,103 Microfiltration (0.2 μm) and nanofiltration technology together with various pre-treatment steps also have the potential to produce recovered products with specific N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios from wastewaters.60,71 Microalgal recovery of wastewater P for re-use in biodiesel production is another bio-based technology, but the economics of this route still remain unfavourable.104,105
P ratios from wastewaters.60,71 Microalgal recovery of wastewater P for re-use in biodiesel production is another bio-based technology, but the economics of this route still remain unfavourable.104,105
3.5 The industrial sector
The waste-based production of white P, a key intermediate for many industrial products (flame retardants, plasticizers, battery ingredients, catalyst ligands, pharmaceuticals, lubricant additives, specialty fertilizers, herbicides and metals and electronics etchants) has been pioneered by Thermphos International and is subsequently being developed by the Recophos consortium (http://www.recophos.org). The raw material is sewage sludge ash (or other P-rich waste), which is converted to white phosphorus in a novel, lean manufacturing setup which shows a clear departure from the classical large scale furnace design practised for over a century. The process will be entering pilot stage in 2015. The processing route involves reduction of phosphates by means of coke to the element (P4), with carbon monoxide and a calcium silicate slag as sellable/usable by-products.Elemental P from this process, or the classical production route, serves as the base for a large number of derivatives. Processing routes include the intermediate product phosphorus trichloride (PCl3), the workhorse for organic P chemistry. The chlorine in this case merely serves as a means to activate the P atom and does not turn up in most – if not all – final products. Therefore, more direct and green routes to such derivatives are needed. Chlorine free routes have been pioneered by CNR-ICCOM, Thermphos International and are now being pursued by the SusPhos project, aimed at green non-phosphate (i.e. white P) based chemistry especially in fine chemistry applications (http://www.susphos.eu). For example, triphenylphosphine (TPP) is commonly used in the Wittig reaction for the industrial production of Vitamin A, carotenoids and many other alkenes. Yet, the P-containing by-product, triphenylphosphine oxide (TPPO), does not currently have a large-scale application, which results in thousands of tonnes of solid P-containing waste.106 SusPhos aims to achieve an economic recycling protocol to create a P waste-free Wittig reaction, which can be applied throughout a wide range of industrial processes. More generally, using white P in an atom efficient way remains a challenge for the non-fertilizer P industry. Designing products to allow re-use over and over again is also a goal for such industries.
High-grade PA is widely used in the chemical and manufacturing industries and a considerable portion could be recovered and recycled from different industrial wastes.11 In terms of quantity, P emitted into steel-making slag is a particularly important secondary P resource and is an input to the P cycle outside the usual PR-based inputs. Coal and iron ore, which are essential raw materials for the manufacture of iron and steel, contain small amounts of P (typically less than 0.03% P2O5 by weight). Since P has detrimental effects on the mechanical properties of steel, it is removed into dephosphorizing slag at concentrations as high as 2–10% P2O5 by weight.107 The global production of iron ore is approximately 2000 Tg year−1. Steel slags therefore contain approximately 0.6 Tg P year−1, which is equivalent to one-fifth of the annual world industrial P demand (2.8 Tg P year−1). Removing P from these slags would allow their iron content to be recovered in the process, adding economic benefits to this P recovery route. As elemental P is reported in some of these cases as a nuisance, its targeted recovery poses a challenge for green chemistry. The separation of iron and other metals from the product (P4 or PA) may be difficult to achieve.
3.6 Losses in the P cycle
Dissolved and particulate P transfer in land (urban and agricultural) runoff represents a considerable loss of P from the cycle. Whilst improvement in land management practices can help reduce these losses, by-product chemical amendments are increasingly viewed as a potential green chemistry strategy for prevention and/or control.108 Applications of moderate-charge-density anionic polyacrylamide (co-polymerised with sodium acrylate) to the soil surface, or in irrigation water, have been highly effective (up to 90%) at reducing sediment and nutrient losses in land runoff from agricultural land and from construction sites.109 The water-soluble polymer works by flocculating soil particles through co-bridging with divalent cations already present in the soil or co-applied. Stabilizing the soil in this way prevents surface crusting, increases water infiltration rates and reduces the risk of soil erosion, which is the main process of P loss in many situations. High P solubility in over-fertilized soils is also a significant eutrophication risk, which can be reduced by application of by-product chemical amendments that bind P. For example, coal combustion by-products from technologies to improve air quality, such as fluidized bed combustion fly ash and flue gas desulfurization gypsum, have been shown to be effective (20–40%) at reducing soluble P concentrations in soils without affecting plant P availability, or increasing soil or runoff contamination from heavy metals and arsenic.110 When targeted at the small critical source areas in catchments that generate the majority of the P loss, by-product re-use provides a cost-effective green chemistry approach to preventing P release to runoff. Similarly a range of natural materials (e.g. Fe-rich sand), synthetic filtration materials (e.g. calcinated clay) and industrial by-products (e.g. steel slag, gypsum, red mud) with high P binding capacity can be used to remove P in land runoff, providing the runoff can be channelled, the flow velocity is not too high and is sufficiently P-rich.102,108 For example from livestock hard standing areas.111 The recovered P is either directly suitable for re-use as a fertilizer, or the P is stripped from the binding agent using acid–base technology and the binding agent re-used. However, the amounts of recoverable P at each site are usually very small. Direct recovery of P from eutrophic waters using algae or aquatic plants, has also been considered with the advantage of also lowering eutrophication risk.112 Whether this is a feasible and worthwhile P recovery route in itself still needs to be established. The synergy between P removal and potentially using the harvested material as P source is the main advantage here.4. Designing a new food system
Progress towards greening the global P cycle also requires consideration of sector or process design, and utilization efficiency.22 Food and industry production systems could be better designed for maximum efficiency and to optimize recovery of secondary P as a substitute for primary PR; a key green chemistry goal. Agriculture dominates regional and global P flows, but it is an input-driven rather than a demand-led sector that overuses P unnecessarily because the P demands of the food chain have no governance. This leads to unsustainable P surpluses, continued P accumulation in soils and increased eutrophication risk, as is currently being evidenced most dramatically in China.113 Animal product consumption accounts for 72% of global dietary P demand and P demands could be reduced, and P efficiency substantially increased, by reducing meat consumption.36,114 For example, in Europe and the USA average dietary P intake is double actual P requirements due to a high proportion of meat in the diet.15,115 It may also be possible to reduce crop P requirements by 20–30% through breeding more P efficient plants.116,117 Reducing the intake and improving the utilization of P in animal feeds, for example through precision (phase) feeding, phytase addition and feeding low phytate crops, could reduce both P inputs and excretion rates by up to a third.118,119 The addition of the phytase enzyme allows the phytate in crops to be more effectively utilized by monogastrics without the need for inputs of highly-soluble feed P supplements. Refining livestock (and human) feeds to improve digestibility through other green biotechnologies could also help to reduce the volumes of livestock (and municipal) manure that requires recovery and recycling. For example the use of biorefinery to separate out the main constituents of feeds (proteins, enzymes and phosphates) to increase their digestibility and absorption.55 The microfiltration of liquid feeds to manipulate their P content might be another green chemistry option.Similarly fertilizer use efficiency could be increased at the field scale through improved product formulation design and by more precise application methods targeting the crop rather than the soil.120,121 The synthesis of nano-particle P fertilizers that are able to supply P to crops efficiently and with lower leaching risk represents a significant advance. Stable hydroxyapatite nanoparticles (15.5 nm in diameter) have been successfully synthesized using sodium carboxymethyl cellulose solution and used to fertilize soybean.122 Fertilizer P nanoparticles (28.2 nm in diameter) have also been recently biosynthesized from tricalcium phosphate using mycelium from the Aspergillus tubingensis fungus.123 The green engineering advantage of these nanoparticles is that they can be potentially be (bio)-synthesized from a range of secondary P products, and engineered so that the particle size (and hence P uptake rate) can be matched to the P uptake patterns of different crops, thereby improving P efficiency.
It has been suggested that society could potentially substitute at least 50% of its PR processing requirement based on recovered secondary P at the regional scale.15,18,45,77 Such predictions belie the huge financial and social challenges in developing and marketing recovered products that have suitable physical and chemical consistency, good P availability, are safe to use and are economically viable. In view of these constraints, progress in greening the global P cycle will be more realistically achieved in the short term through sector and regional level initiatives.21,29,53 For example, green chemistry principles have already been adopted in regional government policies towards circular and bio-based economies,13 and in sustainable food production initiatives such as Origin Green in Ireland.124 New smarter, more diversified and customer-focused business models are rapidly emerging in the mining industries that take account of the their wider social responsibilities, the need to raise safety and environmental standards and reduce wastage.27 For example, under a government-industry covenant, Amsterdam-based ICL has committed to substituting its entire PR feedstock, amounting to 0.5 Tg year−1 with secondary P, initially from human wastewater. Yet more ambitious are the aspirations of leading edge Chinese producer Wengfu, who have taken a top-down policy-led decision to reach zero waste by 2015, which includes 100% re-use of PG as ammonium sulphate and calcium carbonate.63 This has entailed restructuring the company from being a fertilizer only company to having three divisions, fertilizers, chemical products and construction materials. It has also started to recover a wide range of materials from phosphate ores, including 100 t year−1 of iodine.
5. Conclusions
Although it has taken at least one generation to realize the unintended consequences of PR processing, there is compelling economic, environmental and ethical justification for more efficient and sustainable use of P to safeguard PR resources and the environment for future generations. We conclude that the potential opportunities for green chemistry in achieving planetary P sustainability goals are large and fully consistent with societal migration to bio-based and circular economies based on smart science, practical policies and innovative technologies. We define three major areas for progress: (a) maximizing the economic and resource value of PR in fertilizer and other uses, (b) recover and recycle P from the vast array of secondary P resources though innovative green technologies so that they can increasingly be re-used as renewable materials in the future, and with minimum waste, and (c) provide P governance in the food chain and define more precisely what societal (end user) P requirements are to provide a firm foundation for designing and investing in new smarter demand-driven production systems that use only what is needed and with maximum efficiency and minimum P losses to the oceans.Acknowledgements
The authors are grateful to Prof. Dr E Meers, Ghent University, Belgium (Biorefine project), and Dr C. Slootweg and M. de Boer of Vrije Universiteit, Amsterdam, The Netherlands (SusPhos project) for helpful information in the preparation of this manuscript.Notes and references
- T. Lougheed, Environ. Health Perspect., 2011, 119(5), A208–A213 CrossRef PubMed.
- L. Reijnders, Resour., Conserv. Recycl., 2014, 93, 32–49 CrossRef PubMed.
- J. J. Elser, T. J. Elser, S. R. Carpenter and W. A. Brock, PLoS One, 2014, 9, e93998 Search PubMed.
- J. Cooper, R. Lombardi, D. Boardman and C. Carliell-Marquet, Resour. Conserv. Recycl., 2011, 57, 78–86 CrossRef PubMed.
- P. J. A. Withers, K. C. van Dijk, T.-S. S. Neset, T. Nesme, O. Oenema, G. H. Rubæk, O. F. Schoumans, A. L. Smit and S. Pellerin, AMBIO, 2015, 44(Suppl. 2), S163–S179 Search PubMed.
- L. Hein and R. Leemans, AMBIO, 2012, 41, 341–349 CrossRef CAS PubMed.
- V. H. Smith and D. W. Schindler, Trends Ecol. Evolut., 2009, 24, 201–207 CrossRef PubMed.
- B. Qin, G. Zhu, G. Gao, Y. Zhang, W. Li, H. W. Paerl and W. W. Carmichael, Environ. Manage., 2010, 45(1), 105–112 CrossRef PubMed.
- D. F. Millie, G. L. Fahnenstiel, J. D. Bressie, J. Dyble, R. J. Pigg, R. R. Rediske, D. M. Klarer, P. A. Tester and R. W. Litaker, Aquat. Ecol., 2009, 43(4), 915–934 CrossRef CAS.
- J. Elser and E. Bennett, Nature, 2011, 478, 29–31 CrossRef CAS PubMed.
- W. Schipper, Eur. J. Inorg. Chem., 2014, 1567–1571 CrossRef CAS.
- R. W. Scholz, A. H. Roy, F. S. Brand, D. T. Hellums and A. E. Ulrich, Sustainable Phosphorus Management: A Global Transdisciplinary Roadmap, Springer, Dordrecht, 2014 Search PubMed.
- E. C., Memo 14/377, http://europa.eu/rapid/press-release_IP-14-599_en.htm, 2014.
- M. A. Sutton, A. Bleeker, C. M. Howard, M. Bekunda, B. Grizzetti and W. de Vries, et al., Our Nutrient World: The challenge to produce more food and energy with less pollution, The Centre for Ecology and Hydrology, Edinburgh, U. K., 2013 Search PubMed.
- K. C. Van Dijk, J. P. Lesschen and O. Oenema, Sci. Tot. Environ. Search PubMed , In press.
- R. B. Chowdhury, G. A. Moore, A. J. Weatherley and M. Arora, Resour., Conserv. Recycl., 2014, 83, 213–228 CrossRef PubMed.
- D. Cordell, A. Rosemarin, J. J. Schroder and A. L. Smit, Chemosphere, 2011, 84, 747–758 CrossRef CAS PubMed.
- H. Ohtake and K. Okano, Global Environ. Res., 2015, 19(1), 1–30 Search PubMed.
- J. Hilton and C. Dawson, Waste Resour. Manage., 2012, 165(4), 179–189 CAS.
- V. Smil, Ann. Rev. Energy Environ., 2000, 25, 25–53 Search PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, UK, 1998 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 94A CrossRef.
- R. A. Sheldon, Chem. Commun., 2008, 3352–3365 RSC.
- W. Jiao, W. Chen, A. C. Chang and A. L. Page, Environ. Pollut., 2012, 168, 44–53 CrossRef CAS PubMed.
- K. Syers, A. E. Johnston and D. Curtin, FAO Fertilizer and Plant Nutrition Bulletin 18, Food and Agriculture Organization of the United Nations, Rome, Italy, 2008 Search PubMed.
- D. Seyhan, Resour., Conserv.. Recycl., 2009, 53(12), 698–709 CrossRef PubMed.
- R. Batterham, Procedia Eng., 2014, 83, 8–15 CrossRef PubMed.
- E. C., COM/2014/0398, http://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=CELEX:52014DC0398, 2014.
- J. Voorthuis and C. Gijbels, Sustainability, 2010, 2, 371–382 CrossRef PubMed.
- J. Hilton, A. E. Johnston and C. J. Dawson, The Phosphate Life-Cycle: Rethinking the options for a finite resource, Proceedings 668, International Fertiliser Society, York, U. K., 2010 Search PubMed.
- J. S. Guest, S. J. Skerlos, J. L. Barnard, M. B. Beck, G. T. Daigger and H. Hilger, et al. , Environ. Sci. Technol., 2009, 43(16), 6126–6130 CrossRef CAS.
- R. Sylvester-Bradley and P. J. A. Withers, Innovation in Crop Nutrition, Proceedings 700, International Fertiliser Society No. 700, Leek, UK, 2013 Search PubMed.
- J. Peñuelas, B. Poulter, J. Sardans, P. Ciais, M. van der Velde and L. Bopp, et al. , Nat. Commun., 2013, 4, 2934, DOI:10.1038/ncomms3934.
- K. Senthilkumar, T. Nesme, A. Mollier and S. Pellerin, Nutr. Cycling Agroecosyst., 2012, 92, 145–159 CrossRef.
- M. E. Schipanski and E. M. Bennett, Ecosystems, 2012, 15, 256–268 CrossRef CAS.
- G. S. Metson, E. M. Bennett and J. J. Elser, Environ. Res. Lett., 2012, 7, 044024 CrossRef.
- C. J. Dawson and J. Hilton, Food Policy, 2011, 36, S14–S22 CrossRef PubMed.
- M. Prud'Homme, World Phosphate Rock Flows, Losses and Uses, in Proceedings of Phosphate 2010 International Conference, Brussels, 2010 Search PubMed.
- L. Bouwman, K. K. Goldewijka, K. W. Van Der Hoekc, A. H. W. Beusena, D. P. Van Vuurena, J. Willemsa, M. C. Rufinoe and E. Stehfesta, Proc. Natl. Acad. Sci. U. S. A., 2011, 110(52), 20882–20887 CrossRef PubMed.
- H. Lamprecht, D. J. Lang, C. Binder, R. Scholz and W. Roland, GAIA, 2011, 20(2), 112–121 Search PubMed.
- A. C. Edwards and P. J. A. Withers, Soil Use Manage., 1998, 14(Suppl. 1), 124–130 CrossRef PubMed.
- A. Sharpley, H. P. Jarvie, A. Buda, L. May, B. Spears and P. Kleinman, J. Environ. Qual., 2013, 42, 1308–1326 CrossRef CAS PubMed.
- G. K. MacDonald, E. M. Bennett, P. A. Potterc and N. Ramankuttyd, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(7), 3086–3091 CrossRef CAS PubMed.
- H. Li, G. Huang, Q. Meng, L. Ma, L. Yuan, F. Wang, W. Zhang, Z. Cui, J. Shen, X. Chen, R. Jiang and F. Zhang, Plant Soil, 2011, 349, 157–167 CrossRef CAS.
- R. H. E. M. Koppelaar and H. P. Weikard, Global Environ. Change, 2013, 23, 1454–1466 CrossRef PubMed.
- E. M. Bennett, S. R. Carpenter and N. F. Caraco, Bioscience, 2001, 51(3), 227–234 CrossRef.
- S. Sattari, A. Bouwman, K. E. Giller and M. van Ittersum, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 6348–6353 CrossRef CAS PubMed.
- B. Ringeval, B. Nowak, T. Nesme, M. Delmas and S. Pellerin, Global Biogeochem. Cycles, 2014, 28, 743–756 CrossRef CAS.
- G. Villalba, Y. Liu, H. Schroder and R. U. Ayres, J. Ind. Ecol., 2008, 12(4), 557–569 CrossRef PubMed.
- G. van Drecht, A. F. Bouwman, J. Harrison and J. M. Knoop, Global Biogeochem. Cycles, 2009, 23, Gb0a03, DOI:10.1029/2009GB003458.
- T. Karak, R. M. Bhagat and P. Bhattacharyya, Crit. Rev. Environ. Sci. Technol., 2012, 42(15), 1509–1630 CrossRef CAS.
- W. J. Schipper, A. Klapwijk, B. Potjer, W. H. Rulkens, B. G. Temmink, F. D. G. Kiestra and A. C. M. Lijmbach, Environ. Technol., 2001, 22, 1337–1345 CrossRef CAS PubMed.
- O. Oenema, W. Chardon, P. Ehlert, K. van Dijk and O. Schoumans, Phosphorus Fertilizers from By-Products and Wastes, Proceedings 717, International Fertiliser Society, Leek, UK, 2012 Search PubMed.
- H. Ohtake, in Biochemical Engineering and Bioprocessing, Wiley, 2015, vol. 3, pp. 1–15 Search PubMed.
- O. F. Schoumans, F. Bouraoui, C. Kabbe, O. Oenema and K. van Dijk, AMBIO, 2015, 44(Suppl. 2), 180–192 CrossRef PubMed.
- E. Desmidt, K. Ghyselbrecht, Y. Zhang, L. Pinoy, B. van der Bruggen, W. Verstraete, K. Rabaey and B. Meesschaert, Crit. Rev. Environ. Sci. Technol., 2015, 45, 336–384 CrossRef CAS.
- P. Westerhoff, S. Lee, Y. Yang, G. W. Gordon, K. Hristovski, R. U. Halden and P. Herckes, Environ. Sci. Technol., 2015 DOI:10.1021/es505329q.
- J. H. Clark, L. A. Pfaltzgraff, V. L. Budarin, A. J. Hunt, M. Gronnow, A. S. Matharu, D. J. Macquarrie and J. R. Sherwood, Pure Appl. Chem., 2013, 85(8), 1625–1631 CrossRef CAS.
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- M.-P. Zacharof and R. W. Lovitt, Waste Biomass Valorization, 2013, 4, 557–581 CrossRef CAS PubMed.
- J. Hilton, B. K. Birky and M. Moussaid, Comprehensive Extraction, a Key Requirement for Social Licensing of NORM Industries? Proceedings, NORM 7, Beijing, China, 2013 Search PubMed.
- P. Christmann, Procedia Eng., 2014, 83, 19–26 CrossRef CAS PubMed.
- P. Zhang, Procedia Eng., 2014, 83, 37–51 CrossRef CAS PubMed.
- Anon, JDC Phosphate starts hot operations, Fert. Int., 2014, 458, 40–41 Search PubMed.
- IAEA, Radiation Protection and Management of NORM Residues in the Phosphate Industry, Safety Reports Series No. 78, International Atomic Energy Agency, Vienna, Austria, 2013 Search PubMed.
- G. H. Rubaek, K. Kristensen, S. E. Olesen, H. S. Østergaard and G. Heckrath, Geoderma, 2013, 209–210, 241–250 CrossRef CAS PubMed.
- A. R. Richardson and R. J. Simpson, Plant Physiol., 2011, 156, 989–996 CrossRef CAS PubMed.
- L. Ramaekers, R. Remans, I. M. Rao, M. W. Blaier and J. Vanderleyden, Field Crop Res., 2010, 117, 169–176 CrossRef PubMed.
- A. Bateman, H. van der Horst, D. Boardman, A. Kansal and C. Carlliel-Marquet, Resour., Conserv. Recycl., 2011, 55, 1146–1153 CrossRef PubMed.
- K. Suzuki, Y. Tanaka, K. Kuroda, D. Hanajima, Y. Fukumoto, T. Yasuda and M. Waki, Bioresour. Technol., 2007, 98, 1573–1578 CrossRef CAS PubMed.
- M. L. Gerardo, M. P. Zacharof and R. W. Lovitt, Water Res., 2013, 47, 4833–4842 CrossRef CAS PubMed.
- P. J. A. Kleinman, A. N. Sharpley, A. M. Wolf, D. B. Beegle and P. A. Moore Jr., Soil Sci. Soc. Am. J., 2002, 66, 2009–2015 CrossRef CAS.
- T. Wang, M. Camps-Arbestain and M. Hedley, Plant Soil, 2014, 375, 61–74 CrossRef CAS.
- Z. Liu and R. Balasubramanian, Appl. Energy, 2014, 114, 857–864 CrossRef CAS PubMed.
- K. S. Ro, K. B. Cantrell, P. G. Hunt, T. F. Ducey, M. B. Vanotti and A. A. Szogi, Bioresour. Technol., 2009, 100, 5466–5471 CrossRef CAS PubMed.
- C. J. De Vos and L. Heres, Risk Anal., 2009, 29(4), 541–557 CrossRef PubMed.
- C. Kabbe, in Factor X. Re-source – Designing the Recycling Society, Eco-Efficiency in Industry and Science, ed. M. Angrich, A. Burger and H. Lehmann, Springer, Dordrecht, The Netherlands, 2013, vol. 30, pp. 261–273 Search PubMed.
- A. Giesen, Environ. Technol., 1999, 20(7), 769–775 CrossRef CAS.
- D. Gunders, Wasted: How America is Losing Up to 40 Percent of Its Food from Farm to Fork to Landfill, NRDC, Washington, USA, 2012 Search PubMed.
- J. Parfitt, M. Barthel and S. Macnaughton, Philos. Trans. R. Soc. London, Ser. B, 2010, 365, 3065–3081 CrossRef PubMed.
- L. A. Pfaltzgraff, M. de Bruyn, E. C. Cooper, V. Budarin and J. H. Clark, Green Chem., 2013, 15, 307–314 RSC.
- S. R. Smith, Philos. Trans. R. Soc. London, Ser. A, 2009, 367, 4005–4041 CrossRef CAS PubMed.
- H. A. Elliott and G. A. O'Connor, Soil Biol. Biochem., 2007, 39, 1318–1327 CrossRef CAS PubMed.
- W. Pronk and D. Kone, Desalination, 2009, 248(1–3), 360–368 CrossRef CAS PubMed.
- B. Etter, et al. , Water Res., 2011, 45(2), 852–862 CrossRef CAS PubMed.
- J. R. Mihelcic, L. M. Fry and R. Shaw, Chemosphere, 2011, 84(6), 832–839 CrossRef CAS PubMed.
- J. Lienert and T. A. Larsen, Environ. Sci. Technol., 2009, 44(2), 556–566 CrossRef PubMed.
- C. Adam, B. Peplinski, M. Michaelis, G. Kley and F. G. Simon, Waste Manage., 2009, 29, 1122–1128 CrossRef CAS PubMed.
- Z. Tan and A. Lagerkvist, Renewable Sustainable Energy Rev., 2011, 15, 3588–3602 CrossRef CAS PubMed.
- R. Hirota, A. Kuroda, J. Kato and H. Ohtake, J. Biosci. Bioeng., 2010, 109, 423–432 CrossRef CAS PubMed.
- A. Kuroda, N. Takiguchi, T. Gotanda, K. Nomura, J. Kato, T. Ikeda and H. Ohtake, Biotechnol. Bioeng., 2002, 78, 333–338 CrossRef CAS PubMed.
- P. L. Bond, L. Keller and L. L. Blackall, Biotechnol. Bioeng., 1999, 63, 507–515 CrossRef CAS.
- Y. Cao and A. Pawlowski, Renewable Sustainable Energy Rev., 2012, 16, 1657–1665 CrossRef CAS PubMed.
- A. Petterssona, L. E. Amand and B. M. Steenari, Biomass Bioenergy, 2008, 32, 224–235 CrossRef PubMed.
- S. Donatello and C. R. Cheeseman, Waste Manage., 2013, 33, 2328–2340 CrossRef CAS PubMed.
- Y. Song, P. G. Weidler, U. Berg, R. Nüesch and D. Donnert, Chemosphere, 2006, 63, 236–243 CrossRef CAS PubMed.
- N. Marti, L. Pastor, A. Bouzas, J. Ferrer and A. Seco, Water Res., 2010, 44, 2371–2379 CrossRef CAS PubMed.
- K. Okano, M. Uemoto, J. Kagami, K. Miura, T. Aketo, M. Toda, K. Honda and H. Ohtake, Water Res., 2013, 47, 2251–2259 CrossRef CAS PubMed.
- K. Okano, S. Miyamaru, A. Kitao, H. Takano, T. Aketo, M. Toda, K. Honda and H. Ohtake, Sep. Prif. Technol., 2015, 144, 63–69 CrossRef CAS PubMed.
- D. R. Kioussis, F. W. Wheaton and P. Kofinas, Agric. Eng., 2000, 23, 315–332 Search PubMed.
- I. Midorikawa, H. Aoki, A. Ohmori, T. Shimizu, Y. Kawaguchi, K. Kassai and T. Murakami, Proc. 5th IWA Leading-Edge Conference on Water and Wastewater Technologies, 2008.
- P. Loganathan, S. Vigneswaran, J. Kandasamy and N. S. Bolan, Crit. Rev. Environ. Sci. Technol., 2014, 44, 847–907 CrossRef CAS.
- B. E. Rittmann, B. Mayer, P. Westerhoff and M. Edwards, Chemosphere, 2011, 84, 846–853 CrossRef CAS PubMed.
- J. K. Pittman, A. P. Dean and O. Osundeko, Bioresour. Technol., 2011, 102, 17–25 CrossRef CAS PubMed.
- W. Mulbry, S. Kondrad, C. Pizarro and E. Kebede-Westhead, Bioresour. Technol., 2008, 99, 8137–8142 CrossRef CAS PubMed.
- S. P. Marsden, Nat. Chem., 2009, 1, 685–687 CrossRef CAS PubMed.
- E. Yamasue, K. Matsubae, K. Nakajima, S. Hashimoto and T. Nagasaka, J. Ind. Ecol., 2013, 17, 722–730 CrossRef CAS PubMed.
- A. R. Buda, G. F. Koopmans, R. B. Bryant and W. J. Chardon, J. Environ. Qual., 2012, 41, 621–627 CrossRef CAS PubMed.
- J. A. Entry and R. E. Sojka, Trans. Am. Soc. Agric. Eng., 2003, 46, 75–83 CAS.
- W. L. Stout, A. N. Sharpley and J. Landa, J. Environ. Qual., 2000, 29, 1239–1244 CrossRef CAS.
- C. Penn, J. McGrath, J. Bowen and S. Wilson, J. Soil Water Conserv., 2014, 69(2), 51A–56A CrossRef.
- R. S. Quilliam, M. A. van Niekerk, D. R. Chadwick, P. Cross, N. Hanley, D. L. Jones, A. J. A. Vinten, N. Willby and D. M. Oliver, J. Environ. Manage. DOI:10.1016/j.jenvman.2015.01.046.
- L. Ma, G. L. Velthof, F. H. Wang, W. Qin, W. F. Zhang, Z. Liu, Y. Zhang, J. Wei, J. P. Lesschen, W. Q. Ma, O. Oenema and F. S. Zhang, Sci. Total Environ., 2012, 434, 51–61 CrossRef CAS PubMed.
- S. D. Donner, Global Environ. Change, 2007, 17, 105–113 CrossRef PubMed.
- A. Flynn, T. Hirvonen, G. B. M. Mensink, M. C. Ocke, L. Serra-Majem, K. Stos, L. Szponar and I. Tetens, et al. , Food Nutr. Res., 2009, 53, 1–51 Search PubMed.
- T. J. Rose, J. Pariasca-Tanakaa, M. T. Rosea, Y. Fukutab and M. Wissuwaa, Field Crops Res., 2010, 119, 154–160 CrossRef PubMed.
- V. Raboy, Plant Sci., 2009, 177, 281–296 CrossRef CAS PubMed.
- R. O. Maguire, D. A. Crouse and S. C. Hodges, J. Environ. Qual., 2007, 36, 1235–1240 CrossRef CAS PubMed.
- E. Kebreab, A. V. Hansen and A. B. Strathe, Curr. Opin. Biotechnol., 2012, 23, 872–877 CrossRef CAS PubMed.
- P. J. A. Withers, R. Sylvester-Bradley, D. L. Jones, J. R. Healey and P. J. Talboys, Environ. Sci. Technol., 2014, 48, 6523–6530 CrossRef CAS PubMed.
- M. J. McLaughlin, T. M. McBeath, R. Smernik, S. P. Stacey, B. Ajiboye and C. Guppy, Plant Soil, 2012, 349, 69–87 CrossRef.
- R. Q. Liu and R. Lal, Sci. Rep., 2014, 4, 5686, DOI:10.1038/srep05686.
- J. C. Tarafdar, R. Raliya and I. Rathore, J. Bionanosci., 2012, 6, 1–6 CrossRef PubMed.
- Origin Green, http://www.origingreen.ie, 2014.
| This journal is © The Royal Society of Chemistry 2015 |
