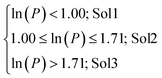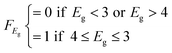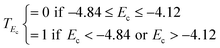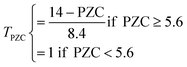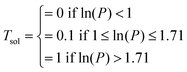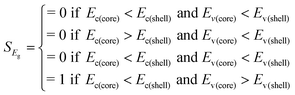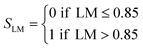Computational design of safer nanomaterials
E.
Burello
Computational Chemistry Group, Risk Analysis for Products in Development (RAPID) Department, TNO, Utrechtseweg 48, 3704 HE Zeist, The Netherlands. E-mail: enrico.burello@tno.nl
First published on 31st July 2015
Abstract
Nanomaterials are expected to find applications in numerous consumer products, posing the challenge to guarantee their safety and environmental sustainability before they can be transferred from research labs to end-consumer products. One emerging solution, called safe design, relies on the implementation, throughout the R&D phase, of key aspects related to the safety and sustainability of nanomaterials, in this way anticipating potential negative health effects. This article proposes a computational screening approach to design safer nanomaterials. The work is based on the calculation of key physicochemical properties of nanomaterials that are related to their safety, functionality and synthetic feasibility. These properties are then used to select a pool of promising structures for further experimental testing and development. The concept is demonstrated on a set of core@shell metal oxide nanoparticles for transparent UV-protecting coating applications.
Nano impactSafe design is a promising approach to pursue the sustainable development of nanotechnology. Currently, a number of initiatives are devoted to explore this idea, which requires a dynamic network of different players to address this complex challenge. Computational chemistry can play a key role in rationalizing the selection of promising nanomaterials in the early stages of a safe design strategy. In this work, we present a computational screening approach to design safe and functional core@shell nanoparticles. |
Introduction
Safe design is a concept that aims at implementing in innovation trajectories aspects related to the safety and sustainability of products and processes. In this way, environmental, health and safety concerns are addressed in the early stages of the innovation chain, with the result of maximizing resource use and increasing the chances of transferring safe and functional materials from research labs to end-consumer products. The safe design of nanomaterials is currently the focus of a growing number of research initiatives, where different players, from industry to research institutes and academia, create a dynamic network that can address the numerous and complex aspects of discovering, producing and marketing nanomaterials in compliance with socio-environmental constraints.Computational chemistry methods can play a key role in rationalizing the selection of promising materials in the early stages of a safe design strategy. Today, computational chemistry is extensively applied in the areas of material design, drug discovery and toxicology to predict the physicochemical properties of chemicals and materials and their behaviour in the human organism and in the environment.1,2 Its growing importance for providing key information is reflected in a number of regulatory frameworks (e.g., REACH) where in silico approaches are considered acceptable methods for filling in knowledge of untested substances. In the long term, these methodologies can contribute to improve occupational and consumer safety, foster product development and innovation strategies, and reduce in vivo testing.
This work is an effort towards the development and integration of computational methods to design safe and functional nanomaterials. The study is based on a computational screening procedure to select nanomaterial structures that comply with the desired characteristics of product safety and functionality. The approach relies on the calculation of key physicochemical properties of nanomaterials that are related to their inhalation toxicity, product application and synthetic feasibility, and the final ranking of nanomaterial structures with respect to these key features. The proposed methodology is conceptually similar to the drug design practice adopted by pharmaceutical industries, where fast computational tools are employed to screen large datasets of molecules in search for lead compounds. In this way, a large portion of the chemical space can be sampled, increasing the chances of obtaining good candidates. For this reason, we use theoretical equations and quantitative structure–activity relationship (QSAR) models to obtain key physicochemical properties of large datasets of nanomaterial structures.
This approach complements with high-throughput screening methods in the early stages of research and development, where a set of potential candidates that display the desired characteristics of functionality and safety are initially identified: the proposed structures are then optimized in subsequent R&D phases to yield a single final product for commercialization. It follows that these computational methodologies convey knowledge and models that can facilitate the decision making process in product R&D.
Results and discussion
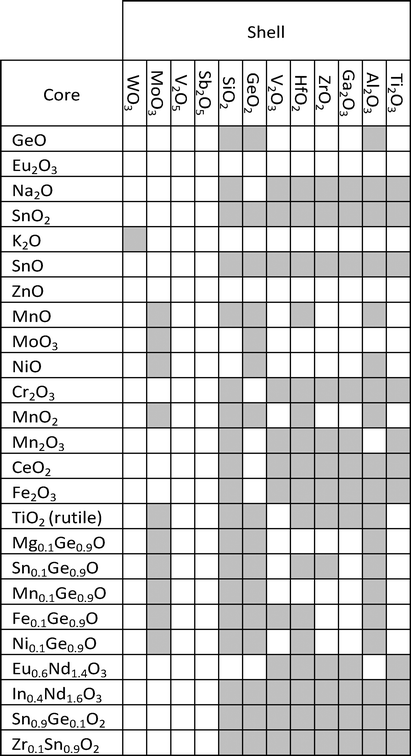
In this article we present a computational strategy for designing safe and functional nanomaterials. The approach is based on a screening procedure that can select from a large number (n > 104) of possible candidates, a focused library of nanomaterials that comply with specific requirements of product safety, functionality and synthetic feasibility. The concept is demonstrated on a set of nanomaterial structures with core@shell architectures for transparent UV-protecting coating applications. The core of the nanoparticles is composed of simple and mixed binary oxide materials whereas the shell is based of simple oxide structures. The selection of structures starts by calculating a number of key physicochemical properties of nanomaterials that are related to their inhalation toxicity, absorption in the UV range and synthetic feasibility in terms of core@shell architecture. The nanomaterials are then ranked according to a total score (S, eqn (20)) that accounts for functionality, safety and synthetic feasibility contributions of the core and shell structures. All scoring functions are parametrized to fit experimental observations and scaled to the [0,1] interval.The strategy we employ to design core@shell nanoparticles is based on the selection of core structures that satisfy functionality requirements, while the material composing the shell is designed to bear the lowest toxicity possible.3 This approach has been proposed also by other authors as a promising way to reduce the toxicity of nanoparticles:4 by encapsulating the core material with an inert or biocompatible shell, a physical barrier confines the core (and often reactive) material and prevents a direct contact with the biological machinery. We proceed by calculating a set of physicochemical properties and their associated scoring values for the core and the shell materials, respectively (eqn (20)–(23)). In addition, a number of supplementary conditions are used to guarantee that the final core@shell architecture is synthesizable and chemically unreactive. All these conditions with their corresponding scoring values are outlined in Table 1.
| Structure | Property ranges | Value of scoring function |
|---|---|---|
| a F = functionality score. b The radius of the core is implemented in the calculation of transmittance. c T = toxicity score. d Fixed value (total size of core@shell nanoparticle is 100 nm). e S = synthesis score. | ||
| Core | 3 ≤ Eg ≤ 4 | F score = 1/2 |
| E g < 3 or Eg > 4 | F score = 0 | |
| Max(T) | F score = T/2 | |
| r = 25 nm | —b | |
| Shell | E c < −4.84 or Ec > −4.12 | T score = 1/3 |
| E c > −4.84 or Ec < −4.12 | T score = 0 | |
| PZC < 5.6 | T score = 1/3 | |
| PZC ≥ 5.6 | T score = (14 − PZC)/25.2 | |
| Sol3 | T score = 1/3 | |
| Sol2 | T score = 0.1/3 | |
| Sol1 | T score = 0 | |
| Thickness = 25 nm | —d | |
| Core@shell | LM > 0.85 | S score = 1/2 |
| LM ≤ 0.85 | S score = 0 | |
| E c(C) < Ec(S) and Ev(C) > Ev(S) | S score = 1/2 | |
| Other (Ec(C), Ev(C)) vs. (Ec(S), Ev(S)) | S score = 0 | |
We choose oxide materials as potential candidates because of their relevant physicochemical properties and the possibility to compare predictions with available experimental studies.
Design of core structures
The functionality of core structures depends on two material's properties: the band gap (Eg) and the transmittance (T). We calculate these two properties for a set of 78 simple oxide structures.A specific band gap value is required to ensure that the material will absorb at a particular range of wavelengths: for optimal UV absorption, we select materials with an Eg value comprised between 3 and 4 eV. The band gap of oxide materials is calculated from their experimental enthalpy of formation using eqn (6). The predicted Eg values agree with measured ones (R2 = 0.81, n = 59) and are constant until a specific particle size threshold, which can be different for each oxide. For example, for ZnO and TiO2, quantum confinement arises when the size of the particles is around 5 and 2 nm, respectively.5 Theoretically, it is possible to estimate the band gap variation as a function of particle size using, e.g., the Brus equation,6 once the effective mass of the excited electron and hole are known. Because here we assume that the core size should be larger than 25 nm, the band gap value can be considered independent of the core size.
A high transmittance is desired to ensure the transparency of a material to visible light. The transmittance is calculated from the index of refraction (n, measured at λ = 589.3 nm) and the radius (r) of particles using eqn (11): by imposing that r should be 25 nm, we obtain different T values which range from 51% to 88%. This radius is then added to the shell thickness (also set to 25 nm) to obtain a final core@shell particle size of 100 nm. Transmittance is highly affected by the scattering component in eqn (11), which in turn depends on the particle's size. As a rule of thumb, scattering is reduced when nanoparticles with a diameter below 10% of the wavelength are used. In this work, core structures have a diameter of 50 nm, which is roughly the limit for acceptable transmittance. Although, scattering should be calculated on the whole particle (100 nm in diameter), rather than on the core alone, measurements of core@shell nanoparticle transmittance showed that a good transparency and UV absorption can be achieved also for particles as large as 100 nm with a core diameter of around 50 nm.7,8
Besides using these conditions for describing the functionality of the core, we also constrain the material search to oxide structures which do not contain very toxic elements such as As, Pb, Cd and Hg. Although in principle, a toxic cation could be included in the core if this is perfectly isolated by the shell material, one cannot exclude accidental exposure, for example when particles are damaged and release toxic cations.
Using the property ranges defined in Table 1, a number of core materials have been identified from the initial pool of 78 oxides: these materials are listed in Table 2. Among them, some are already used in commercial products as UV-protecting agents, such as TiO2, ZnO and CeO2.9 From this list we also note that some of the materials could be disregarded for other reasons: rare earth elements, for example, are too expensive to be considered as good candidates for use in mass consumer products.
| Material | E g (eV) | T (%) | Functionality score |
|---|---|---|---|
| GeO | 3.4 | 88 | 0.94 |
| Eu2O3 | 3.5 | 80 | 0.90 |
| Na2O | 3.1 | 80 | 0.90 |
| SnO2 | 3.7 | 79 | 0.90 |
| K2O | 3.3 | 78 | 0.89 |
| ZnO | 3.5 | 78 | 0.89 |
| SnO | 3.6 | 77 | 0.89 |
| MnO | 3.8 | 76 | 0.88 |
| Nb2O5 | 3.4 | 71 | 0.86 |
| MoO3 | 3.8 | 69 | 0.85 |
| NiO | 3.8 | 68 | 0.84 |
| Cr2O3 | 3.5 | 66 | 0.83 |
| MnO2 | 3.1 | 66 | 0.83 |
| Mn2O3 | 3.0 | 63 | 0.82 |
| CeO2 | 3.0 | 63 | 0.82 |
| Fe2O3 | 3.4 | 53 | 0.77 |
| TiO2 (rutile) | 3.1 | 51 | 0.76 |
To increase the diversity of the dataset and the chances to discover new functional cores, we have also calculated the band gap and transmittance for additionally 3003 binary oxide materials, assuming that these properties vary linearly as a function of the oxides composition (Vegard's law, eqn (19)). Although the value of a physicochemical property can deviate from the Vegard's law of mixing (indeed, this effect is taken into account by using an additional parameter in eqn (19)), various experimental studies show that such approximation holds true in the case of lattice constants, PZC (point of zero charge) and Eg values.10 In addition, to estimate the propensity of mixing of two oxide structures, we apply the substitutional solid solution rules derived by Hume-Rothery.11 These rules state that substitution occurs if the cations have similar atomic radii and electronegativity, equivalent coordination number, and if the crystal structure of the two oxides match. In Table 3 we lists some of the highest ranking mixed oxide materials selected on the bases of functionality and mixing conditions: for oxides of the type ABO, alloys with germanium display high functionality scores; for ABO3 mixed oxides, rare earth elements form the best candidates, whereas for ABO2 mixed oxides, diverse elements are selected.
| Material | E g (eV) | T (%) | Functionality score |
|---|---|---|---|
| Mg0.1Ge0.9O | 3.80 | 88 | 0.94 |
| Sn0.1Ge0.9O | 3.42 | 87 | 0.94 |
| V0.1Ge0.9O | 3.52 | 87 | 0.94 |
| Nb0.1Ge0.9O | 3.48 | 87 | 0.94 |
| Mn0.1Ge0.9O | 3.44 | 87 | 0.94 |
| Fe0.1Ge0.9O | 3.31 | 86 | 0.93 |
| Ni0.1Ge0.9O | 3.44 | 86 | 0.93 |
| Eu0.6Nd1.4O3 | 3.97 | 80 | 0.90 |
| In0.4Nd1.6O3 | 3.91 | 80 | 0.90 |
| Sn0.9Ge0.1O2 | 3.88 | 79 | 0.90 |
| Zr0.1Sn0.9O2 | 3.87 | 78 | 0.89 |
| Nb0.9Sn0.1O2 | 3.74 | 78 | 0.89 |
As an example, we show the band gap and transmittance calculations for the MgxZn(1−x)O binary oxide (Fscore = 0.89), which is being studied experimentally in various architectures (e.g. composite thin films, nanoparticles, etc.) for its UV-absorbing properties. Since Zn and Mg have similar ionic radii in fourfold coordination geometries (r = 0.57 Å and r = 0.60 Å for Mg2+ and Zn2+, respectively) and comparable electronegativity (χ(Zn) = 1.65 and χ(Mg) = 1.31), there can be some replacement in either structures without changing the original structure when alloying. However, the large crystal lattice dissimilarity between wurtzite-hexagonal ZnO (a = 3.25 Å and c = 5.21 Å) and rock-salt-cubic MgO (a = 4.214 Å) and the different cation's coordination numbers can cause unstable phase mixing, especially when x is approaching 0.5. In Fig. 1A and B we show the predicted transmittance and band gap energies over the whole composition range. Transmittance calculations agree with experimental measurements (both on nanoparticle and thin film materials), where a sharp absorption at wavelengths ranging from 400 to 200 nm has been observed with corresponding transmittance values around 80% and blue-shift as x approaches 1.12 Experimental measurements show that the band gap of MgxZn(1−x)O structures varies linearly as a function of x; moreover, when 0 < x < 40, the oxide has a hexagonal structure, whereas for 60 < x < 100 the cubic phase dominates. When x ≈ 50 ± 10, there are mixed regions of hexagonal and cubic phases with undefined energy band gaps. Overall, a binary oxide structure of MgxZn(1−x)O with 0 < x < 0.2 would lead to a core with high functionality.
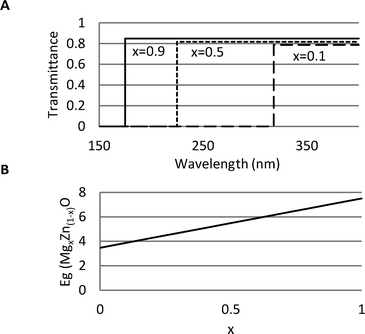 | ||
| Fig. 1 (A) Calculated transmittance for MgxZn(1−x)O core structures with x = 0.1, 0.5 and 0.9. (B) Calculated band gap for MgxZn(1−x)O core structure as a function of Mg composition (x). | ||
Design of shell structures
To estimate the inhalation toxicity of shell materials, we use 4 physicochemical properties: the size, the conduction band energy, the surface charge and the solubility in water and acidic conditions. These properties have been reported to correlate with observed in vitro and in vivo inhalation toxicity studies, as will be explained in more details. We calculate such properties for a pool of 78 oxide materials. All shell materials are then ranked according to the conditions and scoring values outlined in Table 1.A mechanism-of-action based model that predicts the ability of oxide nanoparticles to induce oxidative stress has been proposed by Burello and Worth.13,14 To account for this effect, the model characterizes the reactivity of the nanoparticles by comparing their conduction band energy (Ec, calculated using eqn (1)) to the redox potentials of biological reactions that maintain the cellular redox state, which range from −4.12 to −4.84 eV. When these two energy levels align, electrons can be transferred between the biological material and the oxide nanoparticles, unbalancing the cell's redox equilibrium and producing oxidative stress. This model has been validated experimentally on a high-throughput screening platform for determining the oxidative stress and acute pulmonary inflammation of metal oxide nanoparticles.15 The results of this study concluded also that lung toxicity at both cellular and whole animal levels could be explained by the position of the conduction band energy and the solubility of the oxide nanoparticles.
In another study performed to evaluate which physicochemical properties relate to lung inflammogenicity, Cho and colleagues tested a panel of 15 metal and metal oxide nanoparticles, both in vivo and in vitro, concluding that acute pulmonary inflammogenicity showed a significant correlation with two parameters: the zeta potential for low-solubility nanoparticles, and solubility to toxic species for high-solubility nanoparticles.16 The authors propose 2 possible mechanisms of action where low-solubility nanoparticles with a high and positive zeta potential (measured at pH = 5.6) disrupt the cell membrane of phagolysosomes leading to inflammation, while high-solubility nanoparticles dissolve inside the acidic phagolyosomes and cause inflammation if the ions are toxic. In this study, to include information on the particle's surface charge, we have calculated the point of zero charge (PZC) of shell materials using (eqn (9)). This value is then compared to the environmental pH (here also set to 5.6 (ref. 14)) to determine the net surface charge of the shell: the charge is positive if PZC > 5.6, and negative if PZC < 5.6. In addition, the larger the difference between these two values, the higher the surface charge density of the oxide. The PZCs of pristine oxide surfaces depend mainly on the type and density of surface hydroxyl groups and are very sensitive to sample preparation and environmental conditions. To reproduce the material's structural variability, each PZC value has an associated probability distribution obtained by fitting a Bayesian hierarchical regression model to a large pool of different PZC values obtained from literature. The surface charge of the shell material is also important for the nanomaterial's functionality, because agglomerated nanoparticles will induce more light scattering than single nanoparticles. Although a large difference between the PZC and the environmental pH can provide some degree of electrostatic stabilization in water, often surfactant molecules or polymeric dispersants are used to achieve dispersion in complex formulations. In such cases, one is also confronted with the bonding and wetting properties between the shell material and the organic coating. These properties, in turn, depend on the density of hydroxyl groups on the shell's surface and the ability of surface atoms to bind organic molecules. Agglomeration affects also toxicity, because agglomerated nanoparticles have lower capacity to cross biological barriers. It is extremely complicated, however, to predict (de)-agglomeration of particles in complex biological media; for this reason, we assume that single particles should be equal to or larger than 100 nm, which is a sufficiently large particle size to lower toxicity, as will be explained later.
In this work, we estimate the bulk phase solubility of oxide materials with a classification scheme, as detailed in the methodology section. We use the polarizing power of the oxide's cation (P, eqn (17)) as descriptor to classify the qualitative solubility of 66 oxides into 3 categories: category Sol1 contains the most soluble oxides in water which are defined as reactive, very soluble, soluble and slightly soluble. Category Sol2 contains oxides soluble in diluted acidic and acidic conditions: this category groups oxide materials that can be solubilized inside phagolysosomes. The third category Sol3 is composed of oxides poorly soluble in acidic conditions or insoluble. The classification scheme assigns correctly 82% of the cases, but some false negatives arise for mixed and high valence oxides. Besides chemical composition, particle's solubility depends on other important factors, which we do not consider here, such as the presence of complex-forming and/or redox molecules in the media, and the particle size. These features can change quite drastically the solubility profile of nanoparticles. It should be noted that the dissolution of particles determines also their clearance. In the lungs, 3 clearance mechanisms for deposited particles are possible: one that operates via the mucociliary activity, one that depends on particle solubility and systemic absorption, and a third one relying on the activity of alveolar macrophages, which transport particles to the mucus and lymph nodes. If inhalation of insoluble particles might induce, above a certain exposure level, pulmonary overload and possibly long term retention related effects, very soluble particles could also induce acute toxicity, depending on the intrinsic behaviour of ions in the lung tissue. An option to mitigate particle hazard could be that of controlling its dissolution rate: not too high to induce acute effects nor too slow for the particles to become biopersistent and, above a certain exposure level, induce pulmonary overload. In the case of core@shell nanoparticles, the shell material should be insoluble, otherwise the reactive core can interact with the surrounding media. If a (slowly) dissolving particle is not an option, particle toxicity should be mainly confined to pulmonary overload situations, which in principle can be controlled by keeping the exposure level below a certain threshold.
Last but not least, the size of nanoparticles has also an important effect on toxicity.17 During inhalation exposure, nanoparticles that induce pulmonary inflammation must first deposit in the alveolar region. Nanoparticles with a primary particle size between 10 and 100 nm will deposit more in this region compared to particles with a size between 0.1 and 1 μm.18 In the upper airways, particles must cross the mucus barrier to finally reach the lung epithelial cells. In the mucus layer, the mucin fibers form pores which have a maximum diameter of around 100 nm; therefore, this particle size is an important predictor for steric exclusion, as the diffusion in mucus of particles with diameters above this threshold is sterically impeded.19 Once the particles are in contact with the lung epithelium, cellular uptake occurs, leading to local and/or systemic effects. It is difficult to draw general conclusions on the effect of particle size on cellular uptake and toxicity, because the formation of the biomolecular corona, the agglomeration of nanoparticles and the type of cell studied are all factors that affect these two processes.17 Often, an optimal size for particle uptake around 50 nm is observed independently of particle composition, and in general, small nanoparticles are more likely to cause cytotoxicity than large ones. To account for all these size related processes, we impose that the overall particle diameter, comprising both the core and the shell, should be equal to 100 nm.
Good candidates for shell materials are listed in Table 4. Among them, silica can be considered one of the most promising candidates: compared to oxides with higher valence cations, but same toxicity score, silica has a higher conduction band and a wider band gap: both properties contribute to lower (photo)-reactivity. Compared to other low ranking oxides, silica has a lower PZC and therefore a more negative surface charge. Indeed, silica and silica-coated nanoparticles demonstrated a good degree of biocompatibility20–22 encouraging the idea of developing silica based nanoparticles for drug delivery systems and biosensors.23–25 In addition, this material can coat efficiently on oxide structures even when their lattices do not match: as a result, an amorphous shell can be grown on the core structure with good insulating properties.
| Material | E c (eV) | PZC | Solubility (categorical) | Toxicity score |
|---|---|---|---|---|
| WO3 | −5.7 | 0.6 | 3 | 1 |
| MoO3 | −5.4 | 0.7 | 3 | 1 |
| V2O5 | −5.3 | 3.1 | 3 | 1 |
| Sb2O5 | −5.6 | 4.6 | 3 | 1 |
| SiO2 | −1.9 | 3.9 | 3 | 1 |
| GeO2 | −3.3 | 5.4 | 3 | 1 |
| Ga2O3 | −5.8 | 6.3 | 3 | 0.97 |
| HfO2 | −2.7 | 7.5 | 3 | 0.92 |
| V2O3 | −3.6 | 7.5 | 3 | 0.91 |
| ZrO2 | −2.7 | 7.9 | 3 | 0.91 |
| Al2O3 | −1.2 | 7.9 | 3 | 0.91 |
| Ti2O3 | −2.4 | 9.3 | 3 | 0.85 |
Design of core@shell structures
To guarantee that core@shell structures will not result in a particle with undesirable reactivity and/or structural defects, we introduce two additional conditions. The first is the lattice match (LM, calculated using eqn (18)) between the two oxides, one composing the core and the other the shell. The lattice match accounts for the difference in physical dimensions of the unit cells of the two crystals: the smaller this difference, the better the shell material will grow on top of the core structure. When LM decreases, the shell structure can present defects that will compromise the functionality of the particle and/or enhance its toxicity (e.g., toxic ions can leach from the core or the core's reactive surface can be exposed to the environment). Here, we set that LM should be higher than 0.85.The second condition imposes that the band gap of the core material must be comprised within the bandgap of the shell material. This condition ensures not only that the core structure is coated by a transparent material but also that the shell is chemically inert towards the core (i.e., after UV absorption no electrons are transferred from the core to the shell). This condition fulfils both toxicity and functionality requirements because the nanoparticle is not reactive after UV absorption nor the shell interferes with the functionality of the core.
In Table 5 we list the final structures that fulfil both conditions of lattice match and band gap position for core@shell architectures: core@shell combinations are formed by the core materials identified in Tables 2 and 3, and shell materials from Table 4. The final nanoparticles have a shell thickness of 25 nm and a core diameter of 50 nm.
In addition to addressing the toxicity toward humans, information on the sustainability and environmental impact of the production process of nanomaterials should be included in the safe design process. Anastas and colleagues have put forward the 12 principles of green chemistry which can be directly applied as guidelines to select nanomaterial synthetic processes with low environmental impact. With this respect, an analysis aiming at evaluating the environmental impact and sustainability of the traditional synthesis of several common nanomaterials revealed that their E-Factor, a metric that measures the ratio of all materials used in the production process to the output products, varies over several orders of magnitude and is also quite high compared to other materials.26 Although early approaches to the production of nanomaterials pose a number of concerns for human health and the environment, improvements of the synthetic routes are certainly possible27 and research should focus on all stages of product life cycle.
Conclusions
This work is an effort towards the development and integration of computational methods to design safe and functional nanomaterials. We have proposed a computational screening procedure to select core@shell nanomaterials from a large number of possible architectures. The approach is demonstrated on a set of metal oxide structures for UV-protecting coating applications and is based on the calculation of key physicochemical properties of nanomaterials related to their safety, functionality and synthetic feasibility. The method is suitable to integrate safety aspects in early stages of R&D processes and to complement high-throughput screening methods. In the future, we are planning to focus on additional aspects of product sustainability (e.g. life cycle assessment) and to develop a second screening procedure that uses higher level computational methods to further improve the selection of nanomaterial structures.Methodology
Calculation of physicochemical properties
| Ec = −χoxide + 0.5Eg + 0.059(PZC − pH) | (1) |
| Ev = Ec − Eg | (2) |
The electronegativity of oxides is calculated using eqn (3)–(5):
| χoxide (eV) = 0.45χcation (eV) + 3.36 | (3) |
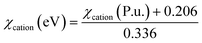 | (4) |
| χcation (P.u.) = 0.274z − 0.15zr − 0.01r + 1 + α | (5) |
Band gap values were either collected from literature or calculated from the experimental standard enthalpy of formation of oxides:
| Eg = Ae0.34EΔH0 | (6) |
| EΔH0 = 2ΔH0f × 2.612 × 1019/NAne | (7) |
| Surface charge = PZC − pHenvironment | (8) |
The PZCs of pristine oxide surfaces depend mainly on the type and density of surface hydroxyl groups and are very sensitive to sample preparation and environmental conditions. To account for this variability, PZC values are calculated using a Bayesian regression method. We first collected several PZC values for different oxides, each oxide having more than one single measured value;28 these measurements were then fitted to a Bayesian hierarchical regression model,29 that included the calculated electronegativity (χ, from eqn (5)) as group-level predictor, and is defined as:
| μi = μ0 + βχi + εi, εi ∼ N(0,τ2) | (9) |
| yij = μi + εij, εij ~ N(0,σ2) | (10) |
| μ0 ~ N(0,102), β ~ N(0,102), σ2 ~ U(0,102), τ2 ~ U(0,102) |
This model was used to predict probability distributions of likely PZC outcomes for every oxide: in this way the variability of experimental PZC values found for each oxide is used to build the distributions around each mean predicted PZC value. The model has an R2 of 0.80, indicating that the electronegativity descriptor is an adequate predictor of the PZC value.
| T = (1 − R)2e−αsx | (11) |
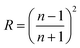 | (12) |
The scattering of the material (αs) is expressed as a function of the number of absorbing/scattering centers per unit volume (N) and the scattering cross-sections (Cs):
| αs = NCs = Nπr2χs | (13) |
| χs = A4β4 | (14) |
| β = 2πr/λ, (in this work, λ = 589.3 nm) | (15) |
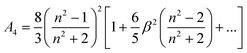 | (16) |
For 66 oxide materials we collected information on their solubility in water and acidic media using 8 discrete solubility levels: r (reactive), vs (very soluble), s (soluble), sl (slightly soluble), sda (soluble in diluted acidic conditions), sa (soluble in acidic conditions), sla (slightly soluble in acidic conditions) and i (insoluble). We then clustered these 8 levels into 3 categories using the logarithm of the polarizing power of the cation (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) as classifier:
P) as classifier:
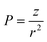 | (17) |
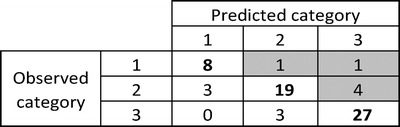
In Table 5 we show the misclassification matrix obtained for the following P intervals:
The percentage of correct classifications for category 1, 2 and 3 are 80%, 73% and 90%, respectively. Because high solubility is related to toxicity and poor functionality, we are mainly interested in false negatives (highlighted in grey in Table 6), e.g. those materials that are predicted to be less soluble than their actual value. Six out of 66 oxides, namely CuO, MgO, Fe2O3, Fe3O4, Co3O4 and Mn2O7 do not follow the classification scheme, with CuO, Fe3O4 and Co3O4 having borderline P values (1.81, 2.08 and 2.07, respectively) and MgO, Fe2O3 and Mn2O7 showing larger errors.
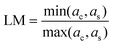 | (18) |
| PCP = xPCPMO1 + (1 − x)PCPMO2 | (19) |
Calculation of scoring functions
The ranking of core@shell nanoparticles is based on a total scoring function (S, eqn (20)) which sums the contribution of functionality (Fscore, eqn (21)), toxicity (Tscore, eqn (22)) and synthetic feasibility (Sscore, eqn (23)) scoring functions: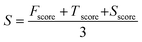 | (20) |
The functionality score Fscore sums over the contribution of band gap (FEg) and transmittance (FT) scores:
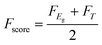 | (21) |
| FT = T |
The toxicity score Tscore sums over the contribution of conduction band (TEc), surface charge (TPZC) and solubility (Tsol) scores:
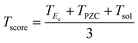 | (22) |
The synthetic feasibility score Sscore sums over the band gap (SEg) and lattice match (SLM) scores:
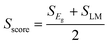 | (23) |
Acknowledgements
This work was funded by the Computational Material Science CMS2014 project from TNO.References
- A. M. Voutchkova, T. G. Osimitz and P. T. Anastas, Chem. Rev., 2010, 110, 5845 CrossRef CAS PubMed.
- E. Burello and A. P. Worth, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2011, 3, 298 CrossRef CAS PubMed.
- G. Moreose, J. Cleaner Prod., 2010, 18, 285 CrossRef PubMed.
- S. Gass, J. M. Cohen, G. Pyrgiotakis, G. A. Sotiriou, S. E. Pratsinis and P. Demokritou, ACS Sustainable Chem. Eng., 2013, 1, 843 CAS.
- N. Satoh, T. Nakashima, K. Kamikura and K. Yamamoto, Nat. Nanotechnol., 2008, 3, 106 CrossRef CAS PubMed.
- L. Burs, J. Phys. Chem. A, 1986, 90, 2555 CrossRef.
- Y. Zhang, L. Yu, S. Ke, B. Shen, X. Meng, H. Huang, F. Lv, J. H. Xin and H. L. W. Chan, J. Sol-Gel Sci. Technol., 2011, 58, 326 CrossRef CAS.
- M. H. Lee, U. M. Patil, S. T. Kochuveedu, C. S. Lee and D. H. Kim, Bull. Korean Chem. Soc., 2012, 33, 3767 CrossRef CAS.
- B. Faure, G. Salazar-Alvarez, A. Ahniyaz, I. Villaluenga, G. Berriozabal, Y. R. De Miguel and L. Bergström, Sci. Technol. Adv. Mater., 2013, 14, 023001 CrossRef.
- H. H. Kung, Transition Metal Oxides: Surface Chemistry and Catalysis, Elsevier Science Publishers B. V., 1989 Search PubMed.
- M. Uichiro, Hume-Rothery Rules for Structurally Complex Alloy Phases, Taylor & Francis, 2010 Search PubMed.
- S. Choopun, R. D. Vispute, W. Yang, R. P. Sharma, T. Venkatesan and H. Shen, Appl. Phys. Lett., 2002, 80, 1529 CrossRef CAS PubMed.
- E. Burello and A. P. Worth, Nanotoxicology, 2011, 5, 228 CrossRef CAS PubMed.
- E. Burello and A. P. Worth, Nanotoxicology, 2015, 9, 16 CrossRef PubMed.
- H. Zhang, Z. Ji, T. Xia, H. Meng, C. Low-Kam, R. Liu, S. Pokhrel, S. Lin, X. Wang, Y.-P. Liao, M. Wang, L. Li, R. Rallo, R. Damoiseaux, D. Telesca, L. Mädler, Y. Cohen, J. I. Zink and A. E. Nel, ACS Nano, 2012, 6, 4349 CrossRef CAS PubMed.
- W. S. Cho, R. Duffin, F. Thielbeer, M. Bradley, I. L. Megson, W. Macnee, C. A. Poland, C. L. Tran and K. Donaldson, Toxicol. Sci., 2012, 126, 469 CrossRef CAS PubMed.
- L. Shang, K. Nienhaus and G. U. Nienhaus, J. Nanobiotechnol., 2014, 12, 5 CrossRef PubMed.
- H. M. Braakhuis, I. Gosens, P. Krystek, J. A. F. Boere, F. R. Cassee, P. H. B. Fokkens, J. A. Post, H. van Loveren and M. V. D. Z. Park, Part. Fiber Toxicol., 2014, 11, 49 CrossRef PubMed.
- R. A. Cone, Adv. Drug Delivery Rev., 2009, 61, 75 CrossRef CAS PubMed.
- T. H. Chung, S. H. Wu and M. Yao, et al. , Biomaterials, 2007, 28, 2959 CrossRef CAS PubMed.
- G. Bhakta, R. K. Sharma and N. Gupta, et al. , Nanomedicine, 2011, 7, 472 CrossRef CAS PubMed.
- G. A. Sotitiou, C. Watson, K. H. Murdaugh, T. H. Darrah, G. Pyrgioakis, A. Elder, J. D. Brain and P. Demokritou, Environ. Sci.: Nano, 2014, 1, 144 RSC.
- W. Sun, N. Fang, B. G. Trewyn, M. Tsunoda, I. I. Slowing and V. S. Lin, et al. , Anal. Bioanal. Chem., 2008, 391, 2119 CrossRef CAS PubMed.
- B. G. Trewyn, S. Giri, I. I. Slowing and V. S. Lin, Chem. Commun., 2007, 3236 RSC.
- Y. Zhao, B. G. Trewyn, I. I. Slowing and V. S. Lin, J. Am. Chem. Soc., 2009, 131, 8398 CrossRef CAS PubMed.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316 CrossRef CAS PubMed.
- X. Wu, C. Lu, Z. Zhou, G. Yuan, R. Xiong and X. Zhang, Environ. Sci.: Nano, 2014, 1, 71 RSC.
- M. Kosmulski, Chemical Properties of Material Surfaces, 2001, Marcel Dekker, New York Search PubMed.
- E. Burello, Comput. Sci. Discovery, 2013, 6, 014009 CrossRef.
- Handbook of Chemistry and Physics ed. D. R. Lide, CRC, 2006 Search PubMed.
- L. Vegard, Z. Phys., 1921, 5, 17 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |