Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot triangular chemoenzymatic cascades for the syntheses of chiral alkaloids from dopamine†
B. R.
Lichman
 a,
E. D.
Lamming
b,
T.
Pesnot
b,
J. M.
Smith
a,
H. C.
Hailes
*b and
J. M.
Ward
*a
a,
E. D.
Lamming
b,
T.
Pesnot
b,
J. M.
Smith
a,
H. C.
Hailes
*b and
J. M.
Ward
*a
aDepartment of Biochemical Engineering, University College London, Bernard Katz Building, Gordon Street, London, WC1H 0AH, UK. E-mail: j.ward@ucl.ac.uk
bDepartment of Chemistry, University College London, Christopher-Ingold Building, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: h.c.hailes@ucl.ac.uk
First published on 18th December 2014
Abstract
We describe novel chemoenzymatic routes to (S)-benzylisoquinoline and (S)-tetrahydroprotoberberine alkaloids using the enzymes transaminase (TAm) and norcoclaurine synthase (NCS) in a one-pot, one-substrate ‘triangular’ cascade. Employment of up to two C–C bond forming steps allows for the rapid generation of molecular complexity under mild conditions.
Introduction
In order to minimise waste products and reduce the usage of finite resources, chemistry must find ‘green’ alternatives to traditional synthetic methods.1 The employment of enzymes as catalysts offers significant potential in this regard as enzymes often demonstrate exquisite chemo- and enantio-selectivity, and operate in mild conditions.2 Furthermore, using enzymes in one-pot cascades can enable the formation of highly complex compounds from cheap starting materials, frequently without the requirement of intermediate isolation or functional group protection strategies.3Benzylisoquinoline alkaloids (BIAs) are a large, diverse family of natural products found in both plants4 and animals.5 Many BIAs have pharmacological activities—these include analgesic,6 antimicrobial7 and antitumor8 effects—and consequently are common synthetic targets.9 A key step in the synthesis of many of these compounds is the formation of the tetrahydroisoquinoline (THIQ) moiety via a Pictet–Spengler condensation.10 Recently, a mild one-pot biomimetic approach to the formation of THIQs was developed, using phosphate buffer as a catalyst.11 There has also been recent progress made in the enzymatic synthesis of diverse chiral THIQs using the plant enzyme norcoclaurine synthase (NCS).12
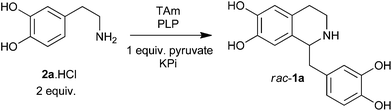
Norlaudanosoline (tetrahydropapaveroline) 1a is a BIA found in mammals: it is the precursor to ‘endogenous’ morphine,5 and also has a number of neuronal effects including roles in Parkinson's disease13 and drug addiction.14 Synthetically, 1a is a versatile BIA precursor, and consequently it has been employed in synthetic biology/metabolic engineering routes to various BIAs.15 BIA 1a can be accessed by a Pictet–Spengler condensation between dopamine 2a and 3,4-dihydoxyphenylacetaldehyde 3a.16
Tetrahydroprotoberberine alkaloids (THPBs, berbines) are a subgroup of the BIAs found in both plants and animals, and include compounds such as canadine17 and spinosine.18a The THPB moiety can be accessed from 1avia a Pictet–Spengler reaction with formaldehyde.18 The two compounds directly formed by this reaction (4 and 5) have been shown to have potential in cancer chemotherapeutics.19 The regioselectivity of this chemical Pictet–Spengler reaction (major product: 10,11-dihydroxy 4) contrasts with the regioselectivity of the plant berberine-bridge enzyme (BBE), which typically forms 9,10-dihydroxy-THPBs from a N-methylated BIA precursor.20 Thus chemical approaches to THPBs provide a complementary method to the reported BBE approach.
Triangular cascade design
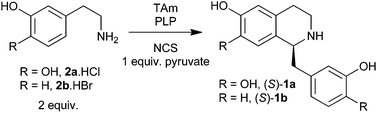
In this work, we present one-pot two-enzyme one-substrate syntheses of (S)-1a and (S)-1b using the enzymes transaminase (TAm) and NCS. The reaction involves in situ generation and utilisation of reactive aldehyde species. We also demonstrate a one-pot, two-enzyme, three-step chemoenzymatic synthesis of the THPB (S)-4. This synthesis uses the same cascade route as for (S)-1a, but involves the sequential addition of formaldehyde to trigger a second Pictet–Spengler cyclisation (Scheme 1).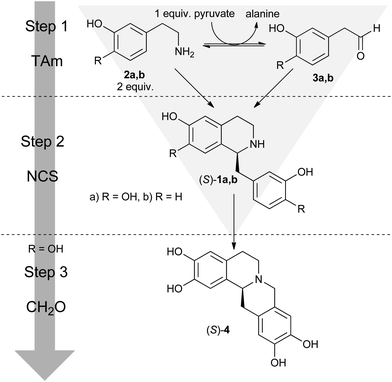 | ||
| Scheme 1 Overview of the biocatalytic and non-enzymatic cascades presented in this work, including the ‘triangular’ cascade. | ||
Previous attempts to achieve this type of cascade in vitro and in vivo have utilised a monoamine oxidase (MAO) as the catalyst in the first step, converting 2a to 3a. Several MAO approaches have focused on forming rac-1a through a spontaneous Pictet–Spengler reaction (typically phosphate catalysis).16 A number of in vivo metabolic cascades forming (S)-reticuline report the use of recombinant NCS as the Pictet–Spengler catalyst. However, it is not clear that NCS is active in these systems: any chirality reported seems to be the result of (S)-selective enzymes downstream of NCS.15a–d
Here, we employ a TAm for the conversion of 2a to 3a (step 1), which then combine to form 1a (step 2). TAms have an advantage over MAOs in this system: the conversion of 2a to 3a with TAms can be controlled by stoichiometrically limiting the quantity of co-substrate (in this instance, pyruvate) available. Furthermore, we avoid the problems associated with driving TAm reactions to completion, as the Pictet–Spengler reaction removes equal quantities of molecules from both sides of the TAm equilibrium. This three-sided ‘triangular’ cascade design results in a system with high atom economy.3
Results and discussion
The first step towards establishing the one-pot cascade system was the identification of a TAm with good activity towards 2a. The screening reaction was conducted in phosphate buffer, which enabled the in situ formation of rac-1a from 2a and 3a (Table 1). The screen identified two TAms with activity towards 2a at pH 7.5: CV2025 (Chromobacterium violaceum)21 and PP_3718 (Pseudomonas putida), providing 21% and 15% conversion of 1a from 50 mM 2a respectively. Trace activities were found in PP_0596 (P. putida), SaV_2612 (Streptomyces avermitilis) and VF_JS17 (Vibrio fluvialis)22 (Table 1, ESI† Fig. S1). We selected the best performing TAm, CV2025, for use in further studies.| Entry | TAm | Organism | Uniprot entry name | Gene name | Conv.b (%) |
|---|---|---|---|---|---|
| a 50 mM 2a, 25 mM pyruvate, 1 mM PLP, 10% v.v−1 TAm, 37 °C, 4 h. b Concentration of rac-1a, determined by analytical HPLC. c n.d. = not detected. See ESI Fig. S1 for reaction time course. | |||||
| 1 | Empty vector | n.d.c | |||
| 2 | BSU_09260 | Bacillus subtilis | YHXA_BACSU | yhxA | n.d. |
| 3 | CV_202521 | Chromobacterium violaceum | Q7NWG4_CHRVO | CV_2025 | 21 |
| 4 | Dgeo_1416 | Deinococcus geothermalis | Q1IYH3_DEIGD | argD/lysJ | n.d. |
| 5 | KPN_00255 | Klebsiella pneumoniae | A6T537_KLEP7 | gabT | n.d. |
| 6 | PP_0596 | Pseudomonas putida | Q88Q98_PSEPK | PP_0596 | Trace |
| 7 | PP_3718 | Pseudomonas putida | Q88GK3_PSEPK | PP_3718 | 14 |
| 8 | SaV_2612 | Streptomyces avermitilis | Q82JZ2_STRAW | SAV_2612 | Trace |
| 9 | SaV_4551 | Streptomyces avermitilis | Q82ER2_STRAW | SaV_4551 | n.d. |
| 10 | VF_JS1722 | Vibrio fluvialis | F2XBU9_VIBFL | JS17 | Trace |
In order to form (S)-1a from 2a two aspects of the previous TAm screening reaction conditions were modified. Changing the buffer from phosphate to HEPES removed most of the background chemical reaction11 and adding purified Δ29TfNCS enabled catalytic formation of the chiral product (S)-1a from 2a. Optimum reaction conditions were determined by varying the concentrations of 2a, NCS and TAm present. A balance between reaction components was crucial to ensure the rates of the two steps were matched; a build-up of 3a would cause an increase in undesirable side-reactions including the non-enzymatic Pictet–Spengler condensation, which would reduce the final enantiomeric excess (ee) of the product.
BIA (S)-1a was produced from 2a with very good conversions and excellent enantioselectivity. In all cases, an increase in TAm concentration resulted in a greater consumption of 2a, generally leading to an increase in product formation (Table 2). However, at lower concentrations of 2a (20 mM) the use of 20% v.v−1 CV2025 lysate, rather than 30%, was optimal. The enantioselectivity was excellent under all conditions. In terms of conversion, the effect of NCS concentration was more apparent with higher concentrations of dopamine. The best conditions identified were those with 20 mM 2a, 500 μg mL−1 NCS and 20% v.v−1 CV2025 lysate, which provided an 87% conversion of (S)-1a from 2a with an ee of 99%.
| Amine [mM] | CV2025 (v.v−1) [%] | NCS [μg mL−1] | Conv.b [%] | eec [%] | |
|---|---|---|---|---|---|
| a General reaction conditions: 2 equivalents 2a or 2b, 1 equivalent pyruvate, purified Δ29TfNCS and CV2025 lysate, 50 mM HEPES pH 7.5, 37 °C, 3 h. b Determined by analytical HPLC. Conversions in brackets refer to depletion of primary amine. c Determined by chiral HPLC of the crude product. | |||||
| 2a | 20 | 10 | 100 | 74 (74) | 99 |
| 2a | 20 | 20 | 100 | 86 (89) | 99 |
| 2a | 20 | 30 | 100 | 82 (93) | 99 |
| 2a | 20 | 10 | 500 | 77 (82) | 99 |
| 2a | 20 | 20 | 500 | 87 (92) | 99 |
| 2a | 20 | 30 | 500 | 84 (95) | 98 |
| 2a | 50 | 10 | 100 | 36 (42) | 98 |
| 2a | 50 | 20 | 100 | 55 (66) | 97 |
| 2a | 50 | 30 | 100 | 70 (78) | 99 |
| 2a | 50 | 10 | 500 | 42 (48) | 98 |
| 2a | 50 | 20 | 500 | 65 (73) | 98 |
| 2a | 50 | 30 | 500 | 72 (85) | 96 |
| 2b | 20 | 20 | 500 | 56 (57) | 90 |
To demonstrate the versatility of this system, it was used to synthesise (S)-1b from 2-(3-hydroxyphenyl)ethylamine 2b. The para-hydroxyl group of 2a is not required for the Pictet–Spengler condensation, and thus 2b is a suitable substrate for NCS.12b Using the optimum conditions determined previously for 2a, (S)-1b was formed from 2b with a fair conversion of 56% and a high ee of 90%. The lower conversion and ee compared to (S)-1a is possibly reflective of a poorer affinity of TfNCS towards for 2b compared to the natural substrate 2a.
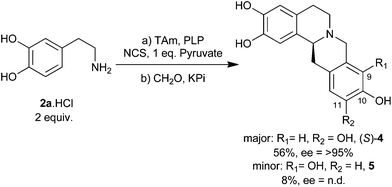
In order to form THPBs (S)-4 and (S)-5, a one-pot synthesis of (S)-1a was conducted (as described above) and formaldehyde was added to the reaction after 3 hours. This triggered the second Pictet–Spengler condensation and resulted in the formation of (S)-4 and (S)-5 in a ratio of approximately 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 2). This cascade features 2 enzymes, 3 steps and the formation of 4 bonds, including 2 C–C bonds. The overall reaction occurred with good conversion: the second Pictet–Spengler step alone (from (S)-1a) provided 74% conversion (64% and 9% for (S)-4 and (S)-5 respectively), which translates as an overall 64% conversion (56% and 8%) from 2a. As the chirality of the system is established by NCS, the subsequent addition of formaldehyde does not affect the ee: only the (S)-enantiomer of the major product 4 was observed by chiral HPLC.
1 (Scheme 2). This cascade features 2 enzymes, 3 steps and the formation of 4 bonds, including 2 C–C bonds. The overall reaction occurred with good conversion: the second Pictet–Spengler step alone (from (S)-1a) provided 74% conversion (64% and 9% for (S)-4 and (S)-5 respectively), which translates as an overall 64% conversion (56% and 8%) from 2a. As the chirality of the system is established by NCS, the subsequent addition of formaldehyde does not affect the ee: only the (S)-enantiomer of the major product 4 was observed by chiral HPLC.
The major regioisomer 4 formed by this method has 10,11-dihydroxy regiochemistry. BBE, a plant enzyme which catalyses the formation of (S)-THPBs en route to berberine, typically forms products with 9,10-dihydroxy regioselectivity.20 This enzyme has recently been used in a novel in vitro deracemisation cascade to produce (S)-THPBs.20b The synthesis presented here and the BBE catalysed cascade can be seen as complementary methods, providing efficient chemoenzymatic routes to 10,11-dihydroxy-(S)-THPBs and 9,10-dihydroxy-(S)-THPBs respectively.
Finally, to demonstrate the preparative potential of our cascades, we performed syntheses of (S)-1a and (S)-4 on a 0.5 mmol scale. The concentrations of components in these cascades were the same as those used in the micro-scale cascades (Scheme 2). The synthesis scaled with no complications: conversion of 2a to (S)-1a was achieved in 2 hours with 86% conversion and 62% isolated yield (>95% ee). (S)-4 was formed from 2a in 2.5 hours with 47% conversion and 42% isolated yield (>95% ee). The identities of these products were verified by NMR.
Conclusions
In summary, we have developed one-pot syntheses of chiral alkaloids, using the enzymes TAm and NCS in a ‘triangular’ cascade. BIA (S)-1a was formed from 2a with very good conversion and excellent enantioselectivity in only 2 hours. (S)-1b was formed in a similar manner with fair conversion and a high ee. A chemical extension to the cascade enabled the formation of (S)-4 in a one-pot three-step reaction from the low-cost starting material 2a, again with excellent enantioselectivity and an overall reaction time of less than 3 hours.The cascades presented here exhibit high atom economy, the in situ generation of reactive intermediates, and the rapid accumulation of molecular complexity through C–C bond forming steps. Overall, these syntheses demonstrate the remarkable potential of in vitro biocatalysis for the formation of complex chiral compounds.
We gratefully acknowledge the Wellcome Trust for studentship funding to B. R. L., EPSRC and GlaxoSmithKline for studentship funding to E. D. L., and the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/G014426/1) for funding T. P.
Notes and references
- (a) J. H. Clark, Green Chem., 1999, 1, 1–8 RSC; (b) R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233–1246 CrossRef CAS.
- B. M. Nestl, S. C. Hammer, B. A. Nebel and B. Hauer, Angew. Chem., Int. Ed., 2014, 53, 3070–3095 CrossRef CAS PubMed.
- For reviews see: (a) E. Ricca, B. Brucher and J. H. Schrittwieser, Adv. Synth. Catal., 2011, 353, 2239–2262 CrossRef CAS; (b) R. C. Simon, N. Richter, E. Busto and W. Kroutil, ACS Catal., 2014, 4, 129–143 CrossRef CAS.
- J. M. Hagel and P. J. Facchini, Plant Cell Physiol., 2013, 54, 647–672 CrossRef CAS PubMed.
- (a) C. Poeaknapo, J. Schmidt, M. Brandsch, B. Dräger and M. H. Zenk, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14091–14096 CrossRef CAS PubMed; (b) C. Boettcher, M. Fellermeier, C. Boettcher, B. Dräger and M. H. Zenk, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 8495–8500 CrossRef CAS PubMed; (c) N. Grobe, M. Lamshöft, R. G. Orth, B. Dräger, T. M. Kutchan, M. H. Zenk and M. Spiteller, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 8147–8152 CrossRef CAS PubMed.
- A. J. Goodman, B. Le Bourdonnec and R. E. Dolle, ChemMedChem, 2007, 2, 1552–1570 CrossRef CAS PubMed.
- K. Iwasa, M. Moriyasu, Y. Tachibana, H. Kim, Y. Wataya, W. Wiegrebe, K. F. Bastow, L. M. Cosentino, M. Kozuka and K.-H. Lee, Bioorg. Med. Chem., 2001, 9, 2871–2884 CrossRef CAS.
- P. Jabbarzadeh Kaboli, A. Rahmat, P. Ismail and K.-H. Ling, Eur. J. Pharmacol., 2014, 740, 584–595 CrossRef CAS PubMed.
- M. Chrzanowska and M. D. Rozwadowska, Chem. Rev., 2004, 104, 3341–3370 CrossRef CAS PubMed.
- J. Stöckigt, A. P. Antonchick, F. Wu and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 8538–8564 CrossRef PubMed.
- T. Pesnot, M. C. Gershater, J. M. Ward and H. C. Hailes, Chem. Commun., 2011, 47, 3242–3244 RSC.
- (a) A. Bonamore, I. Rovardi, F. Gasparrini, P. Baiocco, M. Barba, C. Molinaro, B. Botta, A. Boffi and A. Macone, Green Chem., 2010, 12, 1623–1627 RSC; (b) T. Pesnot, M. C. Gershater, J. M. Ward and H. C. Hailes, Adv. Synth. Catal., 2012, 354, 2997–3008 CrossRef CAS; (c) B. M. Ruff, S. Bräse and S. E. O'Connor, Tetrahedron Lett., 2012, 53, 1071–1074 CrossRef CAS PubMed; (d) M. Nishihachijo, Y. Hirai, S. Kawano, A. Nishiyama, H. Minami, T. Katayama, Y. Yasohara, F. Sato and H. Kumagai, Biosci. Biotechnol. Biochem., 2014, 78, 701–707 CrossRef CAS PubMed; (e) J. J. Maresh, S. O. Crowe, A. a. Ralko, M. D. Aparece, C. M. Murphy, M. Krzeszowiec and M. W. Mullowney, Tetrahedron Lett., 2014, 55, 5047–5051 CrossRef CAS PubMed.
- M. A. Collins, Neurotoxicology, 2004, 25, 117–120 CrossRef CAS.
- L. Yao, P. Fan, M. Arolfo, Z. Jiang, M. F. Olive, J. Zablocki, H.-L. Sun, N. Chu, J. Lee, H.-Y. Kim, K. Leung, J. Shryock, B. Blackburn and I. Diamond, Nat. Med., 2010, 16, 1024–1028 CrossRef CAS PubMed.
- For examples which synthesise norlaudanosoline in situ: (a) H. Minami, J.-S. Kim, N. Ikezawa, T. Takemura, T. Katayama, H. Kumagai and F. Sato, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7393–7398 CrossRef CAS PubMed; (b) A. Nakagawa, H. Minami, J.-S. Kim, T. Koyanagi, T. Katayama, F. Sato and H. Kumagai, Nat. Commun., 2011, 2, 326, DOI:10.1038/ncomms1327; (c) A. Nakagawa, H. Minami, J.-S. Kim, T. Koyanagi, T. Katayama, F. Sato and H. Kumagai, Bioeng. Bugs, 2012, 3, 49–53 Search PubMed; (d) J.-S. Kim, A. Nakagawa, Y. Yamazaki, E. Matsumura, T. Koyanagi, H. Minami, T. Katayama, F. Sato and H. Kumagai, Biosci. Biotechnol. Biochem., 2013, 77, 2166–2168 CrossRef CAS PubMed; examples which use racemic norlaudanosoline as a feedstock: (e) K. M. Hawkins and C. D. Smolke, Nat. Chem. Biol., 2008, 4, 564–573 CrossRef CAS PubMed; (f) E. Fossati, A. Ekins, L. Narcross, Y. Zhu, J.-P. Falgueyret, G. A. W. Beaudoin, P. J. Facchini and V. J. J. Martin, Nat. Commun., 2014, 5, 3283, DOI:10.1038/ncomms4283.
- (a) P. Holtz, K. Stock and E. Westermann, Nature, 1964, 203, 656–658 CrossRef CAS; (b) L. K. Hoover, M. Moo-Young and R. L. Legge, Biotechnol. Bioeng., 1991, 38, 1029–1033 CrossRef CAS PubMed; (c) A. Nakagawa, C. Matsuzaki, E. Matsumura, T. Koyanagi, T. Katayama, K. Yamamoto, F. Sato, H. Kumagai and H. Minami, Sci. Rep., 2014, 4, 6695, DOI:10.1038/srep06695.
- E. F. Queiroz, F. Roblot, A. Cavé, M. e Q. Paulo and A. Fournet, J. Nat. Prod., 1996, 7, 438–440 CrossRef PubMed.
- (a) K. Iwasa, W. Cui, T. Takahashi, Y. Nishiyama, M. Kamigauchi, J. Koyama, A. Takeuchi, M. Moriyasu and K. Takeda, J. Nat. Prod., 2010, 73, 115–122 CrossRef CAS PubMed; (b) K. D. McMurtrey, L. R. Meyerson, J. L. Cashaw and V. E. Davis, J. Org. Chem., 1984, 49, 947–948 CrossRef CAS.
- W. Cui, K. Iwasa, H. Tokuda, A. Kashihara, Y. Mitani, T. Hasegawa, Y. Nishiyama, M. Moriyasu, H. Nishino, M. Hanaoka, C. Mukai and K. Takeda, Phytochemistry, 2006, 67, 70–79 CrossRef CAS PubMed.
- (a) J. H. Schrittwieser, V. Resch, S. Wallner, W.-D. Lienhart, J. H. Sattler, J. Resch, P. Macheroux and W. Kroutil, J. Org. Chem., 2011, 76, 6703–6714 CrossRef CAS PubMed; (b) J. H. Schrittwieser, B. Groenendaal, V. Resch, D. Ghislieri, S. Wallner, E.-M. Fischereder, E. Fuchs, B. Grischek, J. H. Sattler, P. Macheroux, N. J. Turner and W. Kroutil, Angew. Chem., Int. Ed., 2014, 53, 3731–3734 CrossRef CAS PubMed.
- U. Kaulmann, K. Smithies, M. E. B. Smith, H. C. Hailes and J. M. Ward, Enzyme Microb. Technol., 2007, 41, 628–637 CrossRef CAS PubMed.
- J.-S. Shin, H. Yun, J.-W. Jang, I. Park and B.-G. Kim, Appl. Microbiol. Biotechnol., 2003, 61, 463–471 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc02325k |
| This journal is © The Royal Society of Chemistry 2015 |