Spiers Memorial Lecture
New tools for observing the growth and assembly of colloidal inorganic nanocrystals†
A.
Paul Alivisatos
*abc,
Hoduk
Cho
abc and
Jungwon
Park
de
aDepartment of Chemistry, University of California, Berkeley, CA 94720, USA
bMaterials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
cKavli Energy NanoScience Institute, Berkeley, CA 94720, USA
dDepartment of Applied Physics, Harvard University, Cambridge, MA 02138, USA
eSchool of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA
First published on 2nd July 2015
Abstract
We present two examples of the use of liquid cells to study colloidal inorganic nanocrystals using in situ transmission electron microscopy. The first uses a liquid cell to quantify the interaction potential between pairs of colloidal nanocrystals, and the second demonstrates direct imaging of nanocrystal growth and structure in the liquid cell.
Over the last twenty years, colloidal inorganic nanocrystals have evolved into a major building block for solid state chemistry and the design of materials. Today, such nanocrystals are in practical use in biological imaging, in displays, and in many other technologies.1–4 Further, there is intensive interest in designing new nanocrystals with complex interconnections, spatial arrangements, and topologies, which can be used in more advanced areas of application, in solar to fuel catalysis for example.5 In order to achieve these goals, it is highly desirable to be able to understand the growth and assembly of these major building blocks as they form in solution. The very recent advent of the in situ liquid cell for transmission electron microscopy is just such a tool, and this lecture is dedicated to examining how these liquid cells today are enabling a new period of quantitation of nanocrystal growth and assembly.
In the first segment of this lecture, we review the use of the liquid cell to quantitate the interaction potential between pairs of colloidal nanocrystals.6 Here we use a conventional liquid cell, one fabricated with two thin SiN windows (each 50 nm thick) separated by a liquid layer of approximately 150 nm at its thinnest point. In such a cell, we can load nanocrystals, such as positively charged Au nanorods in a buffered water solution. Due to the force of attraction between the nanocrystals and the SiN window, we find that typically the nanocrystals are confined to a two-dimensional region near one of the windows, and within this thin layer they are free to move about (see Movie 1† and Fig. 1, reproduced from ref. 6). When two nanorods approach each other we can see that they tend to do so end to end, a rather surprising result, since the van der Waals force of attraction will be greatest for side to side attraction. Nonetheless, we can clearly see trajectories where nanocrystals fuse end to end. Further, we find that the nanocrystal aggregation is completely suppressed at lower ionic strengths. In principle, from the trajectories of pairs of nanocrystals in solution, we can reconstruct at each value of the ionic strength the form of the pairwise interaction potential versus angle and distance. Using this approach, we were able to show that for separations greater than around 10 nm, where the van der Waals forces become negligible, the effective interaction potential is dominated by screened Coulombic forces, which is consistent with the classical Derjaguin–Landau–Verwey–Overbeek (DLVO) model.7,8 From this we can understand why it is that the nanocrystals join end to end, because this is the direction for which the repulsive force at long distances is the least. This is just one recent example, showing how it is possible to learn about the forces between nanoparticles in a very direct way using the liquid cell.
 | ||
| Fig. 1 A time series of TEM images showing how Au nanorods approach and attach to each other to give the final tip-to-tip assembled structure. Red arrows highlight the trajectories of nanorods before they attach to the cluster of growing rod assemblies. Scale bar is 100 nm. Reprinted with permission from ref. 6. Copyright 2015 American Chemical Society. | ||
A second example to be considered here concerns the direct imaging of nanocrystal growth and structure in the liquid cell. For this purpose, higher resolution is required, and this is greatly facilitated by using the graphene liquid cell.9 Here a thin layer of liquid is trapped between two layers of graphene, either single layer thickness of graphene, or multilayer, depending on what is needed. Inside such a cell, it is possible to use the electron beam itself to induce the nucleation and growth of nanocrystals, and to observe the trajectories of their growth in the fluid. In this cell, the attraction to the “windows” is much less, so the motion is more three dimensional. We have used the liquid cell to observe the growth of Pt nanocrystals as an example. A significant surprise came when we saw that the Pt nanocrystals form through a somewhat unexpected sequence (Movie 2,† reproduced from ref. 9). At the earliest times, we observe a burst of nucleation of rather small (<1 nm) particles. This period of nucleation is followed by a period of coalescence and growth in which there are many particle fusion events, as well as events where individual particles grow by addition of molecular precursors (Fig. 2). This particle fusion is presumably due to the relatively high surface energy of the Pt nanocrystals as compared to the energy of a grain boundary.10 In such a liquid cell, it also appears to be possible to observe the electron diffraction patterns from individual particles as they rotate freely in the solution, opening the door to one day being able to directly obtain the structure of individual nanocrystals.
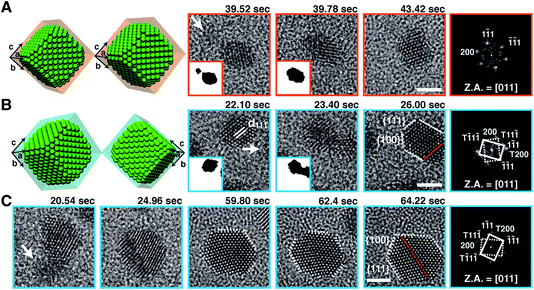 | ||
| Fig. 2 Still snapshots from Movie 2† of Pt nanocrystal growth via coalescence and crystal-structure evolution, observed with atomic resolution in a graphene liquid cell. Schematic illustrations and corresponding TEM images exhibiting nanocrystal coalescence along the 〈111〉 direction, evolving into (A) a single crystalline fcc structure or (B) a twinned (red dotted line) fcc structure. (C) Shape evolution of the Pt nanocrystal by straightening of the twin boundary (red dotted line) and evolution toward a hexagonal shape. The right-hand panel in each sequence shows a FFT of the panel adjacent to it. White arrows denote incoming small nanocrystals (as seen in insets). Scale bars, 2 nm. Z.A., zone axis. Reprinted with permission from ref. 9. Copyright 2012 American Association for the Advancement of Science. | ||
In the two examples given above we can see that the in situ liquid cell for the transmission electron microscope has the potential to provide unprecedented levels of quantitative information about nanocrystal growth and assembly. There are still many aspects of this to be worked out, such as improved understanding of the influence of the electron beam on the chemical environment (we know that heating is negligible), and the nature of the liquid layers that are trapped in such thin cells. Nonetheless, we can see already that a new era of quantitation for nanocrystal building blocks is upon us.
References
- P. D. Howes, R. Chandrawati and M. M. Stevens, Science, 2014, 346, 1247390 CrossRef PubMed.
- G. Konstantatos and E. H. Sargent, Nat. Nanotechnol., 2010, 5, 391 CrossRef CAS PubMed.
- I. J. Kramer and E. H. Sargent, ACS Nano, 2011, 5, 8506 CrossRef CAS PubMed.
- M. G. Panthani and B. A. Korgel, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 287 CrossRef CAS PubMed.
- M. Grzelczak, J. Vermant, E. M. Furst and L. M. Liz-Marzán, ACS Nano, 2010, 4, 3591 CrossRef CAS PubMed.
- Q. Chen, H. Cho, K. Manthiram, M. Yoshida, X. Ye and A. P. Alivisatos, ACS Cent. Sci., 2015, 1, 33 CrossRef CAS.
- B. Derjaguin and L. Landau, Acta Physicochim. URSS, 1941, 14, 633–662 Search PubMed.
- E. J. W. Verwey, J. T. G. Overbeek and K. van Nes, Theory of the Stability of Lyophobic Colloids: The Interaction of Sol Particles Having an Electric Double Layer, Elsevier Publishing Company, 1948 Search PubMed.
- J. M. Yuk, J. Park, P. Ercius, K. Kim, D. J. Hellebusch, M. F. Crommie, J. Y. Lee, A. Zettl and A. P. Alivisatos, Science, 2012, 336, 61 CrossRef CAS PubMed.
- H.-G. Liao, D. Zherebetskyy, H. Xin, C. Czarnik, P. Ercius, H. Elmlund, M. Pan, L.-W. Wang and H. Zheng, Science, 2014, 345, 916 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5fd90056e |
| This journal is © The Royal Society of Chemistry 2015 |
