The osmotic membrane bioreactor: a critical review
Ryan W.
Holloway
a,
Andrea
Achilli
b and
Tzahi Y.
Cath
*a
aColorado School of Mines, Golden, CO, USA. E-mail: tcath@mines.edu; Fax: +(303) 273 3413; Tel: +(303) 273 3402
bHumboldt State University, Arcata, CA, USA
First published on 22nd June 2015
Abstract
The osmotic membrane bioreactor (OMBR) is a hybrid biological-physical treatment process that has been gaining interest for wastewater treatment and water reuse. The OMBR couples semi-permeable forward osmosis (FO) membranes for physiochemical separation with biological activated sludge process for organic matter and nutrient removal. The driving force for water production in OMBR is the osmotic pressure difference across the FO membrane between the activated sludge and a concentrated draw solution, which is made with inorganic or organic salts that have a high osmotic pressure at relatively low concentrations. The draw solution becomes diluted during OMBR treatment and may be reconcentrated using reverse osmosis, membrane distillation, or thermal distillation processes. The combination of processes in the OMBR presents unique opportunities but also challenges that must be addressed in order to achieve successful commercialization. These challenges include membrane fouling, elevated bioreactor salinity that hinders process performance, degradation of the draw solution by chemicals that diffuse through the FO membrane, and the potential for simultaneous water, mineral, and nutrient recovery. In this article, results from past and most recent OMBR studies are summarized and critically reviewed. Information about similar and more established technologies (e.g., traditional porous membrane bioreactors and FO) is included to help compare and contrast state-of-the-art technologies and the novel OMBR approach, and to elucidate practical configurations that should be considered in future OMBR research and development.
Water impactWith increasing water demand and decreasing fresh water resources, special focus is directed towards reclamation and water reuse. Direct potable reuse (DPR) is a desired reuse option over non-potable and indirect potable reuse because existing drinking water infrastructure can be utilized, and treated water is not polluted in currently required environmental buffers. To protect public health when implementing DPR, advanced treatment processes are needed. Osmotic membrane bioreactors (OMBR) are novel multi-barrier systems that produce high quality potable water from impaired streams. Wastewater facilities can be retrofitted to incorporate these technologies without substantial changes to existing treatment processes, eliminating the need to construct new DPR facilities. Considering these benefits, OMBRs have the potential to be a valuable technology to implement DPR. |
1. Introduction
Diminishing fresh water supplies in arid regions and increasing water demand in growing urban centers has prompted increased interest in indirect and direct potable reuse of impaired water.1 Both conventional and advanced treatment processes can be used—alone and in combination—to achieve the required water quality for specific beneficial reuse. Conventional activated sludge processes coupled with tertiary treatment have been used for many years to reclaim water for non-potable reuse. Membrane bioreactors (MBR) are a relatively new wastewater treatment technology that combines activated sludge processes with porous microfiltration (MF) or ultrafiltration (UF) membrane separation as a substitute to clarification (Fig. 1). MBRs are more efficient treatment technologies for non-potable reuse of reclaimed water,2–4 and they can serve as pretreatment ahead of other processes for indirect and direct potable reuse of impaired water.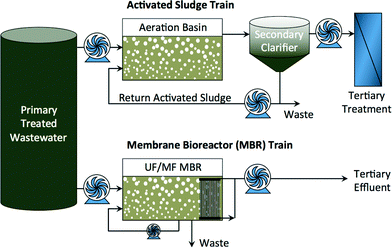 | ||
| Fig. 1 Activated sludge treatment train and MBR treatment train of domestic or industrial wastewater. | ||
The advantages of MBRs over conventional wastewater treatment processes have been thoroughly reviewed.5 These include consistent product water quality, ease of operation and automation, small physical footprint, reduced sludge production due to high biomass concentration in the bioreactor, and very high rejection of suspended solids (TSS rejection >99%) and particles (turbidity rejection >98%), including pathogenic microorganisms (5–8 log removal of total coliform).5,6 For potable reuse, a multiple-barrier treatment approach is required in order to protect public health.1 Porous membranes may not be adequate on their own to provide this protection because of their limited rejection of viruses, ions, and trace organic compounds (TOrCs).7 Yet, MBRs can be coupled with a downstream reverse osmosis (RO) and advanced oxidation processes to comply with more stringent water quality regulations.8–13
Another major problem associated with the operation of MF or UF MBRs is membrane fouling. Membrane fouling occurs on the MBR membranes and may occur on downstream RO membranes.14 Specifically, dissolved organic compounds (DOC) in the MBR permeate can cause severe organic fouling on RO membranes and provide substrate for microbial growth, which may exacerbate biological fouling on RO membranes.15 Membrane fouling lowers productivity, increases energy requirements and operation cost, increases frequency of membrane cleaning and replacement, and may result in deterioration of treated water quality.16
To reduce fouling and enhance rejection of dissolved species and small particles, forward osmosis (FO) membranes can be utilized in MBRs in an osmotic membrane bioreactor (OMBR) configuration. The OMBR is a multiple-barrier technology, well suited for indirect and direct potable water reuse applications.17–26 The OMBR couples activated sludge processes with a dense, semi-permeable FO membrane used for extraction of water from the low-salinity activated sludge into a concentrated draw solution. Compared to MF and UF membranes used in conventional MBRs, the FO membranes in OMBR offer the advantage of much higher rejection (semi-permeable membrane vs. porous membrane) of particles, macromolecules,27 TOrCs,28–31 and ions.32 FO membranes also have lower fouling propensity than MF or UF membranes,33,34 and thus require less air scouring and much less frequent cleaning. When comparing an OMBR system (OMBR followed by RO (Fig. 2)) with a conventional MF or UF MBR followed by an RO system, the high rejection of organic and inorganic constituents by the FO membrane results in an RO influent with lower fouling potential and higher RO permeate quality (two tight semi-permeable membrane barriers).35
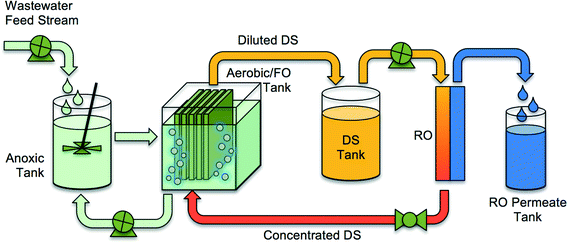 | ||
| Fig. 2 A schematic of an integrated OMBR system containing an FO MBR and RO system for reconcentration of draw solution. Adopted from Holloway et al.21 | ||
The driving force for water flux in OMBRs is the difference in osmotic pressure between the draw solution and the activated sludge. The draw solution is typically made with inorganic salts such as sodium chloride or magnesium chloride, but other inorganic and organic salts with high osmotic pressure at relatively low concentrations can also be used as draw solutions.36,37 The draw solution is diluted in the FO process as water diffuses through the membrane from the activated sludge and absorbs into the draw solution. The diluted draw solution can be returned back to the source reservoir (e.g., ocean) or reconcentrated using a secondary separation process to produce a stream of purified water and a stream of reconstituted draw solution. The draw solution reconcentration step may utilize thermal,38 crystallization,39 or membrane,17,40 processes, or a combination of them. In most cases, the draw solution reconcentration process provides a high-level treatment step, resulting in treated water suitable even for potable reuse.29,41
One of the current inherent limitations of OMBRs is the continuous loss of solutes from the draw solution that have to be replenished to achieve sustainable operation. In FO and OMBR applications solutes reverse diffuse from the draw solution into the feed stream (activated sludge) due to the high concentration difference across the membrane,21,42 and subsequently accumulate in the activated sludge. In OMBRs that utilize RO to reconcentrate the draw solution, more draw solution solutes are lost across the RO membranes with the RO permeate—solutes diffuse across the RO membrane due to the concentration difference and the semi-permeable nature of the RO membrane. Techniques and methods to reduce draw solution solute loss and its adverse impacts (e.g., inhibition of biological activity and decline in osmotic driving force) are further discussed in section 6.2 of this article.
The benefits and limitations of OMBR treatment, outlined above, have been discovered through bench- and small pilot-scale studies. Information about these studies is summarized in Table 1. Benchmarks for OMBR performance (water flux and salt accumulation) and nutrient removal have been established through bench-scale studies operated for relatively short time periods (less than two months) using synthetic feed solutions (after seeding the system with real activated sludge obtained from conventional activated sludge or MBR treatment plants), and nearly all of the studies utilized a cellulose triacetate (CTA) membrane from Hydration Technology Innovations (HTI, Albany, OR). The bench-scale investigations were conducted without a reconcentration step to maintain the draw solution at constant concentration; instead, highly concentrated brine was intermittently dosed into the draw solution reservoir to maintain constant draw solution concentration in the OMBR. These short-term, bench-scale studies conducted with synthetic feed solutions and without draw solution reconcentration step, are a good first step to understanding the performance of OMBR treatment and should serve as a bridge to pilot- and full-scale testing. One long-term pilot-scale study was conducted with a high-pressure RO system to reconcentrate the draw solution.21 This study helped in defining some of the advantages and shortcomings of using RO for draw solution reconcentration. In addition to more pilot-scale studies, more uniform approaches to OMBR design and operation are needed. These include membrane configurations, sludge retention time (SRT), and hydraulic retention time (HRT); and overcoming problems associated with OMBR operation, including salt accumulation in the bioreactor and membrane fouling. Previous articles, including one review article, only minimally discussed OMBR design,43 and they did not focus on problems specific to OMBR design and operation.44–46 This article aims at highlighting the findings from current bench- and pilot-scale studies, defining the benefits, limitations, and current state of OMBR technology, and establishing a preliminary framework for the design and operation of future OMBR systems.
| Operating time (days) | Feed solution | Bioreactor volume (L) | FO membrane | Separation process | HRT (days) | SRT (days) | Ref. |
|---|---|---|---|---|---|---|---|
| a Draw solution concentration was maintained constant using a concentrated-brine dosing system, not a separation process for reconcentration. b Values estimated from reported water flux and tank volumes. Sludge wasting not including in HRT calculation because wasting rate was not provided in the original manuscript. c Values estimated from reported water flux, tank volumes, and sludge wasting rate. | |||||||
| 7 | Synthetic | 5 | CTA | Replenisha | ND | ND | 18 |
| 14 | Synthetic | 7 | CTA | Replenisha | 21–40 | ND | 47 |
| 28 | Synthetic | 14 | CTA | RO | 3.5 | 15 | 17 |
| 30 | Synthetic | 4 | NA | Replenisha | ND | 20 | 48 |
| 32 | Synthetic | 7.6 | CTA | Replenisha | 0.8–2.8a | 10 | 49 |
| 40 | Synthetic | 4.9 | CTA | Replenisha | 0.6 | 50 | 22 |
| 45 | Synthetic | 7.6 | CTA | Replenisha | 0.4–0.6 | 10 | 23 |
| 55 | Synthetic | 4 | TFC | Replenisha | 0.3–1.8 | 10 | 19 |
| 73 | Synthetic | 4 | CTA | Replenisha | ND | 1.4 | 20 |
| 108 | Synthetic | 5 | CTA | Replenisha | ND | 14–24c | 24 |
| 124 | Municipal | 275 | CTA | RO | 1.0–5.2 | 2–19 | 21 |
| 125 | Municipal | 340 | CTA | RO | 1.4–4.6 | 2.3 | 21 |
| 150 | Synthetic | 3.6 | CTA | Replenisha | 3.9–4.6 | 0.6–1.6 | 23 |
2. OMBR configurations
In OMBRs, biological oxidation and reduction of organic (carbon) and inorganic (nitrogen) constituents is achieved in the bioreactor, and solids separation is accomplished using semi-permeable FO membranes. The bioreactors can be arranged in different configurations depending on the treatment goal. The configuration of the membranes with respect to the bioreactors can vary; however, they are most commonly configured as an external membrane skid or a submerged membrane cassette.The volume of the bioreactors is as important as the bioreactor configuration because the volume, influent, and sludge wasting flowrates dictate the time that the water is treated in the system (HRT) and the time that the activated sludge remains in the system (SRT). The HRT and SRT are fundamental treatment parameters that influence carbon and nitrogen removal, and water recovery in biologically active wastewater treatment processes. Accumulation of dissolved constituents in the bioreactors is a unique phenomenon in OMBRs—it is detrimental to the processes and it is a function of the HRT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SRT ratio, but it can be relatively easily controlled in hybrid OMBR systems.
SRT ratio, but it can be relatively easily controlled in hybrid OMBR systems.
Hybrid OMBRs incorporate processes or methods to remove salts from the bioreactors. Examples of processes that have been combined with OMBRs are porous UF and MF membrane systems that extract salts from the bioreactors with the UF or MF permeate.21,23 A method to maintain low bioreactor salinity that has been studied is to use a decant step, which involves settling the sludge and draining the clear supernatant from the system.22 The following section focuses on bioreactor configurations, but due to the importance of HRT and SRT, a detailed discussion of these parameters and implication of these parameters to OMBR operation is provided in section 5.2.
2.1. Bioreactors
The majority of past OMBR studies have been conducted using a single aerated bioreactor coupled with either an external cross-flow membrane cell,18,25,48 a submerged plate-and-frame cassette,17,20–24,26,40,49 or a submerged hollow-fiber membrane bundle.19 A single reactor simplifies the design, but may not be ideal for continuous treatment because ammonia and nitrate present in the activated sludge readily diffuse across FO membranes into the draw solution and may compromise the final product water quality—depending on the draw solution reconcentration process used.21 The concentration of nitrogen species can be reduced in a single reactor design by alternating between anoxic and aerobic conditions (sequencing batch bioreactor (SBR) configuration), but it is difficult to avoid accumulation of nitrogen species in the draw solution if water is continuously being extracted from the activated sludge through the FO membrane in a single reactor.An alternative to the single reactor design that could prevent transport of nitrogen species into the draw solution is to biologically treat the wastewater for carbon and nutrient removal in a batch reactor with the FO membranes submerged in a separate filtration tank or installed in an external cross-flow membrane skid. In this configuration and operating scheme, complete nitrification and denitrification can be achieved in a batch-reactor with alternating anoxic and aerobic phases before water from the activated sludge is extracted through the FO membranes. A similar design has been tested at demonstration-scale with a sequencing-batch MBR (SBMBR) employing UF membranes for filtration.50 It was demonstrated that the SBMBR could achieve near complete nitrogen removal in the bioreactors before being processed through the UF membrane filtration tanks. This batch reactor design is a significant upgrade over the single reactor configurations, which are susceptible to draw solution contamination.
Another typical bioreactor arrangement for nitrogen removal include an anoxic zone for denitrification followed by an aerobic zone for nitrification.51 Water nitrified in the aerobic zone is recirculated to the anoxic zone in which nitrate is reduced to nitrogen gas in the presence of organic carbon. This arrangement is highly efficient for traditional municipal wastewater treatment because no chemical carbon addition is required for denitrification, and aeration requirements are reduced because a portion of the organic carbon load is removed during denitrification. However, this bioreactor configuration must be re-designed for OMBR systems because nitrate will continuously diffuse into the draw solution if the FO membranes extract water from the primary nitrification stage.21 Holloway et al.21 operated an OMBR with an anoxic zone before the aerobic zone (where the FO membrane cassette was installed), in which the organic carbon contained in the incoming raw wastewater was used as the electron donor in the denitrification process in the anoxic tank. They have demonstrated that the draw solution nitrate concentration reaches levels very close to the concentration in the aerobic bioreactor even when denitrification in the pre-anoxic bioreactor was complete. While nitrate is being reduced to nitrogen gas in the anoxic stage, incoming ammonia is transformed to nitrite and nitrate only in the aerobic (FO) tank, thus nitrate is at its highest concentration there and therefore easily diffuses into the draw solution. The presence of nitrate in the primary aerobic stage and in the draw solution, even when there is complete denitrification in the pre-anoxic zone, can be explained by a simple nitrogen mass balance in the primary aerobic stage:
| AccN = (QFO + QREC)CAN-N − QRECCAR-N − BN(CAR-N − CDS-N)Am | (1) |
AccN = (QFO + QREC)CAN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) - -![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N − QRECCAR N − QRECCAR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) - -![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N N | (2) |
Furthermore, if steady-state state conditions are assumed so that AccN = 0 and the equation is rearranged, CAR-N can be solved as a function of QFO, QREC, and CAN-N:
 | (3) |
Thus, in the two-reactor design (anoxic stage followed by aerobic stage), even at very high recirculation rates (QREC ≫ QFO), there will always be nitrate in the aerobic stage that will diffuse into the draw solution over time. An alternative strategy to preventing nitrate diffusion into the draw solution is to have a series of alternating anoxic and aerobic zones (similar to the Bardenpho process52) to remove nitrogen before water is processed through the FO membranes.
A bioreactor and membrane configuration that has recently been tested and that can partially alleviate nitrogen accumulation in the bioreactors is coupling of OMBR with MF or UF membranes.21,23 Holloway et al. investigated a hybrid UF OMBR (UFO-MBR)21 and Wang et al. investigated a hybrid MF-OMBR,23 and both were able to demonstrate more consistent nitrogen removal in these systems compared to other OMBR studies, in part due to the partial removal of ammonia and nitrate through the micro-porous membranes. Another benefit of the hybrid system is that phosphorus, which is well rejected by FO membranes,27,53–55 and other solutes (potassium, magnesium, and calcium) can be harvested from the OMBR for beneficial use.56 Extracting dissolved solids from the OMBR bioreactors through the MF and UF permeate improves FO water flux (reduced concentration difference between the activated sludge and draw solution) and prevents inhibition of nitrifying and denitrifying microorganisms in the activated sludge due to elevated bioreactor salinity. More details on the subject are provided in section 6.2.
2.2. External cross-flow membrane cells and submerged membranes
The concept of external cross-flow and submerged membranes for OMBRs is still new and requires a thoughtful discussion on what will be the most appropriate configuration for pilot- and full-scale instillations in regards to energy demand, fouling prevention (e.g., feed spacer selection and air-scour rates), and draw solution flow channel design. There is limited information on OMBR feed side design; thus, established design parameters for MBRs and preliminary findings from OMBR studies are used in the following section to establish a preliminary feed side design framework for OMBRs. There is even less data on draw solution channel design than there is on the feed side design because operational problems such as head loss through the draw solution channel and vacuum limitations are not relevant in bench-scale testing. Therefore, the discussion on draw solution hydraulic design is focused on foreseeable problems with scaling up the OMBR from bench-scale to pilot- and full-scale.Submerged plate-and-frame or hollow-fiber membrane systems have been investigated at both bench- and pilot-scale and could be well suited for full-scale OMBRs.17,19–24,26,40,49 In this configuration, the membranes are immersed in the activated sludge with coarse-bubble diffusers placed below the membrane modules to airlift activated sludge across the membrane and air-scour foulants from the membrane surface. The spacing between the plates, fiber density in the bundle, and the total suspended solids concentration in the bioreactor also play an important role in preventing membrane fouling in submerged configurations. Two FO membranes (plates and frame) that experienced different intensity of air scouring are illustrated in Fig. 3.
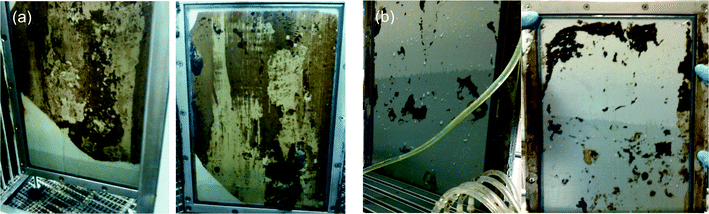 | ||
| Fig. 3 FO CTA membrane fouling during long-term pilot-scale testing (a) with inadequate aeration (b) with sufficient aeration to prevent membrane fouling. Adapted from Coday et al.65 | ||
As shown in Fig. 3, inadequate membrane air scouring may result in severe membrane fouling and loss of productivity (water flux). The aeration intensity required to reduce membrane fouling (specific aeration demand (SAD))66,67 for conventional MF and UF submerged MBRs has been well documented, but has not been thoroughly studied for OMBRs. It was demonstrated that for UF and MF MBRs approximately 29 m3 of air per hour per m2 of membrane area is needed for plate and frame membrane modules and approximately 15 m3 h−1 m−2 of air is needed for submerged hollow-fiber membrane to prevent membrane fouling.3,4 It is important to note that the height of the membrane module plays an important role in controlling the magnitude of the SAD—taller membrane elements will have lower SAD requirements because a unit volume of air is in contact with more membrane area as it travels up through the channels in a membrane cassette or between the fibers in a membrane bundle to the surface of the bioreactor. It is also desired to optimize the membrane air-scouring rate to reduce energy consumption of MBR systems; it has been shown that membrane aeration accounts for close to 70% of the energy consumption of submerged MBRs.3 Most importantly, because air is also used in the bioreactor to oxidize organic matter and nutrients, a thorough design is needed that considers both fouling prevention and aeration requirements for the biological process.
In a recent OMBR air-scour study conducted by Luo et al.,47 the authors demonstrated that the SAD was in fact lower for OMBR-FO membranes compared to traditional MF membranes treating activated sludge operating at similar water fluxes. The OMBR water flux was shown to be constant in their investigation at SADs as low as 8 m3 m−2 h−1 for the OMBR. In another OMBR study by Holloway et al.,21 conducted with a larger plate-and-frame cassette (1.2 m2 of CTA membrane), it was shown that water flux was constant over 125 days of operation at SADs as low as 1.5 m3 m−2 h−1. The difference in the minimum required SAD for OMBRs demonstrated by Luo et al. and Holloway et al. is not completely known but it may be associated with spacing between plates (channel spacing) and air bubble geometry. Channel spacing and bubble geometry (fine- or coarse-bubble) have been shown to be critical in increasing the effectiveness of air-scouring, with smaller channel spacing and bubbles having a diameter similar to the channel size being the most efficient at preventing membrane fouling.68 Luo et al.47 used only one plate that was submerged in the OMBR with no mention of channel spacing between the membrane and another surface. The channel spacing between plates in Holloway et al.21 was approximately 6 mm, which may have improved the effectiveness of the membrane air scouring in preventing fouling. Regardless of the mechanisms controlling the difference in required SAD between these two studies, the lower potential SAD for OMBRs could result in reduced energy demand for membrane air scouring and total OMBR energy use.
In the plate-and-frame configuration the draw solution flows between the membranes and the plate and in hollow-fiber configuration the draw solution flows on the bore side of the fibers. In plate-and-frame skids the draw solution must flow under vacuum (negative pressure) to prevent damage to the membrane and blockage of the feed flow channels due to stretching of the membranes away from the plates. This requirement poses limits on the length of the draw solution flow channels and the type and number of draw solution spacers in the channel—requiring special consideration for the head loss in the draw solution channels (generally, not more than 40 kPa below atmospheric pressure).
The draw solution can be pumped under positive or negative pressure through hollow-fiber or capillary membranes because of their inherent self-supported structure. The draw solution flowrate may be limited due to high head loss developed through the fiber length if the hollow-fiber FO membranes have very small internal diameter (<150 μm). There has been much interest in the development FO hollow-fiber membrane, especially for pressure retarded osmosis (PRO) applications, because of the intrinsic mechanical strength of the hollow-fiber geometry to resist the hydraulic pressure applied in PRO.72–74 One problem with these membranes for OMBR treatment is that the active layer is on the inside (bore side) of the membrane fiber and the internal diameter is relatively small (<1 mm). It would be impractical to pump activated sludge through the bore side of these membranes; therefore, development of membranes with the active layer on the outside (shell side) of the membrane, like those described in Shi et al.,75 will be required for OMBR implementation.
2.3. System integration
Like in all osmotically driven membrane processes, the draw solution used in OMBR is diluted when water is drawn from the activated sludge through the FO membrane—it must be replaced, replenished, or reconcentrated to sustain the driving force for mass transport and the continuous operation of the process. Three common operating strategies are used in FO for draw solution management. These include operation in osmotic dilution mode,25,53 continuous replenishment of the draw solution,18,19 or reconcentration of the draw solution using distillation55,76 or membrane processes.17,21,27,77,78Osmotic dilution for pilot- or full-scale applications has been considered for FO systems coupled with seawater RO desalination.41,77,80 In one implementation, seawater can be diluted using FO upstream of an RO process to dilute the seawater, increasing water recovery and decreasing the specific energy (kWh m−3 water produced) of RO seawater desalination. Alternatively, FO is implemented downstream of the RO desalination process to dilute the RO brine before being discharged to the environment, thereby reducing the potential negative effects of brine discharge.81,82 Cath et al.80 envisioned a combined FO–RO system in which a secondary treated wastewater stream is used to dilute seawater through an FO process prior to RO seawater desalination, and an FO system downstream of the RO system to dilute the concentrated RO concentrate before brine disposal. Achilli et al.83 investigated a combined RO–PRO system in which a secondary treated wastewater stream is used to dilute the concentrated RO reject before brine disposal in order to recover the salinity gradient energy between the two streams and decrease RO specific energy consumption. These coupled processes could effectively be translated to OMBR applications where water reuse and desalination are combined into one integrated treatment system. Two configurations of an integrated FO system that can conveniently incorporate OMBR are illustrated in Fig. 4.
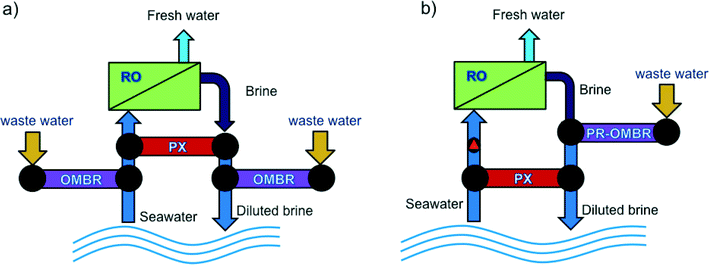 | ||
| Fig. 4 Schematic drawings illustrating two potential integrated FO systems in which OMBR can be incorporated in an osmotic dilution configuration: (a) OMBR-RO-OMBR hybrid system for enhanced water recovery and reuse in seawater desalination, and (b) PR-OMBR-RO hybrid system for water desalination and energy recovery. Adapted from Achilli et al.84 | ||
Another shortcoming of the reconcentration method is the potential high energy consumption of the desalination process. Because high draw solution concentrations must be used to maintain high water fluxes through the current commercial FO membranes, the energy demand of the reconcentration process might be significant. High pressure RO is commonly cited as an appropriate technology for draw solution reconcentration; however, the energy consumption of the RO stage can range from 2 to 4 kWh m−3 to maintain draw solution concentrations of 0.5 to 1.0 M NaCl.87 The loss of salts through the RO membrane, especially at higher draw solution concentrations, could also increase the operating cost of the OMBR because salts continuously diffuse through the RO membranes with the permeating water and must be intermittently added to the draw solution to maintain continuous operation.88 The amount of salts lost per volume of water produced can be minimized by appropriately sizing the RO system. The RO system should be designed for high water flux and element recovery to ensure that the salt concentration in the RO permeate is very low. It can be difficult to design and construct an optimized RO system for smaller pilot-scale OMBRs because commercially available RO elements are oversized (more membrane area and higher water fluxes) compared to external cross-flow FO membrane cells or plate-and-frame FO membrane cassettes that are custom made for OMBRs.
There has been increased interest in using thermal separation process such as membrane distillation (MD) and thermal distillation to reconcentrate the draw solution.38,89–91 These processes could be relatively easily integrated into wastewater treatment facilities that use anaerobic digestion or combustion processes to produce biogas or heat from the carbon contained in the raw wastewater and activated sludge biomass. In MD, hydrophobic, microporous membranes are used to separate a heated brine stream from a cool distillate stream. Water evaporates at the pore entrance and vapors diffuse through the membrane pores, driven by the difference in vapor pressure between the heated brine stream (high vapor pressure) and colder distillate stream (lower vapor pressure).92 The draw solution must be free of ammonia or the MD distillate stream will be contaminated because ammonia will diffuse across MD membranes. Assuming ammonia contamination of the draw solution can be mitigated by ammonia oxidation (nitrification) in the bioreactors, MD has been shown to reject greater than 97% of low molecular weight organics and TOrCs,93,94 making the coupled OMBR-MD system especially suited for potable reuse applications,80 Also, very high draw solution concentrations can be used (upward of 300 g L−1-NaCl) in MD because the vapor pressure driving force is minimally affected by ion concentrations.95 However, the combined thermal and electrical energy demand for the various MD processes (50 to 350 kWh m−3)96 far exceeds the theoretical specific energy contained in the carbonaceous fraction of municipal wastewater (approximately 1.9 kWh m−3 of wastewater).97 Considering the relatively low specific energy contained in municipal wastewater, MD for draw solution reconcentration may only be applicable in locations where low grade, “waste” heat is available in large quantities.
Distillation has also been considered for reconcentration of thermolytic salts draw solutions when thermal energy is available.38,89,90,98 The most commonly used thermolytic draw solution in FO operations is the ammonia–carbon dioxide solution.38,89,90 McCutcheon et al.89 demonstrated that the ammonia–carbon dioxide draw solution could be reconcentrated with distillation processes to much higher osmotic pressures than conventional inorganic draw solutions concentrated with RO, allowing for higher FO water flux. The electrical energy demand of the ammonium bicarbonate distillation system was reported to be 0.24 kWh m−3 by McGinnis and Elimelech,90 well below the theoretical specific energy contained in wastewater. However, using the equations provided in their article for the thermal energy demand and gained output ratio (GOR) (4.4) and enthalpy of evaporation of steam at 40 °C (2408 kJ kg−1), the calculated thermal energy demand for the ammonia–carbon dioxide distillation process is approximately 150 kWh m−3, which is also much higher than the theoretical specific energy contained in wastewater. MD of inorganic salts and thermal distillation of thermolytic salts may be appropriate for certain OMBR scenarios where draw solution having high osmotic pressures or high distillate quality are required, or in locations were low-grade heat is readily available. Otherwise, RO is currently the best option for OMBR draw solution reconcentration due to the relatively low specific energy demand.
3. OMBR membranes: materials and orientations
Most OMBR studies to date have used cellulose triacetate (CTA) flat-sheet FO membranes,17,18,20,21,24–26,48,49 with the exception of one OMBR study that used a thin-film composite (TFC) hollow-fiber membrane19 developed by the investigators at the Singapore Membrane Technology Centre. It is difficult to compare the CTA membrane performance to that of the TFC hollow-fiber membrane tested in Singapore because the TFC membrane was operated with the active layer of the membrane facing the draw solution, which dramatically changes the membrane performance due to differences in concentration polarization and fouling mechanisms. In FO studies, TFC membranes demonstrated much higher water permeability than CTA membranes;99 however, the performance of TFC membranes for OMBR treatment is not known and should be further investigated in future research. Due to the lack of information on TFC membranes in OMBRs, the following discussion is primarily focused on CTA membrane material, benefits, and limitations, and on two membrane orientations that are commonly used in FO and OMBRs studies: FO mode and PRO mode.3.1. Membrane materials
The CTA membrane used in almost all OMBR studies is manufactured by HTI and has been shown to be well suited for osmotically driven membrane processes because it is resistant to fouling, very thin (less than 50 μm), and lacks the thick support layer typical of most semi-permeable, TFC membranes. The mechanical support of the membrane is provided by a very thin polyester mesh over which the CTA is cast.89 It is important that the membrane support layer be thin and porous to reduce internal concentration polarization (ICP) within the pores; high ICP lowers the driving force (osmotic pressure difference between the feed and draw solution) for water flux.79,100,101 A quantitative measurement often used to compare the support layer of different OMBR-FO membranes is the structural parameter (S), which is calculated using the support layer thickness (l), tortuosity (τ), and porosity (ε):102 | (4) |
Although the CTA membrane has a low structural parameter compared to other semi-permeable membranes (e.g., RO membranes) and thus exhibit high water flux at moderate osmotic pressure driving forces, the CTA membrane may not be the most suitable membrane for OMBRs. This is because CTA is potentially biodegradable103,104 and has a narrow pH operating range of 4–6.105 Therefore, the development and implementation of more chemically stable, polyamide-based TFC membranes is important for OMBR applications. Membranes that have shown potential promise for OMBR purposes are new FO TFC membranes developed by the Singapore Membrane Technology Centre,19 Oasys Water, Inc. (Oasys Boston, MA), and HTI.106
3.2. Membrane orientation
Another approach to reducing ICP in FO and OMBRs is to orient the membrane with its active layer facing the draw solution.100,107 This alignment is used in PRO applications and is commonly referred to as the “PRO mode” (sometime called active layer facing draw solution (AL-DS)). The advantage of operating in the PRO mode is that dilutive ICP on the draw solution side of the membrane is substantially reduced; thereby, increasing the water flux.108 However, the main drawback of the PRO mode in OMBRs is that foulants, including organic solids and inorganic salts, are entrapped in the pores of the support layer, resulting in a substantial decline in water flux due to pore clogging (largely irreversible) or concentrative ICP.79,109 Because membrane fouling is increased when operating in the PRO mode, most OMBRs and FO processes treating wastewaters with high fouling potential are operated with the active layer facing the feed solution (FO mode). Membranes operated in FO mode have been shown to be highly resistant to fouling compared to traditional pressure driven membrane processes;34 however, due to the potential advantage of operating an OMBR in PRO mode, the OMBR has been evaluated in both PRO and FO mode. Membrane materials, orientation, and configurations in several past OMBR studies are summarized in Table 2.| Membrane material | Membrane area (cm2) | Membrane orientation | Membrane configuration | Ref. |
|---|---|---|---|---|
| CTA | 110 | FO and PRO | Flow-through cell | 25 |
| CTA | 160 | PRO | Flow-through cell | 18 |
| CTA | 170 | FO | Plate and frame | 17 |
| CTA | 180 | FO and PRO | Plate and frame | 26 |
| TFC | 250 | PRO | Hollow fiber | 19 |
| CTA | 300 | FO | Plate and frame | 47 |
| CTA | 360 | FO | Plate and frame | 24 |
| CTA | 370 | FO | Flow-through cell | 48 |
| CTA | 370 | FO | Plate and frame | 20 |
| CTA | 560 | FO | Plate and frame | 49 |
| CTA | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (1 m2) 000 (1 m2) |
FO | Plate and frame | 21 |
| CTA | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (1.2 m2) 000 (1.2 m2) |
FO | Plate and frame | 21 |
4. Draw solutions
Like in all FO applications, OMBR performance is affected by the choice of draw solutions.36 There are three main criteria for selecting a draw solution for OMBRs, they have to induce high water flux, have low reverse salt flux (RSF) at moderate draw solution concentrations (see section 1), and be nontoxic to microorganisms. RSF is typically lower for draw solutions containing divalent ions with a large hydrated radius (e.g., MgCl2, MgSO4) compared to draw solutions made of monovalent ions (e.g., NaCl); however, draw solutions made of divalent ions tend to have lower water flux than draw solutions made of monovalent ions at similar osmotic pressures.36,42,110,111 The lower RSF and water flux for draw solutions made of divalent ions is due to the lower diffusivity coefficient of divalent ions compared to monovalent ions in solution. Low RSF is important for OMBR applications because salts that reverse diffuse from the draw solution accumulate in the activated sludge, which subsequently decrease the driving force for water flux (concentration difference between feed and draw solution)17,20,112 and may be toxic to the microbial community.18,111,113,1144.1. Inorganic draw solution
NaCl is widely used as an inorganic draw solution in FO and OMBR because it exhibits high water flux at moderate draw solution concentrations, it is easy to dissolve and store, has a relatively low cost, and is readily available.36 However, higher RSF compared to other inorganic draw solutions is one of the main downsides of NaCl as a draw solution. Other draw solution solutes exhibit lower RSF; however, they are generally more expensive and the water flux induced by them is lower at equivalent osmotic pressures compared to NaCl.36 Process performance with various inorganic draw solutions used in past OMBR investigations is summarized in Table 3. These include water flux, RSF, and specific RSF (SRSF,115 which is the RSF normalized by the water flux). Due to the limited number of OMBR studies conducted with draw solutions other than NaCl, the performance of other inorganic draw solutions used in FO are also summarized in Table 3.| OMBR studies | |||||
|---|---|---|---|---|---|
| Solute | Draw solution concentration (M) | Pure water flux (L m−2 h−1) | RSF (g m−2 h−1) | SRSF (g L−1) | Ref. |
| NaCl | 0.51–1.19 | 7.0–14.5 | 4–11 | 0.75 | 17 |
| NaCl | 0.005–0.1 | ND | ND | 0.75 | 116 |
| MgCl2 | 0.51 | 7.5 | ND | ND | 22 |
| MgCl2 | 0.005–0.1 | ND | ND | 0.58 | 116 |
| KCl | 0.005–0.1 | ND | ND | 1.14 | 116 |
| CaCl2 | 0.005–0.1 | ND | ND | 0.82 | 116 |
| NH4HCO3 | 0.005–0.1 | ND | ND | 2.01 | 116 |
| (NH4)2SO4 | 0.005–0.1 | ND | ND | 0.36 | 116 |
| Na2SO4 | 0.005–0.1 | ND | ND | 0.33 | 116 |
| K2SO4 | 0.005–0.1 | ND | ND | 0.40 | 116 |
| FO studies | |||||
|---|---|---|---|---|---|
| Solute | Draw solution concentration (M) | Pure water flux (L m−2 h−1) | RSF (g m−2 h−1) | SRSF (g L−1) | Ref. |
| a Values estimated from reference. ND – References that did not provide data for pure water flux, RSF, and SRSF (no data). | |||||
| NaCl | 0.31–0.87 | 6.22–12.2 | 4.6–9.1 | 0.74–0.75 | 36 |
| NaCl | 0.65 | 8.2a | 5.8a | 0.7a | 111 |
| MgCl2 | 0.21–0.50 | 5.67–9.72 | 3.4–5.6 | 0.59–0.58 | 36 |
| MgSO4 | 0.61–1.17 | 4.25–5.54 | 0.9–1.2 | 0.20–0.21 | 36 |
| MgSO4 | 1.24 | 2.18 | 0.24 | 0.11 | 111 |
| KCl | 0.31–0.94 | 6.73–13.5 | 6.8–15.3 | 1.01–1.14 | 36 |
| CaCl2 | 0.22–0.56 | 6.30–11.6 | 4.8–9.5 | 0.76–0.82 | 36 |
| Ca(NO3)2 | 0.26–0.80 | 5.98–10.7 | 3.7–6.6 | 0.63–0.62 | 36 |
| NH4HCO3 | 0.26–0.87 | 5.47–10.3 | 11.7–20.6 | 2.14–2.01 | 36 |
| (NH4)2SO4 | 0.30–0.83 | 5.94–9.86 | 2.5–3.6 | 0.43–0.36 | 36 |
| NH4Cl | 0.32–0.90 | 6.77–13.0 | 5.3–10.2 | 0.79–0.79 | 36 |
| Na2SO4 | 0.29–0.90 | 5.33–9.22 | 1.9–3.1 | 0.36–0.33 | 36 |
| K2SO4 | 0.28–0.58 | 6.26–9.07 | 2.3–3.7 | 0.37–0.40 | 36 |
| KHCO3 | 0.32–0.99 | 5.33–10.1 | 0.8–2.0 | 0.14–0.20 | 36 |
| KBr | 0.32–0.88 | 6.62–12.9 | 12.3–29.2 | 1.86–2.26 | 36 |
The highest SRSF for inorganic draw solutions tested in an OMBR and in FO were for KCl, KBr, and NH4HCO3 draw solutions, indicating that potassium based salts coupled with a monovalent anion are not well suited for OMBRs. The thermolytic salt ammonia–carbon dioxide (NH4HCO3) has one of the highest SRSF of draw solutions tested in OMBRs and FO.36,116 The RSF of ammonia–carbon dioxide is not likely to increase the bioreactor salinity because both ammonia and bicarbonate can be removed from the bioreactors via nitrification and denitrification; however, if high diffusion rate of carbon dioxide from the draw solution to the bioreactors occurs (depending on solute speciation in the draw solution), the pH of the activated sludge might decline, which is detrimental to the growth of nitrifying microorganisms below pH of 7.117
The lowest SRSF in OMBRs and FO were tested for draw solutions containing sulfate, including MgSO4, Na2SO4, NH4SO4, and K2SO4. Sulfate salts are potentially good draw solutions for OMBRs because of their low RSF; however, they might produce hydrogen sulfide and sulfuric acid in the bioreactors, and may cause scaling on the OMBR-FO and RO membranes (if used for draw solution reconcentration) by sulfate complexes. Zhang et al.118 have shown in a study conducted with a membrane oriented in the PRO mode that FO membranes are susceptible to gypsum scaling (a calcium sulfate precipitate). Gypsum scaling on FO membranes was also demonstrated to be more severe in the presence of organic matter119—calcium is accumulated to relatively high concentrations in OMBRs treating municipal wastewater21 and organic matter is in abundance in the bioreactors, providing the environment for gypsum scaling when using a sulfate rich draw solution.
Achilli et al.36 determined that the most economical draw solutions per liter of water produced in a coupled FO–RO system were NaHCO3 ($0.009 L−1), NaCl ($0.013 L−1), KHCO3 ($0.015 L−1), and MgCl2 ($0.018 L−1). The draw solutions containing bicarbonate (HCO3) are an economical option, but diffusion of bicarbonate from the draw solution to the feed could increase the pH; however, this draw solution may assist in maintaining moderate pH levels in the activated sludge for systems that incorporate biological nitrification processes that involve consumption of alkalinity. Another limitation to using NaHCO3 as a draw solution is that the pKa of the solution is between 6.4 and 10.3, above the pH limit for CTA FO membranes (~7).
Considering the cost and potential drawbacks of past studied draw solutions, NaCl and MgCl2 are probably the two best draw solution options for use in OMBRs. NaCl is inexpensive and has high solubility and MgCl2 is also relatively inexpensive but can complex with organic matter, sulfate, and phosphorus to foul or scale the membrane. However, the fouling potential is much lower compared to calcium.120,121 It is worth noting that magnesium scaling was not observed in the only long-term OMBR evaluation conducted with a MgCl2 draw solution.22
A recent development in inorganic draw solutions is the implementation of mixed salts draw solutions. Holloway et al.110 studied the effect of small additions of MgCl2 and other solutes to a majority NaCl draw solution. The RSF was reduced and the water flux was the same for the mixed MgCl2–NaCl compared to a pure NaCl draw solution at similar draw solution osmotic pressures. Nguyen et al.122 showed a substantial reduction in RSF and only small decline in water flux with the addition of the surfactant Triton X100 to a majority Na3PO4 draw solution. It is likely that these two studies have just scratched the surface of innovative draw solution chemistries that will improve OMBR and FO performance.
4.2. Organic draw solution
Draw solutions made of organic salts have also been considered for OMBRs because organic ions that diffuse into the bioreactors from the draw solution can be biodegraded in the activated sludge;37,111,116 yet, these draw solutions have only been tested in FO mode using deionized water feed and in cultured toxicity tests. Bowden et al.37 investigated the performance (water flux and RSF) of several organic draw solutions in FO mode and attempted to apply these results to OMBR. Ansari et al.111 screened several organic and inorganic draw solutions for anaerobic OMBR treatment by testing the water flux and RSF in FO mode, and by testing microbial inhibition using a biomethane potential apparatus. Nawaz et al.116 investigated several surfactants as draw solutions for OMBR applications; however, their study was extended to the toxicity of the surfactants to E. coli cultures only and did not include any RSF or water flux data. Water flux, RSF, and SRSF for organic draw solutions evaluated by Bowden et al.37 and Ansari et al.111 are summarized in Table 4.| Solute | Draw solution conc. (M) | Pure water flux (L m−2 h−1) | RSF (g m−2 h−1) | SRSF (g L−1) | Ref. |
|---|---|---|---|---|---|
| a Values estimated from reference. | |||||
| EDTA | 0.3 | 2.5a | 0.4a | 0.16a | 111 |
| Glucose | 1.13 | 2.3a | 0.4a | 0.16a | 111 |
| Glycine | 1.31 | 3.2a | 1.5a | 0.48a | 111 |
| Sodium formate | 0.32–1.02 | 5.9–11.7 | 3.8–7.6 | 0.065–0.65 | 37 |
| Sodium formate | 0.72 | 3.9a | 2.3a | 0.58a | 111 |
| Sodium acetate | 0.52–1.69 | 5.8–10.4 | 1.5–3.5 | 0.26–0.34 | 37 |
| Sodium acetate | 0.72 | 3.5a | 0.9a | 0.26a | 111 |
| Sodium propionate | 0.32–1.06 | 5.5–10.7 | 0.8–2.3 | 0.15–0.21 | 37 |
| Magnesium acetate | 0.54–1.85 | 5.7–8.9 | 0.6–1.1 | 0.11–0.12 | 37 |
| Magnesium acetate | 0.84 | 3.8a | 0.6a | 0.17a | 111 |
The data indicate that all the organic salts that they have tested in FO experiments could work well as OMBR draw solutions due to the low SRSF compared to inorganic salts (section 4.1). Although it is imperative that these organic draw solutions be tested in OMBR applications treating activated sludge before any conclusions on the performance (water flux, RSF, and membrane fouling) of organic draw solutions in OMBR systems are made, the methods developed by Bowden et al.,37 Ansari et al.,111 and Nawaz et al.116 are very effective in screening potential draw solutions before conducting expensive and time intensive experiments.
5. OMBR test conditions and models
Experimental OMBR test conditions differ greatly between short- and long-term investigations. Studies assessing specific aspects of the OMBR such as the effect of sludge properties on fouling,26,123 potential application of inorganic and organic draw solutions,37 and microbial toxicity to elevated solute concentrations111,116 generally involve short-term experiments conducted in the absence of a continuously operated activated sludge system. These short-term experiments are insightful but are very unique and difficult to compare.Long-term investigations evaluating water flux, solute flux, fouling, and microbial activity were conducted using activated sludge systems17–24,48,49 that were continuously or intermittently fed with municipal or synthetic wastewater.15–22,46,47 These experiments were conducted at a range of mixed liquor suspended solids (MLSS), HRT, and SRT that impact the biological and physicochemical performance of the OMBR. Test conditions for several long-term OMBR studies are summarized in Table 5.
| Operation (days) | Feed solution | Reactor pH | Sludge wasting | MLSS (g L−1) | HRT (days) | SRT (days) | SRT/HRT | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Values estimated from reported water flux and tank volumes. Sludge wasting not including in HRT calculation because wasting rate was not provided in the original manuscript. b Values estimated from reported water flux, tank volumes, and sludge wasting rate. c Experiments conducted with OMBRs coupled with UF or MF membrane systems used to reduce bioreactor salinity. | ||||||||
| 7 | Synthetic | 6.6–7.9 | ND | 3.4–3.7 | ND | ND | ND | 18 |
| 14 | Synthetic | ND | ND | 7.8–8.8 | 0.9–1.8 | ND | ND | 47 |
| 28 | Synthetic | 7 | Y | 15 | 3.5 | 15 | 4.3 | 17 |
| 30 | Synthetic | 6.6–7.9 | Y | ND | ND | 10 | ND | 48 |
| 30 | Synthetic | 6.6–7.9 | Y | ND | ND | 20 | ND | 48 |
| 32 | Synthetic | ND | Y | <1 | 0.8–2.8a | 10 | 13–3.6 | 49 |
| 40 | Synthetic | ND | Y | <1 | 0.7–3.2a | 15 | 23–4.7 | 49 |
| 40 | Synthetic | ND | Y | 6.53–7.15 | 0.6 | 50 | 83 | 22 |
| 45 | Synthetic | ND | Y | 1.1 | 0.4–0.6 | 10 | 25–16 | 23 |
| 55 | Synthetic | ND | Y | ND | 0.3–1.8 | 10 | 33–5.6 | 19 |
| 63 | Synthetic | ND | Y | ND | 0.6–0.9 | 50 | 83–56 | 22 |
| 73 | Synthetic | 6.5 | Y | ND | 1.4 | 20 | 14 | 20 |
| 108 | Synthetic | 8.5 | Y | ND | 14–24b | 50 | 87–50 | 24b |
| 124 | Municipal | 6–7 | Y | 1.0–5.2 | 2–19 | 70 | 35–3.7 | 21 |
| 125 | Municipal | 6–7 | Y | 1.4–4.6 | 2.3 | 30–60 | 13–26 | 21 |
| 150 | Synthetic | 6.9–7.6 | Y | 3.9–4.6 | 0.6–1.6 | 30 | 50–19 | 23 |
5.1. Feed solution
Synthetic feed solutions are frequently used for continuously operated bench-scale OMBR studies conducted with activated sludge. Achilli et al.17 used a 5 g L−1 meat extract with a C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of approximately 100
P ratio of approximately 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but did not contain appreciable concentration of inorganic salts. Lay et al.20 used a feed stock (C
1, but did not contain appreciable concentration of inorganic salts. Lay et al.20 used a feed stock (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 100
P ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) that included several salts such as MgSO4, FeCl3, and NH4Cl at low concentrations. Zhang et al.19 also used synthetic feed solutions containing inorganic salts, including magnesium and calcium, and observed higher bioreactor salinities compared to Achilli et al.17 operating under similar conditions.
1) that included several salts such as MgSO4, FeCl3, and NH4Cl at low concentrations. Zhang et al.19 also used synthetic feed solutions containing inorganic salts, including magnesium and calcium, and observed higher bioreactor salinities compared to Achilli et al.17 operating under similar conditions.
It is important that synthetic feed solutions used in OMBRs research contain inorganic salts having concentrations similar to the simulated wastewater because salts rejected by the FO membrane accumulate in the bioreactor. A simple method to closely simulate the inorganic salt concentrations of wastewater was developed by Zhang et al.19,48 and Qiu et al.22 who used municipal tap water to make up the synthetic feed solution in long-term OMBR studies. This resulted in more realistic wastewater characteristics.
Although studies conducted with synthetic feed solutions provide useful insight into OMBR bioreactor and membrane performance, these solutions do not adequately represent the complex organic and inorganic composition of real wastewaters. While in all published OMBR studies the bioreactors were seeded with real activated sludge from conventional or MBR wastewater treatment plants, only in two of them was the OMBR continuously fed with real municipal wastewater throughout the study.21,35 Holloway et al.21 demonstrated that in addition to sodium and chloride, calcium, magnesium, and other dissolved salts intrinsic to municipal wastewater accumulate in the OMBR, resulting in increased membrane fouling. Future OMBR research should focus on treatment of real industrial and municipal wastewaters to address the advantages and limitations of OMBR treatment that are otherwise overlooked when treating synthetic feed solutions.
Besides the differences in the inorganic composition between synthetic and real wastewater streams, there are evidence that membrane fouling differs between membrane systems operated with synthetic and real feed streams due to differences in soluble microbial products (SMPs), including proteins, polysaccharides, and humic matter.124 Results published by Villain et al.125 showed that there are substantial differences in membrane fouling in bench-scale MBRs treating synthetic and real wastewater. Increased organic fouling was observed for the MBR treating synthetic wastewater compared to the MBR treating real wastewater; however, the fouling layer that developed on the membrane filtering synthetic wastewater was more reversible. The difference in membrane fouling for MBRs treating synthetic and real wastewater streams has been associated with variations in the protein-to-carbohydrate ratio.126,127 A change in this ratio in the feed stream influences the floc size and hydrophobicity of the extracellular polymeric substances (EPS), resulting in significant differences in the extent and reversibility of the fouling layer. As suggested by Villain et al.,125 MBR studies, and in the current case OMBR studies, should be conducted with real wastewater streams, especially when the primary focus is on evaluating membrane fouling in an activated sludge environment.
5.2. OMBR test conditions: HRT, SRT, and MLSS
OMBRs were tested under a wide range of operating conditions, making it difficult to compare results between individual studies. Many studies also do not provide values for important test parameter including the HRT, SRT, and MLSS concentration. As a starting point to standardize OMBR test conditions, it may be prudent to apply the lessons learned from 30 years of MBR bench-, pilot-, and full-scale operation.Typical operating conditions for MF and UF MBRs treating municipal wastewater also vary; however, several parameters were identified and fine-tuned to optimize MF and UF membrane performance and nutrient removal in MBRs. These include MLSS concentration of 7–16 g L−1, HRT of 2–10 h, and SRT of 10–50 day.124,128–132 Higher MLSS concentrations (compared to conventional activated sludge systems) have been adopted for MBRs to achieve full nutrient removal in a smaller footprint and to minimize sludge wasting (e.g., higher water recovery).133 The maximum suggested MLSS concentration for MBRs is approximately 16 g L−1 due to excessive MF and UF membrane fouling and decreased oxygen transfer rates at higher MLSS concentrations.130 MLSS target concentrations that have been adopted for MF/UF MBRs should be considered for OMBRs to take advantage of the smaller footprint and reduced sludge wasting, which are the main advantages of MF/UF MBRs compared to conventional activated sludge treatment.
The HRT and SRT are decoupled in MBRs and OMBRs because water is processed through the membrane but solids are retained during filtration.133 The decoupling of the HRT and SRT allows for high carbon and nutrient removal at low HRTs, allowing for smaller tank volumes and reduced capital cost. The minimum SRT that has been demonstrated in MF/UF MBRs that achieve complete nitrification is approximately 10 days;129 lower SRTs have been tested (as low as 2 days) in MBRs with results showing that nitrification is incomplete at SRTs of less than 5 days.129,131 At SRTs longer than 50 days biological phosphorus removal begins to deteriorate, and at even longer SRTs (SRT > 80 days) the dewaterability of the sludge is reduced.124,132 Phosphorus removal in OMBR applications may not be necessary because phosphorus is well rejected by FO membranes and can potentially be harvested from the system for beneficial use.22 If phosphorus removal is not required, the SRT may be extended to as long as 80 days before the dewaterability of the sludge is negatively affected. In general, future OMBR studies should extend the SRT beyond 10 days, which is the minimum for establishing good nitrification, to take advantage of some of the marketing points that have been established for commercial MBRs. The current limitation to extending the SRT in OMBRs is the increase in bioreactor salinity.
5.3. Salt accumulation models
Accumulation of salts in the OMBR bioreactors is a function of the RSF, water flux through the FO membrane (Jw), bioreactor influent salt concentration (Cinf), HRT, and SRT.37,112 The HRT and SRT are important parameters influencing salt accumulation in the bioreactor because the only way to control the accumulation of salt in an OMBR, except for hybrid MF/UF and OMBRs, is through sludge wasting. Bowden et al.37 developed an equation for the steady state salt concentration in the bioreactor (Cb) using a mass balance approach and assuming an SRT much longer than the HRT, so that 1/HRT − 1/SRT ≈ 1/HRT, thus: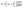 | (5) |
This approach for calculating the expected steady state concentration of salt in the bioreactor is useful but is only appropriate in situations where the water flux and the HRT are constant. In reality, salt accumulation and membrane fouling decrease the water flux over time. As a result, HRT decreases and the bioreactor salt concentration decreases to a value lower than predicted by the model. A model that accounted for the reduction in water flux due to salt accumulation was developed by Xiao et al.:112
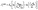 | (6) |
A common conclusion that can be drawn from both models is that the only operating parameter that can be adjusted to reduce the bioreactor salinity is the SRT. This seems like a simple solution to increase process performance; however, increasing sludge wasting (decreasing the SRT) reduces water recovery, increases the volume of sludge that must be stabilized and disposed of, and inhibits nitrification at SRTs shorter than 10 days. In an excellent review by Lay et al.44 on elevated salinity in high rejection MBRs such as the OMBR, the authors suggested that the HRT should be less than 1 day to mitigate excessive capital costs, and the SRT should be maintained between 10 and 30 days to allow for adequate biological activity. It was estimated that the bioreactor TDS concentration under these conditions will be 5 to 15 g L−1, which was assumed to be acceptable to ensure stability of the biological system and not compromise membrane performance (water flux and fouling). It is still questionable if nitrification and denitrification can be sustained at the suggested bioreactor concentrations, but this may be a reasonable starting point for developing future OMBR operating criteria.
6. Experimental results and observations from past OMBR studies
Results and observations from long-term OMBR studies have been primarily related to physiochemical performance parameters, including water flux, membrane fouling, RSF, and salt accumulation. Water flux and salt accumulation for 10 long-term OMBR studies are summarized in Table 6. The removal and rejection of nutrients is not included in Table 6 because the articles provide very limited data for these constituents; thus, nutrient removal and rejection should be a point of emphasis in future studies because nitrogen and phosphorus that are not removed biologically accumulate in the bioreactor due to their high rejection, especially phosphorous, by FO membranes.53| Initial water flux (L m−2 h−1) | Steady-state water flux (L m−2 h−1) | Water flux post cleaning (L m−2 h−1) | Initial bioreactor salt conc. (g L−1) | Steady-state bioreactor salt conc. (g L−1) | Membrane orientation | Draw solution conc. (M) | Ref. |
|---|---|---|---|---|---|---|---|
| a Values estimated from the source. Data originally reported in millisiemens. b Experiment conducted with MgCl2 draw solution. c Anaerobic OMBR evaluation. | |||||||
| 12 | 3 | NA | 0.14 | 4.1 | PRO | 1.0 | 18 |
| 9 | 5 | NA | 0.4 | 12 | FO | 1.0 | 47 |
| 11 | 9 | 9.9 | 0.0 | 4.5 | FO | 0.9 | 17 |
| 9 | 2 | NA | 0.3a | 32a | FO | 1.0 | 49 |
| 9 | 2 | NA | 0.3a | 41a | FO | 1.0 | 49 |
| 8.9 | 6.6 | NA | 9.7a | 21a | FO | 0.8 | 22 |
| 10.5 | 5.5 | NA | 0.3a | 3.2a | FO | 1.0 | 23 |
| 23 | 3.9 | NA | 1.2a | 7.7a | PRO | 0.5 | 19 |
| 7.5 | 6.5 | NA | 0.3a | 9.7a | FO | 0.5 | 22 |
| 3.2 | 2.7 | NA | 0.5a | 8.1a | FO | 0.5 | 20 |
| 9.9 | 5.7 | NA | 0.5a | 18a | FO | 0.8 | 24 |
| 4.3 | 1.6 | 2.5 | 0.5 | 8.2 | FO | 0.5 | 21 |
| 5.3 | 4.0 | NA | 0.4 | 1.2 | FO | 0.5 | 21 |
| 9.9 | 5.7 | NA | 0.5a | 18a | FO | 0.5 | 23 |
6.1. Water flux and membrane fouling and cleaning
A decline in water flux was observed in each of the OMBR studies summarized in Table 6. A portion of the flux decline can be attributed to a reduction in the osmotic pressure driving force due to the increasing bioreactor salinity. Membrane fouling was evident in the studies conducted by Achilli et al.,17 Lay et al.,20 Zhang et al.,19 and Holloway et al.21 The decline in water flux due to membrane fouling as opposed to increasing bioreactor salinity can be observed in these studies because the water flux continued to decline after the bioreactor salinity reached steady state. The most severe membrane fouling was observed for the two studies conducted in PRO mode.18,19 The significant flux decline observed in the PRO orientation illustrates the detrimental effect and ineffectiveness of operating an OMBR with the membrane in PRO mode.Zhang et al.19 examined the extent of organic fouling and scaling in an OMBR operating in PRO mode using sequential cleaning steps, which included deionized water to remove biofouling followed by acid cleaning to remove scaling. Zhang et al. demonstrated that membrane scaling was the primary membrane foulant as approximately 10% of the membrane permeability was recovered after removing the biofouling and 72% of the membrane permeability was recovered after acid cleaning of the membrane to remove inorganic scaling. Membrane scaling in OMBR applications is of particular concern because the ions responsible for scaling (e.g., calcium) are at high concentrations in the bioreactor. Holloway et al.21 demonstrated that the calcium concentrations in a bioreactor fed with municipal wastewater increased 6 fold during long-term OMBR operation.
Studies on organic fouling of FO membrane have been conducted with activated sludge, algae, polysaccharides, proteins, EPS, and transparent exopolymer particles (TEP).109,123,135,137,138 Zhang et al.123 characterized FO fouling using twenty different activated sludge solutions with varying MLSS concentrations, particle sizes, polysaccharides, proteins, EPS, SMPs concentrations, and multiple other constituents. Using a partial least squares regression, it was determined that the concentration of polysaccharide- and protein-bound EPS correlated positively with water flux decline. Likewise, Linares et al.137 conducted FO experiments on secondary effluent and determined that the organic fouling layer on the FO membrane surface was composed of biopolymers and protein like substances. Gu et al.138 and Parida et al.109 also found that the FO membrane was fouled by organic constituents; however, both investigators determined that the fouling was more severe in the presence of cations (calcium and magnesium).
Mi and Elimelech139 investigated organic fouling of FO membranes in the presence of relatively low calcium concentrations (0.5 mmol calcium) using alginate and bovine serum albumin (BSA) as model organic foulants. Findings from the fouling study illustrated that a gel layer of cross-linked organic chains fouled the membrane when alginate was used as the model foulant but the gel layer formed to a much lesser extent when using BSA. It was hypothesized that calcium formed bridges between the carboxylic groups on the alginate but was not effective in bridging BSA because of the lack of carboxylic groups. Similar to the model foulants, calcium has been shown to be critical in forming gel-like layers with SMPs found in activated sludge supernatant.140 The similarity between alginate and SMPs (particularly exopolysaccharides) is the abundance of carboxylic groups that can be bridged by calcium, resulting in rigid and non-deformable gels.141
A unique characteristic of the gel layer is that the structure may change from a non-porous film covering the membrane surface at low to moderate calcium concentrations (<6 mmol calcium) to a much more porous layer at higher calcium concentrations, specifically due to the gel-like structure collapsing on itself.141 Although the structure of the gel-like fouling layer on the FO membrane in high salinity OMBRs is not completely understood, it is likely that the gel-like fouling layer that was observed in long-term OMBR experiments is a result of bridging between calcium and the SMP present in the activated sludge. Membrane cleaning may be periodically required to remove the gel layer from the membrane surface to sustain/recover water flux during continuous OMBR operation.
Cleaning solutions (Alconox, EDTA, and NaOCl) that have been determined to work well for CTA FO membranes are not compatible with polyamide-based, TFC FO membranes. Exposure to Alconox and EDTA increases the water flux and RSF through the TFC membrane due to changes in the polymeric structure of the membrane.149 As an alternative to detergents and EDTA, the polyamide TFC membrane can be cleaned with a basic solution (NaOH) followed by an acid (HCl) mixture without compromising the integrity of the membrane active or support layers.149 Results from Wang et al.149 demonstrated that nearly 100% of the water flux was restored and RSF unchanged after base (0.1% NaOH + 0.1% sodium dodecyl sulfate (SDS)) and acid (0.5% HCl) cleaning a TFC membrane (pH information was not provided). The majority of these cleaning studies were done by applying the cleaning agent to the active side of the membrane that was exposed to the foulant-rich feed stream, except for the NaOCl cleaning of the support side conducted by Linares et al.137 Chemically cleaning the active side of the membrane requires that the membranes cassettes be removed from the bioreactor to an external chemical cleaning tank, which is a major short-coming for pilot- and full-scale OMBR treatment because it is laborious to remove large cassettes from the process and increases treatment down-time.
The severe fouling illustrated in Fig. 5a was a result of inadequate air scouring due to the coarse-bubble diffuser being plugged with sludge. Osmotic backwashing was used in the first attempt of membrane cleaning (Fig. 5b and e) by placing the membrane plates in an external cleaning tank filled with a concentrated salt solution (25 g L−1 of NaCl) and deionized water recirculating on the draw solution side of the membrane for 1 hour. The osmotic backwashing removed the majority of loosely attached biosolids from the membranes, but the membranes were still significantly covered with a biofilm. Following the first cleaning attempt, the system was operated for approximately two weeks in the activated sludge tanks before a chemically enhanced osmotic backwashing was attempted. For the chemically enhanced osmotic backwashing, the membranes remained submerged in the activated sludge. The TFC membranes used in the study were first cleaned with an NaOH base solution at pH of 10–11 followed by an HCl acid solution at pH of 3–4. Results from the chemically enhanced osmotic backwashing cleaning method were very encouraging—most of the fouling layer was removed from the membrane surface without having to remove the membrane skid from the bioreactor. The chemically enhanced osmotic backwashing could be an improvement to existing techniques that require large volumes of chemicals and external chemical cleaning tanks.
6.2. Solute accumulation, driving force, and microbial toxicity
Salt accumulation in the bioreactors is inevitable in OMBR systems treating municipal or industrial wastewater because the FO membrane retains salts that enter the bioreactors with the influent, and salts that make up the draw solution continuously diffuse through the FO membrane from the draw solution to the bioreactors. Elevated bioreactor TDS concentrations results in reduced osmotic pressure driving force for water flux and potential inhibition of microbial growth and functionality. The driving force for water flux (TDS concentration gradient between the activated sludge and draw solution) decreased by approximately 4 g L−1, 8 g L−1, and 17.5 g L−1 in respective long-term OMBR studies conducted by Achilli et al.,17 Lay et al.,20 and Tan et al.24 operating in FO mode. Results presented by Tan et al.24 illustrate that almost all of the reduction in water flux (approximately 43%) was associated with decreased driving force and not membrane fouling. Zhang et al.19 demonstrated that approximately 70% of the reduction in water flux was due to reduced driving force and not membrane fouling in a long-term evaluation operated in PRO mode.Elevated bioreactor salinity may also be detrimental to the microbial community responsible for nitrification, denitrification, and carbon oxidation. Achilli et al.17 noted that ammonia oxidation (nitrification) did not appear to be affected at elevated salt concentrations. Evidence of microbial inhibition occurred in a long-term evaluation conducted by Holloway et al.21 in which nitrification ceased as the bioreactor salinity increased above 6 g L−1 for approximately 20 days before eventually being regained. Similarly, denitrification stopped as the bioreactor salinity initially increased before returning after 20 days of operation in an OMBR study conducted by Tan et al.24 It is not entirely unexpected that the denitrification rate would initially decrease as the bioreactor salinity increases and eventually return after prolonged high-salinity operation. Dinçer and Kargi,150 determined that the denitrification rate in a normally low-salinity activated sludge decreased by 20% when exposed to NaCl concentrations of 5 g L−1. Ye et al.113 also demonstrated that the ammonia-oxidizing bacteria (AOB) responsible for nitrification were inhibited at salt concentrations exceeding 2 g L−1 in evaluation of a sequencing batch reactor treating municipal wastewater. However, given time to acclimate at a sufficiently long SRT, nitrification can return at elevated bioreactor salinities because the microbial community responsible for nitrification shifts to a community populated by salt tolerant AOB and ammonia-oxidizing archaea (AOA).151 Although it is not surprising that the OMBR microbial community shifts with changing salinity, there is still limited understanding of the specific characteristics of the microbial community and should be an emphasis in future OMBR research.
Alturki et al.18 concluded that the ratio of mixed liquor volatile suspended solids (MLVSS) to MLSS decreased from 0.87 to 0.66 in a long-term OMBR investigation. A decrease in this ratio is an indication that biological activity may have deteriorated. Wang et al.23 measured a decrease in DHA, an important enzyme for biological oxidation of organic matter, as the bioreactor salinity increased overtime. Chen et al.23 described a reduction in carbon removal during anaerobic OMBR experiments. Considering the array of results obtained from long-term OMBR testing, carbon and nitrogen removal is likely inhibited during the initial increase in bioreactor salinity. Once salt tolerant microbial communities are established, carbon and nitrogen removal resumes, yet at a lower rate compared to that observed in low salinity activated sludge processes. It is important to note that in all past OMBR studies the activated sludge used to seed the OMBR bioreactors was obtained from conventional activated sludge treatment plants. If the sludge is not allowed to acclimate to the basic OMBR operating conditions (e.g., longer SRT and HRT), results from experiments conducted to determine the effect of increasing salinity on carbon and nutrient removal might be skewed by changes in SRT between the system that the activated sludge was collected from and the OMBR studied. Kimura et al.152 have shown that the characteristics of the activated sludge differed between MBRs operated at different SRTs and Luo et al.47 commented that activated sludge should be acclimated to OMBR operating conditions before assessing the effects of salinity build-up on OMBR performance.
Mitigating salt accumulation to prevent reduced driving force and microbial inhibition requires creative system configurations and draw solution selections. Holloway et al.21 demonstrated that the bioreactor salinity (NaCl equivalent) could be maintained at approximately 1 g L−1 using a hybrid UFO-MBR (Fig. 6). A UF membrane skid, operated in parallel to an FO membrane skid, is used in this configuration to extract solutes from the bioreactor with the UF permeate, leaving the biosolids in the bioreactor. Results from this study demonstrated that operating the OMBR system at low salinity increased the sustainability of OMBR water flux (operated at 6 L m−2 h−2 for 125 days without cleaning) and improved nitrogen removal. Furthermore, as an ancillary benefit, the UF permeate was rich in phosphorus and nitrogen that could potentially be recovered for beneficial use. In a comparable study, Wang et al.23 incorporated a MF membrane into the OMBR to maintain low bioreactor salinities. The water flux declined more substantially, from 10.5 L m−2 h−1 to 5.5 L m−2 h−1, in the MF-OMBR study, but excellent carbon removal and nitrogen oxidation was maintained over the full testing period. The higher decline in water flux in the MF-OMBR may have to do with the higher initial water flux (10.5 L m−2 h−1 compared to 5.3 L m−2 h−1 in Holloway's study). Hybrid OMBRs such as the UFO-MBR and MF-OMBR should be considered in future OMBR research to improve mitigation of salt accumulation in OMBR bioreactors.
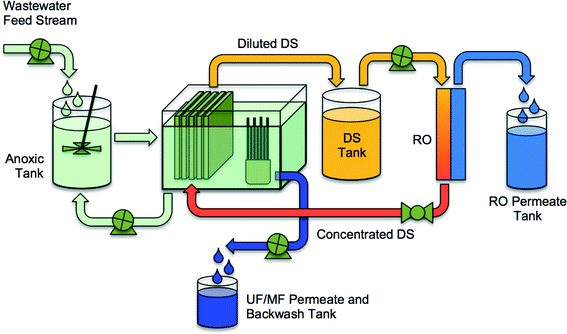 | ||
| Fig. 6 A schematic of an integrated UFO-MBR (or MFO-MBR) system containing an OMBR, a UF-MBR, and an RO system for reconcentration of draw solution. Adapted from Holloway et al.21 | ||
The use of organic draw solutions could be another method for reducing salt accumulation in the bioreactor. Bowden et al.37 concluded that magnesium acetate and sodium propionate could be viable organic draw solutions due to the low SRSF, biodegradation potential, and low capital and replenishment costs. Nawaz et al.116 recommended several different surfactants as potential draw solutions because of the relatively low microbial toxicity and RSF. It is important to note that the organic draw solutions proposed by Bowden et al.37 and Nawaz et al.116 were never tested in an OMBR; therefore, the benefits of using an organic draw solution are still questionable. Additionally, researchers should focus on finding organic draw solutions for OMBR treatment that are compatible with the microbial community in the bioreactor. Ansari et al.111 have recently highlighted that the RSF and microbial toxicity of an organic draw solution solute are interrelated; the RSF of some organic draw solution solutes into the bioreactor may increase microbial activity while others hinder microbial activity.
6.3. Nutrient: removal, rejection, and recovery
The mechanisms for membrane rejection and microbial degradation of nutrients, and potential for nutrient recovery are very unique to the OMBR. Biological nutrient removal has to be carefully evaluated for OMBRs due to the impact of bioreactor salinity on the activity and make-up of the microbial community. The rejection of nutrients through the FO membrane is important because they will accumulate in the bioreactors to very high concentrations that may result in increased diffusion of nutrients, specifically nitrogen, from the feed to the draw solution, and may compromise the product water quality of the draw solution reconcentration process. The accumulation of nutrients in the bioreactors can also be viewed as a benefit if the nutrients can be recovered at high concentrations for beneficial use.A major shortcoming of almost all published long-term OMBR studies is the lack of data for nitrate concentrations. In bioreactors, nitrate is produced via biological oxidation of ammonia and it accumulates in the bioreactors if anoxic zones, where nitrate can be reduced to nitrogen gas, are not incorporated into the bioreactor design. Results from Holloway et al.21 illustrate that nitrate can accumulate in the bioreactors to relatively high concentrations (more than 80 mg L−1) even when the OMBR is coupled with an anoxic bioreactor for denitrification. Subsequently, the draw solution is contaminated with nitrate due to the low nitrate rejection of OMBR-FO membranes.153 Nitrate contamination of the draw solution is further exacerbated when the draw solution reconcentration step has higher nitrate rejection than the OMBR-FO membranes drawing water from the activated sludge. This point is highlighted in the results presented by Holloway et al.;21 the concentration of nitrate in the draw solution was very high, almost equal to the bioreactor concentration, because the tight seawater RO membranes used for draw solution reconcentration had higher nitrate rejection than the OMBR-FO membrane. Although the draw solution was contaminated by nitrate, the system used by Holloway et al.21 was still able to achieve total rejection (FO and RO membrane) of ammonia, nitrate, and TOC of approximately 89%, 82%, and 96%, respectively due to the multiple biological and membrane barriers. A concept that has not been published on but may mitigate excessive accumulation of nitrate in the draw solution is periodic polishing of the draw solution. This could be done by processing the draw solution through a nanofiltration (NF) stage that allows nitrate to pass through the membrane relatively freely but has high retention of the draw solution solute (e.g., MgCl2). Future OMBR research should emphasis the impact of nitrate on system performance, investigate the potential benefits and limitations of the draw solution reconcentration step, and evaluate methods for draw solution polishing.27
6.4. TOrCs: removal and rejection
Rejection of TOrCs by the FO membrane is one of the major advantages of using OMBRs over other traditional treatment processes.28–31,41,80,86,157,158 Alturki et al.18 examined the rejection and microbial degradation of 50 TOrCs in an OMBR operated for approximately seven days. The results from this study demonstrated that 25 of 27 compounds with a molecular weight higher than 266 g mol−1 were rejected by the FO membrane to concentrations that were below or near the detection limit. The author attributed the high rejection to FO membrane selectivity and biological degradation.Rejection of compounds with molecular weights lower than 266 g mol−1 varied from negligible to removal below the detection limit. It was speculated that the removal of these low-weight compounds was not due to physical separation but to biological degradation because TOrCs that are known to have low biodegradability were not removed or rejected in this study. Alturki et al.28 demonstrated the importance of having biological and FO membrane separation to enhance TOrC removal. Similarly, Holloway et al.35 illustrated the effectiveness of using biological and membrane treatment for TOrC removal—it was demonstrated that 19 of 20 measured TOrCs in real wastewater were completely removed and rejected by the biological and membrane barriers, and that the RO reconcentration step provided an additional barrier for TOrC removal. Increased TOrC removal using a reconcentration step has also been illustrated by Linares et al.29 and Hancock et al.41 The total TOrC rejection in these coupled FO–RO systems exceeded 96% and 99%, respectively. One disadvantage of the coupled system is that TOrCs that are not well rejected by the FO membrane as by the RO membrane will accumulate in the draw solution.41,86
7. Future challenges
The OMBR is a promising treatment technology for nutrient recovery and indirect or direct potable reuse applications due to the multiple treatment barriers inherent to its design. Despite the obvious advantages of the OMBR to remove and reject nutrients and TOrCs, there are still many obstacles to overcome before the OMBR could be a viable, commercial treatment technology. Future research should focus on further investigating mass transport in OMBRs, developing new membrane and membrane modules, recovery of nutrients, general evolution of the OMBR and its hybrid configurations (UFO-MBR, MFO-MBR, and other future configurations). These include:• Developing OMBR design parameters such as appropriate cross-flow velocity and spacer design for cross-flow arrangements and the specific aeration demand for submerged configurations.
• Standardizing OMBR operating conditions such as the SRT and HRT to minimize salt accumulation but take advantage of the high SRT operating conditions that have become a hallmark of traditional MF and UF MBRs used in full-scale wastewater treatment.
• Determining the effect of salinity build-up and prolonged high salinity operation on the microbial community during OMBR treatment. Additionally, characterizing the microbial community in an OMBR and how it may differ from conventional low-salinity activated sludge treatment processes.
• Developing membrane cleaning strategies that allow clean in-place; thereby reducing chemical costs and system down time.
• Evaluating the effect (membrane degradation) of long-term exposure of FO CTA and TFC membranes to an activated sludge environment.
• Designing and producing polyamide TFC FO membranes with reasonable water and reverse salt flux characteristics, and that are structurally durable and chemically stable to withstand the rigors of operating in the chemically and microbially aggressive activated sludge environment.
• Designing creative hybrid OMBR systems capable of removing salts from the bioreactor and harvesting nitrogen and phosphorus for beneficial use.
• Selecting and testing organic draw solutions that could be biodegraded and prevent salt accumulation in the bioreactor.
• Evaluating the energy consumption of current OMBR configurations and optimize energy demand of OMBR to be competitive with traditional wastewater treatment technologies.
Lastly, the energy consumption of the OMBR must be competitive with conventional activated sludge (CAS) and MF/UF MBR systems implementing advanced treatment processes for potable and high quality water reuse. The specific energy demand for full-scale CAS and MBR wastewater treatment plants range between 0.3 to 0.6 kWh m−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97,159 and 0.4 to 2.0 kWh m−3,3,4,160 respectively. Considering that the effluent from CAS and MBR treatment must be further treated using advanced treatment technologies such as MF (for CAS), RO, and UVAOP to produce high quality reuse water, an additional energy demand must be included to the cost of CAS and MBR treatment. The reported specific energy demand of a large-scale municipality implementing MF, RO, and UVAOP to treat secondary CAS effluent is 0.6 to 1.2 kWh m−3 for the advanced treatment train.161 Therefore, the combined specific energy demand of CAS followed by advanced wastewater treatment is likely less than 2.0 kWh m−3.
97,159 and 0.4 to 2.0 kWh m−3,3,4,160 respectively. Considering that the effluent from CAS and MBR treatment must be further treated using advanced treatment technologies such as MF (for CAS), RO, and UVAOP to produce high quality reuse water, an additional energy demand must be included to the cost of CAS and MBR treatment. The reported specific energy demand of a large-scale municipality implementing MF, RO, and UVAOP to treat secondary CAS effluent is 0.6 to 1.2 kWh m−3 for the advanced treatment train.161 Therefore, the combined specific energy demand of CAS followed by advanced wastewater treatment is likely less than 2.0 kWh m−3.
The most energy efficient desalination process currently available to reconcentrate the draw solution in OMBRs is RO. The approximate specific energy demand calculated using ROSA system design software (Dow Filmtec, Edina, MN) for a 3.8 million liters per day (1 million gallons per day) RO system, optimized with low-pressure RO membranes, designed to reconcentrate a draw solution of 40 g L−1-NaCl is approximately 1.6 kWh m−3. If a pressure exchanger were to be incorporated into the RO system design, the new calculated specific energy is approximately 1.1 kWh m−3 (calculated from an energy recovery device model developed by Energy Recovery Inc. (ERI, San Francisco, CA)). It is likely, even if an energy recovery devices was incorporated, that the specific energy of the OMBR coupled with RO for draw solution reconcentration would be on the high end or exceed that of current advanced wastewater treatment trains. Thus, the major barrier to full-scale OMBR implementation is to develop creative new reconcentration configurations and methods, and novel draw solutions that can overcome the major energy penalty associated with OMBRs.
List of acronyms
| AOA | Ammonia Oxidizing Archaea |
| AOB | Ammonia Oxidizing Bacteria |
| AOP | Advanced Oxidation Processes |
| BSA | Bovine Serum Albumin |
| CAS | Conventional Activated Sludge |
| CTA | Cellulose Triacetate |
| DOC | Dissolved Organic Carbon (concentration) |
| EDX | Energy Dispersive X-ray |
| EPS | Extracellular Polymeric Substances |
| FO | Forward Osmosis |
| GOR | Gained Output Ratio |
| HRT | Hydraulic Retention Time |
| ICP | Internal Concentration Polarization |
| MBR | Membrane Bioreactor |
| MD | Membrane Distillation |
| MF | Microfiltration |
| MF-OMBR | Microfiltration Osmotic Membrane Bioreactor |
| MLSS | Mixed Liquor Suspended Solids (concentration) |
| MLVSS | Mixed Liquor Volatile Suspended Solids (concentration) |
| NOM | Natural Organic Matter |
| OMBR | Osmotic Membrane Bioreactor |
| PRO | Pressure Retarded Osmosis |
| RO | Reverse Osmosis |
| RSF | Reverse Salt Flux |
| SAD | Specific Aeration Demand |
| SBMBR | Sequencing Batch Membrane Bioreactor |
| SBR | Sequencing Batch Reactor |
| SEM | Scanning Electron Microscope |
| SMP | Soluble Microbial Products |
| SRSF | Specific Reverse Salt Flux |
| SRT | Solids Retention Time |
| TDS | Total Dissolved Solids (concentration) |
| TEP | Transparent Exopolymer Particles |
| TFC | Thin Film Composite |
| TOC | Total Organic Carbon (concentration) |
| TOrC | Trace Organic Compound |
| UF | Ultrafiltration |
| UFO-MBR | Ultrafiltration-Osmotic Membrane Bioreactor |
| UV | Ultraviolet |
Acknowledgements
The authors would like to thank Hydration Technology Innovations, LLC and the National Science Foundation Engineering Research Center Program under Cooperative Agreement EEC-1028968 (ReNUWIt) for supporting this study.References
- NRC, Water reuse : potential for expanding the nation's water supply through reuse of municipal wastewater, National Academies Press, Washington, D.C., 2012 Search PubMed.
- J. Arevalo, G. Garralon, F. Plaza, B. Moreno, J. Perez and M. A. Gomez, Desalination, 2009, 243, 32–41 CrossRef CAS PubMed.
- S. Judd, The MBR book: Principles and applications of membrane bioreactors for water and wastewater treatment, Elsevier, Oxford, 2008 Search PubMed.
- S. Judd, The MBR book: Principles and applications of membrane bioreactors for water and wastewater treatment, Elsevier, 2010 Search PubMed.
- T. Stephenson, S. Judd, B. Jefferson and K. Brindle, Membrane bioreactors for wastewater treatment, IWA Publishing, 2000 Search PubMed.
- T. Melin, B. Jefferson, D. Bixio, C. Thoeye, W. De Wilde, J. De Koning, J. van der Graaf and T. Wintgens, Desalination, 2006, 187, 271–282 CrossRef CAS PubMed.
- J. Ottoson, A. Hansen, B. Bjorlenius, H. Norder and T. A. Stenstrom, Water Res., 2006, 40, 1449–1457 CrossRef CAS PubMed.
- L. S. Tam, T. W. Tang, G. N. Lau, K. R. Sharma and G. H. Chen, Desalination, 2007, 202, 106–113 CrossRef CAS PubMed.
- H. J. Qin, K. A. Kekre, G. H. Tao, M. H. Oo, M. N. Wai, T. C. Lee, B. Viswanath and H. Seah, J. Membr. Sci., 2006, 272, 70–77 CrossRef PubMed.
- A. M. Comerton, R. C. Andrews and D. M. Bagley, Water Res., 2005, 39, 3982–3990 CrossRef CAS PubMed.
- J. Noble and C. Marner, Advanced wastewater treatment with MBR and integrated MBR/RO, http://www.infrastructurenorthafrica.com/presentations/advanced-wastewater-treatment-with-mbr-and-integrated-mbrro.
- S. Dukes and A. von Gottberg, Membrane bioreactors for RO pretreatment, http://www.environmental-expert.com/Files%5C5306%5Carticles%5C13848%5C482.pdf.
- N. Jones, Optimization of an industrial wastewater treatment plant through implementation of a membrane bioreactor and reverse osmosis system for reuse, http://www.membranes.com/docs/papers/NewFolder/AMTA2012Paper-NaomiJonesFINAL.pdf.
- J. S. Vrouwenvelder and D. V. D. Kooij, Desalination, 2001, 139, 65–71 CrossRef CAS.
- B. Wu, T. Kitade, T. H. Chong, T. Uemura and A. G. Fane, Desalination, 2013, 311, 37–45 CrossRef CAS PubMed.
- P. Le-Clech, V. Chen and T. A. G. Fane, J. Membr. Sci., 2006, 284, 17–53 CrossRef CAS PubMed.
- A. Achilli, T. Y. Cath, E. A. Marchand and A. E. Childress, Desalination, 2009, 239, 10–21 CrossRef CAS PubMed.
- A. Alturki, J. McDonald, S. J. Khan, F. I. Hai, W. E. Price and L. D. Nghiem, Bioresour. Technol., 2012, 113, 201–206 CrossRef CAS PubMed.
- J. S. Zhang, W. L. C. Loong, S. R. Chou, C. Y. Tang, R. Wang and A. G. Fane, J. Membr. Sci., 2012, 403, 8–14 CrossRef PubMed.
- W. C. L. Lay, Q. Y. Zhang, J. S. Zhang, D. McDougald, C. Y. Tang, R. Wang, Y. Liu and A. G. Fane, Desalination, 2011, 283, 123–130 CrossRef CAS PubMed.
- R. W. Holloway, A. S. Wait, A. Fernandes da Silva, J. Herron, M. D. Schutter, K. Lampi and T. Y. Cath, Desalination, 2015, 363, 64–74 CrossRef CAS PubMed.
- G. Qiu and Y.-P. Ting, Bioresour. Technol., 2014, 170, 221–229 CrossRef CAS PubMed.
- X. H. Wang, B. Yuan, Y. Chen, X. F. Li and Y. P. Ren, Bioresour. Technol., 2014, 167, 116–123 CrossRef CAS PubMed.
- J.-M. Tan, G. Qiu and Y.-P. Ting, J. Cleaner Prod., 2014, 88, 146–151 CrossRef PubMed.
- E. R. Cornelissen, D. Harmsen, K. F. de Korte, C. J. Ruiken, J. J. Qin, H. Oo and L. P. Wessels, J. Membr. Sci., 2008, 319, 158–168 CrossRef CAS PubMed.
- G. Qiu and Y.-P. Ting, Desalination, 2014, 332, 91–99 CrossRef CAS PubMed.
- N. T. Hancock, P. Xu, M. J. Roby, J. D. Gomez and T. Y. Cath, J. Membr. Sci., 2013, 445, 34–46 CrossRef CAS PubMed.
- A. A. Alturki, J. A. McDonald, S. J. Khan, W. E. Price, L. D. Nghiem and M. Elimelech, Sep. Purif. Technol., 2013, 103, 258–266 CrossRef CAS PubMed.
- R. V. Linares, V. Yangali-Quintanilla, Z. Y. Li and G. Amy, Water Res., 2011, 45, 6737–6744 CrossRef CAS PubMed.
- X. Jin, J. H. Shan, C. Wang, J. Wei and C. Y. Y. Tang, J. Hazard. Mater., 2012, 227, 55–61 CrossRef PubMed.
- J. L. Cartinella, T. Y. Cath, M. T. Flynn, G. C. Miller, K. W. Hunter and A. E. Childress, Environ. Sci. Technol., 2006, 40, 7381–7386 CrossRef CAS.
- K. L. Hickenbottom, N. T. Hancock, N. R. Hutchings, E. W. Appleton, E. G. Beaudry, P. Xu and T. Y. Cath, Desalination, 2013, 312, 60–66 CrossRef CAS PubMed.
- S. Lee, C. Boo, M. Elimelech and S. Hong, J. Membr. Sci., 2010, 365, 34–39 CrossRef CAS PubMed.
- B. X. Mi and M. Elimelech, J. Membr. Sci., 2010, 348, 337–345 CrossRef CAS PubMed.
- R. W. Holloway, J. Regnery, L. D. Nghiem and T. Y. Cath, Environ. Sci. Technol., 2014, 48, 10859–10868 CrossRef CAS PubMed.
- A. Achilli, T. Y. Cath and A. E. Childress, J. Membr. Sci., 2010, 364, 233–241 CrossRef CAS PubMed.
- K. S. Bowden, A. Achilli and A. E. Childress, Bioresour. Technol., 2012, 122, 207–216 CrossRef CAS PubMed.
- J. R. McCutcheon, R. L. McGinnis and M. Elimelech, J. Membr. Sci., 2006, 278, 114–123 CrossRef CAS PubMed.
- D. Y. Kim, B. Gu, J. H. Kim and D. R. Yang, J. Membr. Sci., 2013, 444, 440–448 CrossRef CAS PubMed.
- K. Y. Wang, M. M. Teoh, A. Nugroho and T.-S. Chung, Chem. Eng. Sci., 2011, 66, 2421–2430 CrossRef CAS PubMed.
- N. T. Hancock, P. Xu, D. M. Heil, C. Bellona and T. Y. Cath, Environ. Sci. Technol., 2011, 45, 8483–8490 CrossRef CAS PubMed.
- N. T. Hancock, W. A. Phillip, M. Elimelech and T. Y. Cath, Environ. Sci. Technol., 2011, 45, 10642–10651 CrossRef CAS PubMed.
- W. J. Yap, J. S. Zhang, W. C. L. Lay, B. Cao, A. G. Fane and Y. Liu, Bioresour. Technol., 2012, 122, 217–222 CrossRef CAS PubMed.
- W. C. L. Lay, Y. Liu and A. G. Fane, Water Res., 2010, 44, 21–40 CrossRef CAS PubMed.
- W. H. Luo, F. I. Hai, W. E. Price, W. S. Guo, H. H. Ngo, K. Yamamoto and L. D. Nghiem, Bioresour. Technol., 2014, 167, 539–546 CrossRef CAS PubMed.
- K. Lutchmiah, A. R. D. Verliefde, K. Roest, L. C. Rietveld and E. R. Cornelissen, Water Res., 2014, 58, 179–197 CrossRef CAS PubMed.
- W. Luo, F. I. Hai, W. E. Price and L. D. Nghiem, Sep. Purif. Technol., 2015, 145, 56–62 CrossRef CAS PubMed.
- Q. Y. Zhang, Y. W. Jie, W. L. C. Loong, J. S. Zhang, A. G. Fane, S. Kjelleberg, S. A. Rice and D. McDougald, Water Res., 2014, 58, 141–151 CrossRef CAS PubMed.
- X. Wang, Y. Chen, B. Yuan, X. Li and Y. Ren, Bioresour. Technol., 2014, 161, 340–347 CrossRef CAS PubMed.
- D. Vuono, J. Henkel, J. Benecke, T. Y. Cath, T. Reid, L. Johnson and J. E. Drewes, J. Membr. Sci., 2013, 446, 34–41 CrossRef CAS PubMed.
- Metcalf & Eddy, Wastewater engineering: Treatment and reuse, McGraw-Hill Companies, Incorporated, 2002 Search PubMed.
- J. L. Barnard, Water Res., 1975, 9, 485–490 CrossRef CAS.
- R. W. Holloway, A. E. Childress, K. E. Dennett and T. Y. Cath, Water Res., 2007, 41, 4005–4014 CrossRef CAS PubMed.
- N. C. Nguyen, S. S. Chen, H. Y. Yang and N. T. Hau, Bioresour. Technol., 2013, 132, 224–229 CrossRef CAS PubMed.
- M. Xie, L. D. Nghiem, W. E. Price and M. Elimelech, Environ. Sci. Technol. Lett., 2014, 1, 191–195 CrossRef CAS.
- M. S. Pravin, T. Y. Cath, R. W. Holloway, J. R. Herron, K. Lampi and W. L. Schultz, US Pat. App., 61/876,108, 2013 Search PubMed.
- T. Buer and J. Cumin, Desalination, 2010, 250, 1073–1077 CrossRef CAS PubMed.
- C. Y. Y. Tang, T. H. Chong and A. G. Fane, Adv. Colloid Interface Sci., 2011, 164, 126–143 CrossRef CAS PubMed.
- C. H. Tan and H. Y. Ng, J. Membr. Sci., 2008, 324, 209–219 CrossRef CAS PubMed.
- M. Park and J. H. Kim, J. Membr. Sci., 2013, 427, 10–20 CrossRef CAS PubMed.
- R. Valladares Linares, S. S. Bucs, Z. Li, M. AbuGhdeeb, G. Amy and J. S. Vrouwenvelder, Water Res., 2014, 57, 223–233 CrossRef CAS PubMed.
- Y. N. Wang, F. Wicaksana, C. Y. Tang and A. G. Fane, Environ. Sci. Technol., 2010, 44, 7102–7109 CrossRef CAS PubMed.
- C. Fritzmann, M. Wiese, T. Melin and M. Wessling, J. Membr. Sci., 2014, 463, 41–48 CrossRef CAS PubMed.
- C. Fritzmann, M. Hausmann, M. Wiese, M. Wessling and T. Melin, J. Membr. Sci., 2013, 446, 189–200 CrossRef CAS PubMed.
- B. D. Coday, P. Xu, E. G. Beaudry, J. Herron, K. Lampi, N. T. Hancock and T. Y. Cath, Desalination, 2014, 333, 23–35 CrossRef CAS PubMed.
- Z. F. Cui, S. Chang and A. G. Fane, J. Membr. Sci., 2003, 221, 1–35 CrossRef CAS.
- F. Wicaksana, A. G. Fane and V. Chen, J. Membr. Sci., 2006, 271, 186–195 CrossRef CAS PubMed.
- L. Bohm, A. Drews, H. Prieske, P. R. Berube and M. Kraume, Bioresour. Technol., 2012, 122, 50–61 CrossRef PubMed.
- H. Zhang, S. Cheng and F. Yang, Desalination, 2014, 347, 112–119 CrossRef CAS PubMed.
- Y. C. Kim and M. Elimelech, Environ. Sci. Technol., 2012, 46, 4673–4681 CrossRef CAS PubMed.
- A. P. Straub, N. Y. Yip and M. Elimelech, Environ. Sci. Technol. Lett., 2014, 1, 55–59 CrossRef CAS.
- L. Luo, P. Wang, S. Zhang, G. Han and T. S. Chung, J. Membr. Sci., 2014, 461, 28–38 CrossRef CAS PubMed.
- G. Han and T. S. Chung, AIChE J., 2014, 60, 1107–1119 CrossRef CAS PubMed.
- S. Zhang, P. Sukitpaneenit and T. S. Chung, Chem. Eng. J., 2014, 241, 457–465 CrossRef CAS PubMed.
- L. Shi, S. R. Chou, R. Wang, W. X. Fang, C. Y. Tang and A. G. Fane, J. Membr. Sci., 2011, 382, 116–123 CrossRef CAS PubMed.
- M. Xie, L. D. Nghiem, W. E. Price and M. Elimelech, Environ. Sci. Technol., 2013, 47, 13486–13493 CrossRef CAS PubMed.
- C. Boo, M. Elimelech and S. Hong, J. Membr. Sci., 2013, 444, 148–156 CrossRef CAS PubMed.
- V. Yangali-Quintanilla, Z. Y. Li, R. Valladares, Q. Y. Li and G. Amy, Desalination, 2011, 280, 160–166 CrossRef CAS PubMed.
- C. Y. Y. Tang, Q. H. She, W. C. L. Lay, R. Wang and A. G. Fane, J. Membr. Sci., 2010, 354, 123–133 CrossRef CAS PubMed.
- T. Y. Cath, N. T. Hancock, C. D. Lundin, C. Hoppe-Jones and J. E. Drewes, J. Membr. Sci., 2010, 362, 417–426 CrossRef CAS PubMed.
- J. Zhou, V. W. C. Chang and A. G. Fane, Desalination, 2013, 308, 233–241 CrossRef CAS PubMed.
- M. Latorre, Desalination, 2006, 189, 324–324 CrossRef CAS PubMed.
- A. Achilli, J. L. Prante, N. T. Hancock, E. B. Maxwell and A. E. Childress, Environ. Sci. Technol., 2014, 48, 6437–6443 CrossRef CAS PubMed.
- A. E. Childress and A. Achilli, Encyclopedia of Membrane Science and Technology, John Wiley & Sons, Inc., 2013 Search PubMed.
- T. Y. Cath, M. Elimelech, J. R. McCutcheon, R. L. McGinnis, A. Achilli, D. Anastasio, A. R. Brady, A. E. Childress, I. V. Farr, N. T. Hancock, J. Lampi, L. D. Nghiem, M. Xie and N. Y. Yip, Desalination, 2013, 312, 31–38 CrossRef CAS PubMed.
- A. D'Haese, P. Le-Clech, S. Van Nevel, K. Verbeken, E. R. Cornelissen, S. J. Khan and A. R. D. Verliefde, Water Res., 2013, 47, 5232–5244 CrossRef PubMed.
- A. Altaee, G. Zaragoza and H. R. van Tonningen, Desalination, 2014, 336, 50–57 CrossRef CAS PubMed.
- S. M. J. Zaidi, F. Fadhillah, Z. Khan and A. F. Ismail, Desalination, 2015, 368, 202–213 CrossRef CAS PubMed.
- J. R. McCutcheon, R. L. McGinnis and M. Elimelech, Desalination, 2005, 174, 1–11 CrossRef CAS PubMed.
- R. L. McGinnis and M. Elimelech, Desalination, 2007, 207, 370–382 CrossRef CAS PubMed.
- S. Zhang, P. Wang, X. Z. Fu and T. S. Chung, Water Res., 2014, 52, 112–121 CrossRef CAS PubMed.
- A. Alkhudhiri, N. Darwish and N. Hilal, Desalination, 2012, 287, 2–18 CrossRef CAS PubMed.
- K. C. Wijekoon, F. I. Hai, J. Kang, W. E. Price, W. Guo, H. H. Ngo, T. Y. Cath and L. D. Nghiem, Bioresour. Technol., 2014, 159, 334–341 CrossRef CAS PubMed.
- M. Khayet and J. I. Mengual, Desalination, 2004, 168, 373–381 CrossRef CAS PubMed.
- K. L. Hickenbottom and T. Y. Cath, J. Membr. Sci., 2014, 454, 426–435 CrossRef CAS PubMed.
- L. M. Camacho, L. Dumee, J. H. Zhang, J. D. Li, M. Duke, J. Gomez and S. Gray, Water, 2013, 5, 94–196 CrossRef PubMed.
- P. L. McCarty, J. Bae and J. Kim, Environ. Sci. Technol., 2011, 45, 7100–7106 CrossRef CAS PubMed.
- C. Boo, Y. F. Khalil and M. Elimelech, J. Membr. Sci., 2015, 473, 302–309 CrossRef CAS PubMed.
- B. D. Coday, N. Almaraz and T. Y. Cath, J. Membr. Sci., 2015, 488, 40–55 CrossRef CAS PubMed.
- J. R. McCutcheon and M. Elimelech, J. Membr. Sci., 2006, 284, 237–247 CrossRef CAS PubMed.
- S. A. F. Zhao and L. D. Zou, J. Membr. Sci., 2011, 379, 459–467 CrossRef CAS PubMed.
- A. Tiraferri, N. Y. Yip, W. A. Phillip, J. D. Schiffman and M. Elimelech, J. Membr. Sci., 2011, 367, 340–352 CrossRef CAS PubMed.
- A. P. Murphy, C. D. Moody, R. L. Riley, S. W. Lin, B. Murugaverl and P. Rusin, J. Membr. Sci., 2001, 193, 111–121 CrossRef CAS.
- J. H. Choi, K. Fukushi and K. Yamamoto, Sep. Purif. Technol., 2007, 52, 470–477 CrossRef CAS PubMed.
- N. Y. Yip, A. Tiraferri, W. A. Phillip, J. D. Schiffman and M. Elimelech, Environ. Sci. Technol., 2010, 44, 3812–3818 CrossRef CAS PubMed.
- B. D. Coday, D. M. Heil, P. Xu and T. Y. Cath, Environ. Sci. Technol., 2013, 47, 2386–2393 CrossRef CAS PubMed.
- G. T. Gray, J. R. McCutcheon and M. Elimelech, Desalination, 2006, 197, 1–8 CrossRef CAS PubMed.
- A. Achilli, T. Y. Cath and A. E. Childress, J. Membr. Sci., 2009, 343, 42–52 CrossRef CAS PubMed.
- V. Parida and H. Y. Ng, Desalination, 2013, 312, 88–98 CrossRef CAS PubMed.
- R. W. Holloway, R. Maltos, J. Vanneste and T. Y. Cath, J. Membr. Sci., 2015, 491, 121–131 CrossRef CAS PubMed.
- A. J. Ansari, F. I. Hai, W. Guo, H. H. Ngo, W. E. Price and L. D. Nghiem, Bioresour. Technol., 2015, 191, 30–36 CrossRef CAS PubMed.
- D. Xiao, C. Y. Tang, J. Zhang, W. C. L. Lay, R. Wang and A. G. Fane, J. Membr. Sci., 2011, 366, 314–324 CrossRef CAS PubMed.
- L. Ye, C. Y. Peng, B. Tang, S. Y. Wang, K. F. Zhao and Y. Z. Peng, Desalination, 2009, 246, 556–566 CrossRef CAS PubMed.
- A. Uygur, Process Biochem., 2006, 41, 61–66 CrossRef CAS PubMed.
- N. T. Hancock and T. Y. Cath, Environ. Sci. Technol., 2009, 43, 6769–6775 CrossRef CAS.
- M. S. Nawaz, G. Gadelha, S. J. Khan and N. Hankins, J. Membr. Sci., 2013, 429, 323–329 CrossRef CAS PubMed.
- P. Antoniou, J. Hamilton, B. Koopman, R. Jain, B. Holloway, G. Lyberatos and S. A. Svoronos, Water Res., 1990, 24, 97–101 CrossRef CAS.
- M. M. Zhang, D. X. Hou, Q. H. She and C. Y. Y. Tang, Water Res., 2014, 48, 387–395 CrossRef CAS PubMed.
- Y. L. Liu and B. X. Mi, J. Membr. Sci., 2014, 450, 153–161 CrossRef CAS PubMed.
- H. Mo, K. G. Tay and H. Y. Ng, J. Membr. Sci., 2008, 315, 28–35 CrossRef CAS PubMed.
- C. Jarusutthirak, S. Mattaraj and R. Jiraratananon, J. Membr. Sci., 2007, 287, 138–145 CrossRef CAS PubMed.
- H. T. Nguyen, S.-S. Chen, N. C. Nguyen, H. H. Ngo, W. Guo and C.-W. Li, J. Membr. Sci., 2015, 489, 212–219 CrossRef CAS PubMed.
- H. Zhang, Y. Ma, T. Jiang, G. Zhang and F. Yang, J. Membr. Sci., 2012, 390–391, 270–276 CrossRef CAS PubMed.
- M. Kraume and A. Drews, Chem. Eng. Technol., 2010, 33, 1251–1259 CrossRef CAS PubMed.
- M. Villain, I. Bourven, G. Guibaud and B. Marrot, Bioresour. Technol., 2014, 155, 235–244 CrossRef CAS PubMed.
- W. J. Gao, M. N. Han, X. Qu, C. Xu and B. Q. Liao, Bioresour. Technol., 2013, 128, 207–214 CrossRef CAS PubMed.
- S. Arabi and G. Nakhla, J. Membr. Sci., 2008, 324, 142–150 CrossRef CAS PubMed.
- H. Itokawa, C. Thiemig and J. Pinnekamp, Water Sci. Technol., 2008, 58, 2319–2327 CrossRef CAS PubMed.
- X. J. Fan, V. Urbain, Y. Qian, J. Manem, W. J. Ng and S. L. Ong, Water Sci. Technol., 2000, 42, 289–294 CAS.
- M. Kraume, U. Bracklow, M. Vocks and A. Drews, Water Sci. Technol., 2005, 51, 391–402 CAS.
- G. A. Soriano, M. Erb, C. Garel and J. M. Audic, Water Environ. Res., 2003, 75, 225–231 CrossRef CAS.
- C. B. Ersu, S. K. Ong, E. Arslankaya and Y. W. Lee, Water Res., 2010, 44, 3192–3202 CrossRef CAS PubMed.
- M. Gander, B. Jefferson and S. Judd, Sep. Purif. Technol., 2000, 18, 119–130 CrossRef CAS.
- Q. She, Y. K. W. Wong, S. Zhao and C. Y. Tang, J. Membr. Sci., 2013, 428, 181–189 CrossRef CAS PubMed.
- S. Zou, Y.-N. Wang, F. Wicaksana, T. Aung, P. C. Y. Wong, A. G. Fane and C. Y. Tang, J. Membr. Sci., 2013, 436, 174–185 CrossRef CAS PubMed.
- W. R. Thelin, E. Sivertsen, T. Holt and G. Brekke, J. Membr. Sci., 2013, 438, 46–56 CrossRef CAS PubMed.
- R. Valladares Linares, V. Yangali-Quintanilla, Z. Li and G. Amy, J. Membr. Sci., 2012, 421–422, 217–224 CrossRef CAS PubMed.
- Y. Gu, Y.-N. Wang, J. Wei and C. Y. Tang, Water Res., 2013, 47, 1867–1874 CrossRef CAS PubMed.
- B. Mi and M. Elimelech, J. Membr. Sci., 2008, 320, 292–302 CrossRef CAS PubMed.
- X. M. Wang and T. D. Waite, Environ. Sci. Technol., 2009, 43, 9341–9347 CrossRef CAS PubMed.
- Y. Xin, M. W. Bligh, A. S. Kinsela, Y. Wang and T. David Waite, J. Membr. Sci., 2015, 475, 395–405 CrossRef CAS PubMed.
- R. V. Linares, Z. Y. Li, V. Yangali-Quintanilla, Q. Y. Li and G. Amy, Desalin. Water Treat., 2013, 51, 4821–4824 CrossRef PubMed.
- A. Sagiv and R. Semiat, Desalination, 2005, 179, 1–9 CrossRef CAS PubMed.
- G. Ramon, Y. Agnon and C. Dosoretz, J. Membr. Sci., 2010, 364, 157–166 CrossRef CAS PubMed.
- J. J. Qin, M. H. Oo, K. A. Kekre and B. Liberman, J. Membr. Sci., 2010, 346, 8–14 CrossRef CAS PubMed.
- H. Yoon, Y. Baek, J. Yu and J. Yoon, Desalination, 2013, 325, 30–36 CrossRef CAS PubMed.
- C. R. Martinetti, A. E. Childress and T. Y. Cath, J. Membr. Sci., 2009, 331, 31–39 CrossRef CAS PubMed.
- B. D. Coday, N. Almaraz and T. Y. Cath, J. Membr. Sci., 2015 DOI:10.1016/j.memsci.2015.03.059.
- Z. Wang, J. Tang, C. Zhu, Y. Dong, Q. Wang and Z. Wu, J. Membr. Sci., 2015, 475, 184–192 CrossRef CAS PubMed.
- A. R. Dincer and F. Kargi, Enzyme Microb. Technol., 2001, 28, 661–665 CrossRef CAS.
- Y. J. Wu, L. M. Whang, T. Fukushima and S. H. Chang, J. Biosci. Bioeng., 2013, 115, 424–432 CrossRef CAS PubMed.
- K. Kimura, T. Naruse and Y. Watanabe, Water Res., 2009, 43, 1033–1039 CrossRef CAS PubMed.
- G. J. Irvine, S. Rajesh, M. Georgiadis and W. A. Phillip, Environ. Sci. Technol., 2013, 47, 13745–13753 CrossRef CAS PubMed.
- N. O. Nelson, R. L. Mikkelsen and D. L. Hesterberg, Bioresour. Technol., 2003, 89, 229–236 CrossRef CAS.
- K. N. Ohlinger, T. M. Young and E. D. Schroeder, J. Environ. Eng. Div., 1999, 125, 730–737 CrossRef CAS.
- J. D. Doyle and S. A. Parsons, Water Res., 2002, 36, 3925–3940 CrossRef CAS.
- J. L. Cartinella, T. Y. Cath, M. T. Flynn, G. C. Miller, K. W. Hunter and A. E. Childress, Environ. Sci. Technol., 2006, 40, 7381–7386 CrossRef CAS.
- M. Xie, L. D. Nghiem, W. E. Price and M. Elimelech, Water Res., 2012, 46, 2683–2692 CrossRef CAS PubMed.
- R. Goldstein and W. Smith, Water & sustainability (volume 4): US electricity consumption for water supply & treatment-the next half century, Electric Power Research Institute, 2002 Search PubMed.
- B. Barillon, S. M. Ruel, C. Langlais and V. Lazarova, Water Sci. Technol., 2013, 67, 2685–2691 CrossRef CAS PubMed.
- H. L. Leverenz and T. Asano, in Treatise on Water Science, ed. P. Wilderer, Elsevier, Oxford, 2011, pp. 63–71 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




