Open Access Article
Open Access ArticleEnhanced disinfection by-product formation due to nanoparticles in wastewater treatment plant effluents†
Jacob W.
Metch
,
Yanjun
Ma
,
Amy
Pruden
and
Peter J.
Vikesland‡
*
Department of Civil and Environmental Engineering, Virginia Tech, 418 Durham Hall, Blacksburg, VA 24061, USA. E-mail: pvikes@vt.edu; Tel: (540) 231 3568
First published on 13th July 2015
Abstract
Nanoparticles (NPs) are increasingly being incorporated into consumer products and are being used for industrial applications in ways that will lead to their environmental dissemination via wastewater treatment plants (WWTPs). Many NPs possess catalytic properties that could potentially enhance undesired chemical reactions such as the formation of disinfection by-products during disinfection of wastewater effluent. In this effort, silver (AgNPs), titanium dioxide (TiO2), ceria (CeO2), and nano zero valent iron (NZVI) NPs were investigated for their potential to enhance trihalomethane (THM) formation in three different disinfection regimes: UV alone, free chlorine, and UV+free chlorine. Of the test nanomaterials, only AgNPs demonstrated the capacity to enhance THM formation and thus they were subjected to additional study. AgNPs enhanced THM formation at all concentrations examined (1, 10, and 20 mg L−1) even though the AgNPs were chemically unstable in the presence of free chlorine. The transformation of the AgNPs and the production of non-metallic silver species was observed via UV-vis spectroscopy. The capacity for AgNPs to enhance THM formation was considerably increased in the UV+free chlorine disinfection regime. Although not the focus of the study, formation of AgNPs during UV disinfection of Ag+ in the effluent was also observed. This study illustrates the potential for NPs to catalyze unfavorable chemical reactions during WWTP effluent disinfection. Such a result could prove detrimental to aquatic receiving environments and is especially of concern in water reuse scenarios where aggressive disinfection regimes may be utilized.
Water impactIncreased incorporation of nanomaterials in consumer products may lead to their dissemination to the environment via wastewater treatment. The presence of nanomaterials within waste streams likely has unintended consequences on wastewater treatment processes and effluent quality due to the antimicrobial and catalytic properties of some nanoparticles. The research described herein investigated the potential for nanoparticles to enhance disinfection by-product formation during wastewater disinfection. We observed that effluents containing silver nanoparticles had higher chloroform levels when compared to effluents containing silver ions or background controls. This observation is potentially concerning as wastewater reuse is increasingly utilized and it often employs aggressive disinfection regimes for pathogen inactivation. This study highlights the need for further investigation into the potential for nanoparticles to enhance undesirable reactions in aquatic systems. |
1 Introduction
The rapid development of nanotechnology has led to the incorporation of nanomaterials in numerous industrial and commercial products. Nanomaterials are being employed in these products due to their exceptional antimicrobial,1 photocatalytic,2 and optical3 properties, amongst others. The widespread application of nanomaterials in industrial and consumer products increases the likelihood that they will be released into the environment in ways that are uncontrolled and possibly detrimental.The manner by which nanomaterial containing products are consumed or cleaned is expected to result in significant nanomaterial inputs to wastewater treatment plants (WWTPs).4 For example, consumer products, such as clothing and medical textiles, that contain embedded silver nanoparticles (AgNPs) have been found to release these nanomaterials during laundering.5,6 Nanomaterials such as titanium dioxide (TiO2) applied in food, lotions, and sunscreens have similar potential for transmission to sewer collection systems simply through industrial and consumer use.7 Ceria (CeO2) is used as a polishing agent in industry,8 and as a fuel additive to reduce harmful emissions from diesel engines.9 Nano zero valent iron (NZVI) is also being developed for the oxidation of several water contaminants10 and therefore may be introduced to the sewer system as well. The combination of their unique properties (relative to bulk materials) and the strong potential for nanomaterial dissemination to sewer systems has led to increased concern regarding the possible detrimental effects of nanomaterials to WWTP performance. Past studies by our group and others have shown that one unintended biological consequence of nanomaterial fluxes into WWTPs is a shift in the activated sludge microbial communities11,12 and specifically, decreases in nitrifying bacteria.11,13 To date, however, the potential implications of nanomaterials on the chemical processes used for wastewater disinfection have yet to be examined.
As a result of their unique properties, nanomaterials are widely applied as catalysts that can enhance the rates of a diverse array of chemical transformations.14 For example, Vejerano et al. (2013) discovered that during waste incineration, total polycyclic aromatic hydrocarbon emissions were approximately six times higher in waste containing nanomaterials as compared to bulk controls.15 Of concern to the present study was the potential for nanomaterials to catalyze undesirable reactions within a WWTP. Our particular focus was to evaluate the potential effects of nanomaterials on wastewater disinfection.
It is well known that high concentrations of organic matter can lead to elevated concentrations of toxic disinfection by products (DBPs) in chlorinated WWTP effluents.16 DBPs form when strong oxidants such as free chlorine oxidize organic matter, bromide, and iodide in water.17 Similarly, UV irradiation is known to photorearrange organic matter and possibly increase its reactivity towards free chlorine,18 thus potentially increasing the DBP formation potential. Although it is acknowledged that DBP occurrence in WWTP effluents is important to water sustainability, and that NPs will be transported to the environment via WWTPs, currently there are no studies investigating the effect of NPs on the formation of DBPs in WWTP effluents.
In this study, we assessed the potential for four common nanomaterials: Ag, TiO2, NZVI, and CeO2 to catalyze DBP formation with a focus on trihalomethanes (THMs) and chloropicrin. THMs are of immediate concern because of their relative ease of formation in chlorinated water, their mutagenicity and genotoxicity, as well as the regulatory standards placed on them by the US EPA.19 Chloropicrin is a concern due to its formation potential in chlorinated waters and its genotoxicity to mammalian cells.19 Because AgNPs demonstrated an increased THM formation potential, follow up experiments were conducted to gain further insight into the mechanism by which AgNPs increased THM formation. These experiments monitored free chlorine demand, assessed THM formation at various AgNP concentrations, and used UV-vis spectroscopy to investigate AgNP stability and material speciation throughout the disinfection process.
2 Methods
2.1 Nanoparticle source, synthesis, and characterization
AgNPs were synthesized by sodium citrate reduction, as described elsewhere.20 NZVI dispersions were purchased with organic and inorganic stabilizers from NANO IRON s.o.r. (NANOFER 25S, Rajhrad, Czech Republic). Anatase TiO2 and CeO2 nanopowders were purchased from Sigma-Aldrich (St. Louis, Missouri, USA) and were suspended into nanopure water by sonication for 20 minutes (90 W, 20 KHz, 20 °C). Nanoparticle suspensions and bulk or ionic controls were prepared as stock suspensions of 100 mg L−1 and stored in the dark until use. The bulk or ionic controls for AgNP, TiO2-NP, CeO2-NP, and NZVI were silver nitrate (Fisher, Suwanee, GA), TiO2 (≈44 μm) and CeO2 (≈5 μm) bulk powders (Sigma-Aldrich, Saint Louis, MO), and ferrous sulfate (Fisher), respectively.The average sizes of the nanoparticles were determined by transmission electron microscopy (TEM) as previously described.21 Nanoparticle suspensions were diluted 100×, then drop-cast onto a 200 mesh, lacey-carbon-coated copper TEM grid. Hydrocarbons that would cause interference were removed by heating under vacuum at 120 °C for 3 h. A JEM 2100 TEM (JEOL Corporation) operated at 200 kV, equipped with an energy dispersive X-ray spectrometer and diffractometer was employed. Average nanoparticle sizes were determined by counting at least 70 particles per TEM image and then sizing via use of ImageJ software. Using this approach the AgNPs were determined to have a diameter of 52 ± 12 nm, NZVI was 46 ± 10 nm, TiO2-NPs were 21 ± 12 nm, and CeO2-NPs were 33 ± 12 nm.
2.2 WWTP effluent collection
Plant One (WWTP 1) received primarily domestic wastewater flow, while Plant Two (WWTP 2) received approximately 15% industrial wastewater flow along with domestic wastewater flow. Both WWTPs were conventional activated sludge plants and at the time of sample collection were not practicing denitrification. WWTP samples were collected immediately prior to disinfectant addition, transported immediately to the lab and stored at 4 °C until use (3 days or less). For general wastewater characteristics see ESI† Tables S1 and S2.2.3 UV254 disinfection
UV254 disinfection was carried out using a collimated beam apparatus using our previously described setup.22 A low pressure mercury lamp was used to produce UV light at a wavelength of 254 nm. A fluence dose of 200 mJ cm−2 was achieved by measuring the fluence rate with a UVX Radiometer with a UVX-25 (UV254) sensor (UVP, LLC, Upland, California, USA), and then calculating the contact time based on the fluence rate. Wastewater disinfection was carried out in 65 mL volumes in 9 cm diameter glass Petri dishes (depth of approximately 1 cm) with continuous mixing throughout the irradiation period.2.4 Free chlorine disinfection
Three conditions (background control, bulk or ionic control, and NP) were subjected to free chlorine disinfection in triplicate using 20 mL amber vials. The free chlorine concentration of a commercial chlorine stock was determined via the colorimetric DPD method (HACH, Colorado, USA). A 2000 mg L−1 free chlorine stock was prepared fresh for each experiment and then diluted to achieve an initial free chlorine concentration of 20 mg L−1 in the reaction vials. Although this chlorine dose is greater than what is practiced at most WWTPs, this dose was chosen to represent a worst case scenario and to investigate the potential DBP enhancing capability of the nanoparticles. Final concentrations of the bulk or ionic controls as well as the nanoparticle conditions were set at 20 mg L−1. For metal oxide particles this was the mass of the nanoparticles and for the zero valent metal particles, with respect to the concentration of the metal. As a background control the same volume of nanopure water was used to dilute the reaction matrix to the same volume (20 mL). In the experiments examining sequential disinfection by UV followed by free chlorine, the product of the UV disinfection step was pipetted into 20 mL amber reaction vials in triplicate for each condition and then subjected to free chlorine disinfection. Following free chlorine addition the reaction vials were capped and mixed by inversion and then incubated at room temperature for 30 minutes. Excess citric acid was used to quench the reaction.Because the AgNPs enhanced DBP formation at 20 mg L−1, the experiments were repeated at lower NP doses and also repeated at 20 mg L−1. In these follow-up experiments, the chlorine disinfection protocol was modified slightly as in previous studies.23 Here a 5.6–6% sodium hypochlorite solution (Sigma Aldrich, St. Louis, Missouri, USA) was used as a chlorinating agent and diluted to prepare a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 free chlorine solution (concentration verified using DPD method as described above). A large batch of the background control, bulk or ionic control, or NP condition (180 mL) was prepared in an Erlenmeyer flask and mixed with a magnetic stir bar. In the UV+free chlorine disinfection regime; the product of the UV disinfection step was put into an Erlenmeyer flask. While mixing, the free chlorine solution was added to a final concentration of 20 mg L−1, and the vial was then capped immediately. After 30 seconds of mixing, 20 mL amber vials were filled and capped with no head space, in triplicate, and incubated for 30 minutes at room temperature. Citric acid was added to the head space analysis vials so when the sample was added after 30 minutes, the reaction was quenched.
000 mg L−1 free chlorine solution (concentration verified using DPD method as described above). A large batch of the background control, bulk or ionic control, or NP condition (180 mL) was prepared in an Erlenmeyer flask and mixed with a magnetic stir bar. In the UV+free chlorine disinfection regime; the product of the UV disinfection step was put into an Erlenmeyer flask. While mixing, the free chlorine solution was added to a final concentration of 20 mg L−1, and the vial was then capped immediately. After 30 seconds of mixing, 20 mL amber vials were filled and capped with no head space, in triplicate, and incubated for 30 minutes at room temperature. Citric acid was added to the head space analysis vials so when the sample was added after 30 minutes, the reaction was quenched.
2.5 THM analysis
Four THMs (trichloromethane, bromodichloromethane, dibromochloromethane, and tribromomethane) and chloropicrin were quantified using a Thermo Finnigan TraceGC Ultra gas chromatograph (Thermo Finnigan, San Jose, California, USA) with a SPB-624 Supelco fused silica capillary column (Sigma Aldrich, St. Louis, Missouri, USA) and a headspace autosampler and an electron capture detector as reported previously.24 Standards were diluted to known concentrations in headspace free glass syringes to limit loss of standard to volatilization, and concentrated stock standards were stored under water for up to one week. Samples were transferred from the reaction vials to 20 mL headspace vials and crimp sealed using an aluminum seal with septa (Restek Corporation, Bellefronte, Pennsylvania, USA). THM standards were purchased as a mixture (Restek Corporation, Bellefronte, Pennsylvania, USA). Chloropicrin was purchased (VWR International Inc., Suwanee, GA) and combined with the THM standards during dilution. THMs were identified based upon elution times and quantified using calibration curves with reference to an internal standard of 1,2 dibromopropane (Crescent Chemical Company Inc., Islandia, New York, USA).2.6 UV-vis spectrum analysis
The UV-vis spectrum was analyzed during the follow up experiments of varying concentrations of AgNPs. Samples were collected in 10 mL amber vials for background control, bulk/ion control, and NP conditions at four intervals of the follow up experiments (no disinfection, free chlorine disinfection, UV disinfection, and UV+free chlorine disinfection). Samples were stored at 4 °C for no longer than 2 days until they were analyzed using a Beckman DU-640 spectrophotometer (Beckman Instruments Inc., Fullerton, CA, USA).2.7 Free chlorine demand
Free chlorine demand experiments were conducted in the follow up experiments of varying AgNP concentrations. Samples for no disinfection and after UV disinfection were collected in 20 mL amber vials in triplicate for background control, bulk/ion control, and NP conditions. These samples were chlorinated with sodium hypochlorite at 50 mg L−1, after 24 hours of incubation in the dark at room temperature free chlorine was measured according to Standard Method 4500-Cl25 using a DR2700 spectrophotometer (HACH, Loveland, CO). Chlorine demand was calculated as the difference between the initial free chlorine concentration and the final free chlorine concentration.3 Results and discussion
3.1 Formation of disinfection by-products in the presence of nanoparticles
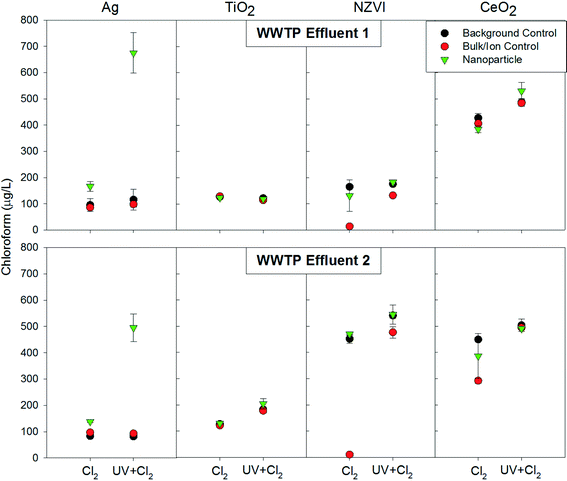
To ascertain whether our four tested nanomaterials were capable of enhancing DBP formation, our initial survey employed a high nanomaterial dose of 20 mg L−1. Chloroform was the most abundant THM in all samples tested and therefore will be the focus of the following discussion as it drove THM trends. Of the four nanoparticles investigated, only the AgNPs resulted in increased chloroform formation relative to the bulk control, ion control, or the background controls (Fig. 1). Chloropicrin was not quantifiable in most samples and no trends could be observed (ESI† Tables S3–S6). We note that WWTP effluent variations likely caused background formation of chloroform to vary greatly between experiments as DBP formation has been found to vary temporally.26 Interestingly, neither CeO2 nor TiO2 resulted in increased chloroform formation relative to the control conditions. This result was unexpected given the well-established capability of TiO2 to catalyze a variety of reactions via UV light mediated formation of reactive oxygen species, as has been applied for wastewater disinfection.27 Although not as reactive as TiO2, CeO2 has also demonstrated photocatalytic properties;28,29 however, no such properties were displayed in this study. Similarly, there was also no significant increase in chloroform formation in effluent containing nano zero-valent iron relative to the background control. However, there was a significant decrease in chloroform formation in the ion control (ferrous sulfate). This result is likely because ferrous sulfate incurred a significant free chlorine demand,30 thus limiting the opportunity for free chlorine to react with organic matter and produce chloroform.Interestingly, formation of chloroform in the presence of the AgNPs was considerably greater when subjected to UV+free chlorine disinfection relative to the free chlorine only condition. There is considerable understanding of the photocatalytic properties of silver doped titanium dioxide;31 however less is known about the photocatalytic ability of silver itself. Subsequent experiments attempted to identify the mechanisms driving this phenomenon.
3.2 AgNP enhanced chloroform production
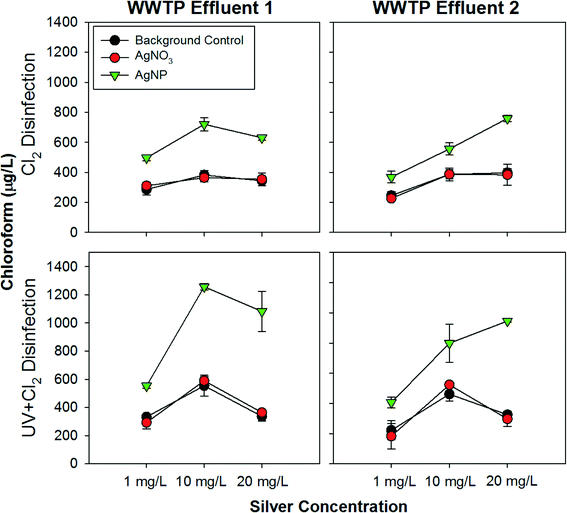
A second series of experiments focused specifically on further evaluation of the capability of AgNPs to catalyze disinfection by-product formation at 20 mg L−1 and at lower concentrations of 10 mg L−1 and 1 mg L−1. We note these concentrations are still higher than EPA freshwater discharge guidelines of 3.2 μg L−1 for silver,32 however, it is important to investigate the relationship between enhanced chloroform formation and the concentration of AgNPs. Chloroform production was found to be enhanced in wastewater effluent amended with all three doses of AgNPs (Fig. 2). Enhanced chloroform production by AgNPs was consistently observed in the highly aggressive UV+free chlorine disinfection regime. Low amounts of chloropicrin were produced in experiments examining 1 mg L−1 silver; in experiments with either 10 or 20 mg L−1 of silver, chloropicrin was not quantifiable. No trends were discernible for AgNP mediated enhancement of chloropicrin formation. Brominated THMs were also produced in all samples tested with AgNPs having increased bromodichloromethane in some situations, however, this was not consistent and no trend could be discerned (ESI† Tables S8–S10).The results of this study suggest that in most cases UV light influences wastewater chemistry in a manner that enhances disinfection by-product formation when the water is subsequently chlorinated. UV disinfection has been previously shown to photo-rearrange natural organic matter (NOM), which could increase its reactivity towards chlorine.18 Also, it is known that NOM can form a thin layer or corona around metallic nanoparticles under many environmental conditions.33 Specifically, NOM has been found to associate with both citrate coated nanoparticles34 and AgNPs.35 Therefore, it is possible that NOM coats the AgNPs and that this close proximity results in increased photo-rearrangement of NOM during UV disinfection and thus increases its overall reactivity towards free chlorine.
Of interest in this study was the potential formation of disinfection by-products within the background matrix of treated wastewater effluent. Wastewater effluent is relatively rich in organic matter, relative to treated drinking water, which could enhance disinfection by-product formation. In this study, two WWTP effluents were examined, in order to capture a range of possible matrix effects. When the AgNPs were dosed to WWTP Effluent 1, there was no apparent trend between the concentration and the enhancement of chloroform production. Although there was increased enhancement in the 10 mg L−1 condition as compared to the 1 mg L−1 condition, there was not a significant increase in chloroform generation between the 10 mg L−1 and 20 mg L−1 conditions. When dosed to WWTP Effluent 2, however, there was a clear trend of an increase in chloroform production with an increase in AgNP dose. The differing trends observed with the two WWTP Effluent matrices indicates that variations in wastewater chemistry can influence the magnitude of the enhancement in chloroform formation caused by AgNPs. Based on the general characteristics of the effluents (ESI† Table S1 and S2), the most striking difference between the effluents is that the ionic strength was much higher in WWTP Effluent 2 (1,013 μS cm−1) compared to WWTP Effluent 1 (594 μS cm−1). Ionic strength has been demonstrated to affect nanoparticle stability and surface chemistry,36 which will likely impact reactivity and could account, at least in part, for the differing patterns of chloroform production between the two effluents.
3.3 Potential effects of nanomaterial coatings
Tugulea et al. (2013) recently investigated the effect of polyvinylpyrrolidone (PVP) coated AgNPs on DBP formation during drinking water disinfection and found they had no effect on the formation of several DBPs, including THMs.37 This conclusion is in direct contrast to the findings of the present study, which indicated a significant increase in chloroform production in the presence of citrate coated AgNPs. Given that the main difference between the two studies is the AgNP coating, this difference suggests that the coating of the particle could play a crucial role in enhancing the formation of chloroform.To investigate whether free citrate could be acting independently of the AgNPs to enhance formation of DBPs, an experiment was conducted to isolate the effect of the citrate. Citrate is oxidized during AgNP formation and is converted into a diverse suite of oxidation products such as formate and acetoacetate.38 It is therefore difficult to discern the possible effects of these oxidation products on THM formation, however an experiment was conducted to evaluate how free citrate itself participates in THM formation. Citrate was dosed to both WWTP effluents and controls and subjected to the same disinfection regimes applied in the nanoparticle survey. No significant increase in chloroform formation was observed in either effluent in the presence of citrate (ESI† Table S3). However, a parallel experiment was conducted with citrate coated gold nanoparticles and a similar effect was observed as has been described above for the AgNPs (ESI† Table S10). These results support the conclusion that it is not free citrate in and of itself that is driving the enhanced formation of chloroform, rather it is an interactive effect of the association of citrate and its oxidation products with the AgNP surface that enhances chloroform formation.
3.4 Role of free chlorine demand
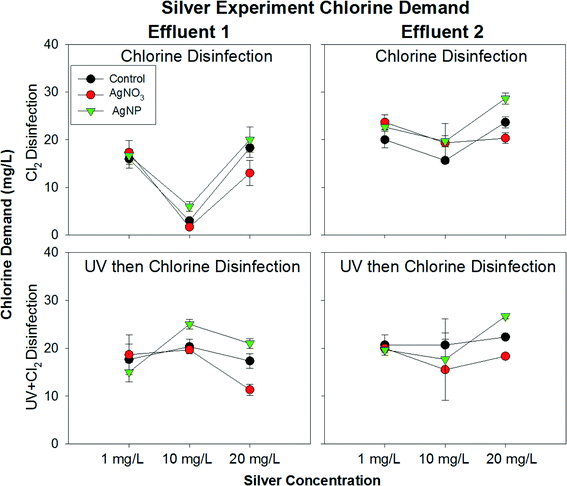
Changes in chlorine demand mediated by AgNPs could potentially have played a role in the enhanced formation of DBPs. To explore this possibility, chlorine demand was measured while conducting the THM formation experiments with varying concentrations of AgNPs. However, there was no obvious difference in the chlorine demand of the controls relative to the AgNP condition at 1 mg L−1 and 10 mg L−1 (Fig. 3). This is likely due to the fact that the majority of the chlorine demand in this system could be attributed to other materials present in the effluents, with minimal change in chlorine demand attributable to the presence of silver. In the 20 mg L−1 AgNP condition, however, a slight increase in chlorine demand was detected, likely resulting from chlorine directly reacting with AgNPs. In contrast, the effluent containing AgNO3 had decreased chlorine demand, which was most likely due to the formation of silver sulfide complexes and therefore decreased reactivity with chlorine. This was especially apparent in the UV+free chlorine disinfection regime, in which silver nanoparticle formation likely occurred.3.5 Insight into physiochemical changes of AgNPs via UV-vis absorbance spectra
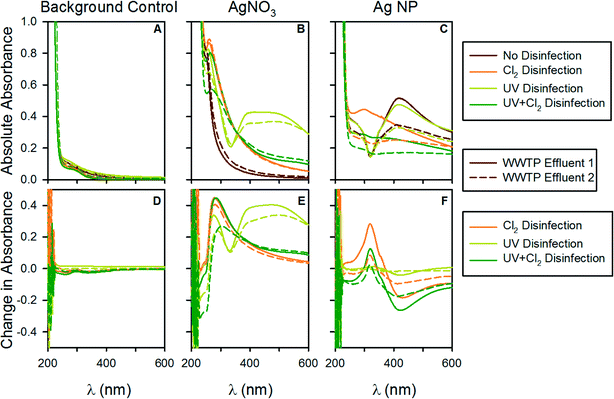
To gain deeper insight into the complex interactions of the AgNPs during the disinfection treatments we collected UV-vis absorbance spectra for the samples both prior to and following disinfection. Past studies by our group and others have shown that UV-vis spectral analysis is an appropriate means to capture the complexities of nanoparticle systems.39 AgNPs exhibit a localized surface plasmon resonance (LSPR) that gives rise to a characteristic absorption band whose spectral location and width is a function of the nanoparticle size, nanoparticle aggregation state, and the solution chemistry.40As indicated in Fig. 4, the AgNPs exhibit a characteristic absorption peak at ~400 nm whose intensity scales with the nanoparticle concentration. This peak was stable following UV disinfection; however, following free chlorine addition the peak was either completely eliminated, as observed when AgNP concentrations were 1 mg L−1 and 10 mg L−1 (ESI† Fig. 1 and 2) or was dramatically diminished as for the AgNP concentration of 20 mg L−1 (Fig. 4).
Yuan et al. (2013) investigated the chemical stability of polyvinyl alcohol (PVA) coated AgNPs during disinfection and observed that, at neutral pH, the AgNPs were stable following UV disinfection.41 However, this stability was greatly reduced under more acidic or alkaline pH conditions. The effluents applied in the present study were near neutral in pH (ESI† Table S2) and although the AgNP coating differed, they behaved similarly with respect to their stability during UV disinfection.
During chlorine disinfection; however, it was apparent that the AgNPs were not chemically stable, as indicated by the drastic reduction of the AgNP band at 400 nm. Previous studies investigating the stability of PVA41 and PVP37 coated AgNPs similarly concluded that AgNPs are not chemically stable during free chlorine disinfection and likely dissolve to produce other silver species. We believe the observed formation of UV-vis peaks in the vicinity of ~300 nm following free chlorine disinfection is consistent with such a phenomenon as its temporal evolution is consistent with AgNP dissolution. The lack of chemical stability of the AgNPs during chlorine disinfection in conjunction with the increased formation of chloroform suggests that the AgNPs are transformed during the reaction and therefore should not be considered as catalysts that enhance the kinetics of chloroform formation. Interestingly, even though the AgNPs act as a sink for free chlorine, there was still an increase in chloroform production. This result indicates the participation of AgNPs in the chloroform production, or at least in the alteration of NOM leading to the increased formation of chloroform.
Evaluation of the UV-vis spectra also provides insight into why the AgNPs may have exhibited different patterns of DBP formation within the two WWTP effluent matrices. Differences in the spectra were most noticeable in the 10 and 20 mg L−1 conditions and were characterized by different shaped AgNP peaks for WWTP Effluents 1 and 2. The spectra suggest that the AgNPs were less stable in WWTP Effluent 2, without disinfection, as indicated by the smaller AgNP peak for WWTP Effluent 2 relative to WWTP Effluent 1. This could be related to the higher ionic strength of WWTP Effluent 2, as indicated above.
An unexpected discovery of this study occurred in the ionic silver controls. Spontaneous formation of AgNPs occurred when the AgNO3 control was exposed to UV light, as indicated by the formation of a LSPR band at ~400 nm. The large breadth of this peak suggests that the particles formed via this mechanism were highly polydisperse. We note that various methods for generating AgNPs exist and that AgNPs have previously been synthesized using by UV254 irradiation of AgNO3 in the presence of PVP.42 It is therefore not surprising that UV treatment can reduce the silver ions to AgNPs in situ. Because these nanoparticles exhibit a LSPR band they must consist at least partially of AgNPs. Were they fully sulfidized (i.e., present as AgS2 NPs) they would not be expected to exhibit a LSPR band.43,44 It is interesting that such a reaction can occur unintentionally and that the organic matter in the effluents was able to stabilize the particles. Importantly, similar to the pre-synthesized citrate coated AgNPs, the spontaneously generated particles were chemically unstable during free chlorine disinfection (as evidenced by the decrease in the LSPR band), but they did not lead to increased chloroform production. This observation further supports the conclusion that the citrate coating plays a key role in the observed formation of chloroform.
4 Conclusions, environmental implications
The present study determined that citrate coated AgNPs have the capacity to enhance chloroform formation in WWTP effluents at all concentrations of AgNPs investigated (1, 10, and 20 mg L−1). It is hypothesized that the citrate coating plays a crucial role in the reactions enhancing chloroform formation, given that a similar study using PVP coated AgNPs observed no enhanced chloroform formation.37 Citrate coatings are commonly used in studies investigating nanoparticle behavior as citrate is often used to reduce metal salts to nanoparticles in industrial applications before the particles are coated with another substance. Based on the UV-vis spectrum, AgNPs are not stable during free chlorine disinfection and are likely cycled from particulate to soluble forms.This study demonstrates that it is possible that nanomaterials present in WWTP effluents could enhance the formation of disinfection by products. This enhancement of DBP formation could be of concern to receiving environments due to DBP toxicity to aquaculture as well as a concern for downstream municipalities. As indirect wastewater reuse now supplies downstream cities with a significant source of drinking water,45 increased DBPs in WWTP effluents will pose a challenge to water sustainability strategies. Such potential increases in DBP levels could also have implications in water reuse scenarios in which rigorous disinfection, including UV followed by free chlorine treatment, is commonly employed as an added safeguard.46 This scenario may lead to violations in DBP regulations, or increased toxicity to persons exposed to reclaimed irrigation water vapors as inhalation has been identified as an important DBP exposure route.47
While the presence of AgNPs in the WWTP effluent enhanced the formation of disinfection by-products, it was encouraging that three of the four nanoparticles tested did not increase THM formation. However, it is important to consider that the conclusions of this study are limited to the conditions that were tested and that different outcomes are possible with changes in water chemistry and nanoparticle characteristics, such as surface coating. Further, this study focused primarily on THM formation, whereas there are over 500 disinfection by-products that have been identified to date.17 Therefore, increased levels of DBPs other than THMs could potentially be formed by these nanoparticles, which would not be revealed in the present study.
Collectively the experiments described in this work illustrate the labile nature of silver in wastewater systems. Although it was not the initial focus of this effort, the UV-vis results indicate that UV exposure can convert Ag+ into AgNPs and that free chlorine addition can reverse this process. The cycling of silver between its soluble form and nanoparticulate form during both wastewater and drinking water disinfection requires additional study.
Acknowledgements
Funding for this study was provided by the U.S. Environmental Protection Agency Star Grant #834856, the National Science Foundation (NSF) Center for the Environmental Implications of Nanotechnology (CEINT) (EF-0830093), and the Virginia Tech Institute for Critical Technology and Applied Science (ICTAS). The findings do not reflect the views of the sponsors. The authors would also like to thank Jody Smiley for her assistance in method development.References
- C. Marambio-Jones and E. M. V. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- F. Han, V. S. R. Kambala, M. Srinivasan, D. Rajarathnam and R. Naidu, Appl. Catal., A, 2009, 359, 25–40 CrossRef CAS PubMed.
- S. Eustis and M. el-Sayed, Chem. Soc. Rev., 2006, 35, 209–217 RSC.
- A. Keller and A. Lazareva, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- T. M. Benn and P. Westerhoff, Environ. Sci. Technol., 2008, 42, 4133–4139 CrossRef CAS.
- D. M. Mitrano, E. Rimmele, A. Wichser, M. Height and B. Nowack, ACS Nano, 2014, 8, 7208–7219 CrossRef CAS PubMed.
- A. Weir, P. Westerhoff, L. Fabricius, K. Hristovski and N. von Goetz, Environ. Sci. Technol., 2012, 46, 2242–2250 CrossRef CAS PubMed.
- L. Wang, K. Zhang, Z. Song and S. Feng, Appl. Surf. Sci., 2007, 253, 4951–4954 CrossRef CAS PubMed.
- J. Lahaye, S. Boehm, P. H. Chambrion and P. Ehrburger, Combust. Flame, 1996, 104, 199–207 CrossRef CAS.
- F. Fu, D. D. Dionysiou and H. Liu, J. Hazard. Mater., 2014, 267, 194–205 CrossRef CAS PubMed.
- Y. Ma, J. W. Metch, E. P. Vejerano, I. J. Miller, E. C. Leon, L. C. Marr, P. J. Vikesland and A. Pruden, Water Res., 2015, 68, 87–97 CrossRef CAS PubMed.
- Y. Yang, J. Quensen, J. Mathieu, Q. Wang, J. Wang, M. Li, J. M. Tiedje and P. J. J. Alvarez, Water Res., 2014, 48, 317–325 CrossRef CAS PubMed.
- X. Zheng, Y. Chen and R. Wu, Environ. Sci. Technol., 2011, 45, 7284–7290 CrossRef CAS PubMed.
- K. Hemalatha, G. Madhumitha, A. Kajbafvala, N. Anupama, R. Sompalle and S. M. Roopan, J. Nanomater., 2013, 2013, 1–23 CrossRef.
- E. P. Vejerano, A. L. Holder and L. C. Marr, Environ. Sci. Technol., 2013, 47, 4866–4874 CrossRef CAS PubMed.
- S. W. Krasner, P. Westerhoff, B. Chen, B. E. Rittmann and G. Amy, Environ. Sci. Technol., 2009, 43, 8320–8325 CrossRef CAS PubMed.
- S. Richardson, TrAC, Trends Anal. Chem., 2003, 22, 666–684 CrossRef CAS.
- M. L. Magnuson, C. A. Kelty, C. M. Sharpless, K. G. Linden, W. Fromme, D. H. Metz and R. Kashinkunti, Environ. Sci. Technol., 2002, 36, 5252–5260 CrossRef CAS.
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. Demarini, Mutat. Res., 2007, 636, 178–242 CAS.
- A. J. Kennedy, M. S. Hull, A. J. Bednar, J. D. Goss, J. C. Gunter, J. L. Bouldin, P. J. Vikesland and J. A. Steevens, Environ. Sci. Technol., 2010, 44, 9571–9577 CrossRef CAS PubMed.
- E. P. Vejerano, E. C. Leon, A. L. Holder and L. C. Marr, Environ. Sci.: Nano, 2014, 1, 133–143 RSC.
- C. W. McKinney and A. Pruden, Environ. Sci. Technol., 2012, 46, 13393–13400 CrossRef CAS PubMed.
- C. W. Li, M. M. Benjamin and G. V. Korshin, Environ. Sci. Technol., 2000, 34, 2570–2575 CrossRef CAS.
- I. Montesinos and M. Gallego, Anal. Bioanal. Chem., 2012, 402, 2315–2323 CrossRef CAS PubMed.
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater. (American Water Works Association and Water Environment Federation, 1998) Search PubMed.
- D. Baytak, A. Sofuoglu, F. Inal and S. C. Sofuoglu, Sci. Total Environ., 2008, 407, 286–296 CrossRef CAS PubMed.
- H. Foster, I. B. Ditta, S. Varghese and A. Steele, Appl. Microbiol. Biotechnol., 2011, 90, 1847–1868 CrossRef CAS PubMed.
- M. D. Hernández-Alonso, A. B. Hungría, A. Martínez-Arias, M. Fernández-García, J. M. Coronado, J. C. Conesa and J. Soria, Appl. Catal., B, 2004, 50, 167–175 CrossRef PubMed.
- P. Ji, J. Zhang, F. Chen and M. Anpo, Appl. Catal., B, 2009, 85, 148–154 CrossRef CAS PubMed.
- P. J. Vikesland and R. L. Valentine, Environ. Sci. Technol., 2002, 36, 662–668 CrossRef CAS.
- K. Awazu, M. Fujimaki, C. Rockstuhl and J. A. Tominaga, J. Am. Chem. Soc., 2008, 130, 1676–1680 CrossRef CAS PubMed.
- U.S. EPA. National Recommended Water Quality Criteria; Office of Water, Office of Science and Technology: Washington, DC, 2006 Search PubMed.
- G. R. Aiken, H. Hsu-kim and J. N. Ryan, Environ. Sci. Technol., 2011, 46, 3196–3201 CrossRef PubMed.
- D. P. Stankus, S. E. Lohse, J. E. Hutchison and J. A. Nasan, Environ. Sci. Technol., 2010, 45, 3238–3244 CrossRef PubMed.
- M. Delay, T. Dolt, A. Woellhaf, R. Sembritzki and F. H. Frimmel, J. Chromatogr. A, 2011, 1218, 4206–4212 CrossRef CAS PubMed.
- K. L. Garner and A. J. Keller, J. Nanopart. Res., 2014, 16, 2503 CrossRef.
- A.-M. Tugulea, D. Bérubé, M. Giddings, F. Lemieux, J. Hnatiw, J. Priem and M.-L. Avramescu, Environ. Sci. Pollut. Res., 2014, 21, 11823–11831 CrossRef CAS PubMed.
- C. H. Munro, W. E. Smith, M. Garner, J. Clarkson and P. C. White, Langmuir, 1995, 11, 3712–3720 CrossRef CAS.
- M. Y. Chan and P. J. Vikesland, Environ. Sci. Technol., 2014, 48, 1532–1540 CrossRef CAS PubMed.
- H. Wei, S. M. Hossein Abtahi and P. J. Vikesland, Environ. Sci.: Nano, 2015, 2, 120–135 RSC.
- Z. Yuan, Y. Chen, T. Li and C. P. Yu, Chemosphere, 2013, 93, 619–625 CrossRef CAS PubMed.
- H. H. Huang, X. P. Ni, G. L. Loy, C. H. Chew, K. L. Tan, F. C. Loh and J. F. Deng, Langmuir, 1996, 12, 909–912 CrossRef CAS.
- J. Yang and J. Ying, Chem. Commun., 2009, 3187 RSC.
- R. Chen, N. T. Nuhfer, L. Moussa, H. R. Morris and P. M. Whitmore, Nanotechnology, 2008, 19, 455604 CrossRef PubMed.
- J. Rice, A. Wutich and P. Westerhoff, Environ. Sci. Technol., 2013, 47, 11099–11105 CrossRef CAS PubMed.
- U.S. Envrionmental Protection Agency (EPA), Guidelines for Water Reuse (EPA/600/R-12/618), 2012 Search PubMed.
- C. C. Wang, Z. G. Niu and Y. Zhang, J. Hazard. Mater., 2013, 262, 179–188 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ew00114e |
| ‡ Current address: Department of Civil Environmental Engineering, 418 Durham Hall, Virginia Tech, Blacksburg, VA 24061. |
| This journal is © The Royal Society of Chemistry 2015 |