Open Access Article
Open Access ArticlePlasmonic colorimetric and SERS sensors for environmental analysis
Haoran
Wei
abc,
Seyyed M.
Hossein Abtahi
abc and
Peter J.
Vikesland
*abc
aDepartment of Civil and Environmental Engineering, Virginia Tech, Blacksburg, Virginia, USA. E-mail: pvikes@vt.edu; Tel: (540) 231 3568
bVirginia Tech Institute of Critical Technology and Applied Science (ICTAS) Sustainable Nanotechnology Center (VTSuN), Blacksburg, Virginia, USA
cCenter for the Environmental Implications of Nanotechnology (CEINT), Duke University, Durham, North Carolina, USA
First published on 10th March 2015
Abstract
The potential for water pollution outbreaks requires the development of rapid, yet simple detection methods for water quality monitoring. Plasmonic nanostructures such as gold (AuNPs) and silver (AgNPs) nanoparticles are compelling candidates for the development of highly sensitive biosensors due to their unique localized surface plasmon resonances (LSPRs). The LSPR of AuNPs and AgNPs lies in the visible and infrared light range and is sensitive to the composition, size, shape, surrounding medium, and aggregation state of these NPs. This plasmonic behavior provides the basis for fabrication of colorimetric sensors for environmental analyses. Furthermore, the LSPR also enhances the electromagnetic field near the NP surface, which provides the basis for surface-enhanced Raman spectroscopy (SERS) based detection. Organic or inorganic pollutants and pathogens can be detected and differentiated based upon the finger-print spectra that arise when they enter SERS-active hot spots. In this tutorial review, we summarize progress made towards environmental analysis based on LSPR-based colorimetric and SERS detection. The problems and challenges that have hindered the development of LSPR-based nanosensors for real-world environmental pollutant monitoring are extensively discussed.
Nano impactThe localized surface plasmon resonance (LSPR) of gold (AuNP) and silver nanoparticles (AgNP) enables rapid identification and detection of environmental pollutants. Using a LSPR-based colorimetric assay it is possible to detect contaminants either visually or via spectroscopic approaches. For even greater sensitivity, LSPR enabled surface-enhanced Raman spectroscopy (SERS) makes single molecule or single pathogen detection achievable. |
1. Introduction
One notorious side effect of global development is the ever-increasing number of gaseous and aqueous pollutants that pose ecosystem and human-health risks. Rapid pollutant recognition is vitally important in some emergent situations. For example, in the 2014 Elk River, WV incident in excess of 7500 gallons of 4-methylcyclohexanemethanol (4-MCHM) rapidly leaked into the Elk River such that the drinking water distribution system for the greater Charleston, WV area was heavily contaminated.1 Similarly, in the summer of 2014 a massive algal bloom led to closure of the Toledo, OH drinking water treatment plant due to the contamination of the water by microcystin toxins.2 In addition to outbreaks caused by chemicals, outbreaks of waterborne pathogens are also problematic. For example, the 1993 Milwaukee Cryptosporidium outbreak in drinking water caused 104 deaths in only two weeks.3 In November 2010, Cryptosporidium infected ≈27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people in Östersund, Sweden via contaminated drinking water, and in December 2012 an outbreak of waterborne norovirus caused acute gastrointestinal illness in a district containing 368 families in Denmark.4,5 In addition to waterborne contaminants, airborne contaminants, such as dioxins from garbage incineration plants or potentially pandemic bird flu, also threaten people's health.6,7
000 people in Östersund, Sweden via contaminated drinking water, and in December 2012 an outbreak of waterborne norovirus caused acute gastrointestinal illness in a district containing 368 families in Denmark.4,5 In addition to waterborne contaminants, airborne contaminants, such as dioxins from garbage incineration plants or potentially pandemic bird flu, also threaten people's health.6,7
To prevent contaminants from causing environmental catastrophes it would be ideal to detect such contamination events as quickly as possible in order to rapidly initiate remedial strategies. Unfortunately, the most commonly used detection methods for water and airborne contaminants such as gas/liquid chromatography-mass spectrometry, inductively coupled plasma mass spectroscopy, and quantitative polymerase chain reaction, although very sensitive and quantitative, require either laborious sample preparation procedures or onerous analysis methods and are thus very time-consuming. Besides, they all require expensive instruments and high level of expertise and thus cannot be conducted on site. Plasmonic nanostructures such as gold and silver nanoparticles (AuNPs and AgNPs) provide a promising avenue for the development of rapid, cost-effective and highly sensitive sensor platforms, which also exhibit the potential for on site detection.8 Many of the sensing capabilities enabled by AuNPs and AgNPs rely upon localized surface plasmon resonance (LSPR). When excited by light of a specific wavelength, the conduction electrons on the nanoparticle surface collectively oscillate and generate a significantly enhanced electromagnetic field or LSPR.8–10 LSPR is an extremely sensitive optical transducer, which is dependent on the type, size, shape and aggregation state of plasmonic nanoparticles as well as the refractive index of the surrounding environment.11–13 Changes in the LSPR result in color changes of the colloid suspension. Based on this phenomenon, LSPR-based colorimetric sensors have been developed.14–16
When the incident light wavelength is coupled with the LSPR of plasmonic NPs the electromagnetic field near the NP surface is significantly enhanced.17,18 When analytes closely associate with the NP surface, their Raman scattering cross-section increases substantially and this phenomenon is the basis for surface-enhanced Raman scattering (SERS).18 SERS is an ultrasensitive sensing technique that has been shown to enable the detection of single molecules.19–22 Compared with fluorescent techniques, SERS has greater potential for multiplex analysis due to the narrower peak widths in the collected Raman spectra. Because SERS is a vibrational spectroscopy method it provides chemical bonding information that facilitates differentiation of highly similar molecules and different molecular orientations.23,24 Unlike other environmental analysis techniques such as inductively coupled plasmon atomic emission spectroscopy and gas chromatography-mass spectroscopy, SERS does not require complex sample pretreatment, sophisticated analytical method optimization, or advanced analyst training. During the last decade, the rapid development of nanotechnology has created a number of novel nanostructures that have the potential for ultrasensitive SERS detection of environmental contaminants.25–27
Ultrasensitive chemical analysis via SERS was reviewed in the late 1990s, with the focus on the mechanisms responsible for “single molecule detection”.28,29 Subsequently, many review papers have appeared that describe the fundamental theories, material fabrication methods, and applications of SERS.17,18,21,30–36 Reviews on colorimetric sensors that monitor the LSPR band location have also been produced.37–39 However, relatively few of these reviews focus explicitly on environmental applications of LSPR based sensing. A number of recent reviews discuss nanomaterial-based sensors for environmental monitoring.40–45 However, these reviews covered either a broad suite of nanoparticles and sensing techniques or focused exclusively on SERS-based sensors. Herein we focus on the application of AuNPs and AgNPs for environmental sensing via either colorimetric or SERS approaches because these two related methods dominate much of the current literature. Readers interested in SPR sensors based on refractive index sensing are referred elsewhere.46–48 This review is organized into five parts (including this introduction). The second part briefly introduces the photonic behavior responsible for LSPR-based colorimetric and SERS sensors. The third and fourth parts summarize recent progress in environmental analysis with colorimetric and SERS sensors, respectively. In the SERS portion of the review, we focus on organic pollutants, biomolecules, and pathogen detection. For SERS detection of inorganic analytes the reader is referred elsewhere.49 The concluding part of this tutorial review discusses the extant challenges associated with ultimate application of these sensors in environmental samples.
2. Background on photonics
Colloidal gold and silver nanoparticles exhibit intense colors due to a phenomenon known as surface plasmon resonance.12,50–52 This phenomenon occurs when conduction band electrons undergo coherent oscillations following excitation by an electromagnetic field. The interaction between the electric field of the incoming light and NPs with dimension smaller than the incident wavelength causes polarization of the electrons in the nanoparticle relative to its heavier ionic core.53 This net charge difference is confined to the nanoparticle surface and acts as a restoring force that causes the collective oscillation of the surface electrons (i.e., a surface plasmon).53 The frequency at which these surface plasmons oscillate is known as the LSPR.The LSPR bands for gold and silver are within the visible portion of the electromagnetic spectrum. For example, the LSPR of spherical 50 nm gold nanoparticles is at ≈530 nm, which falls into the green light range (495–570 nm). Accordingly, green light is absorbed and red light is transmitted thus causing suspensions of this size AuNP to exhibit red colors under visible light excitation. Similarly, the LSPR of spherical 50 nm silver nanoparticles is at ≈430 nm, which falls in the violet light range, leading suspensions of this size AgNP to exhibit green colors.12,52 The exact location of the LSPR band is highly dependent on the identity, size, shape, and aggregation state of the noble metal nanoparticle, and the chemistry of the suspension medium.12,50 Increases in size result in red-shifts (an absorption peak shift to a longer wavelength), while changes in shape result in more complicated effects. For example, the peak LSPR wavelength of 100 nm edge-length silver triangles is approximately 100 nm larger than that for 100 nm silver pentagons (pentagon length is defined as the distance between opposite corners), which is in turn 100 nm greater than that of 50 nm diameter silver spheres (Fig. 1).12 Asymmetric gold nanorods exhibit two LSPR bands – one that corresponds to the longitudinal direction and the other the transverse direction of the rods.54,55
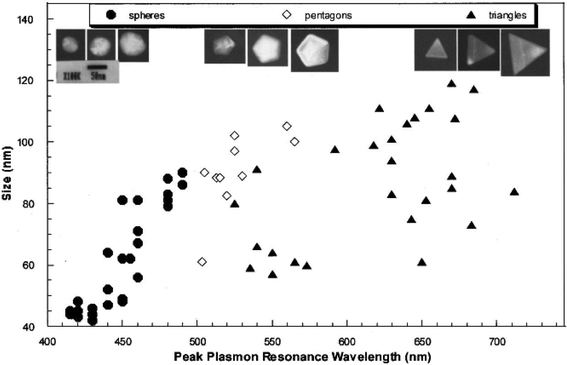 | ||
| Fig. 1 TEM images of silver spheres, pentagons, and triangles with different size (above) and their size-dependent peak LSPR wavelength. The size of a silver triangle is its edge length; the size of a silver pentagon is the distance between its opposite corners; the size of a silver sphere is its diameter.12 Reprinted with permission from J. Mock, M. Barbic, D. Smith, D. Schultz and S. Schultz, J. Chem. Phys., 2002, 116, 6755–6759. Copyright 2014 American Institute of Physics. | ||
In addition to shape mediated effects, changes in aggregation result in quantifiable red-shifts or blue-shifts.56,57 The potential development of secondary LSPR bands at longer wavelengths has been observed in end-to-end assembly of gold nanorods and at shorter wavelengths in side-by-side assembly of gold nanorods.56 Although the physics are quite complex, in simplistic terms the new LSPR band is the result of dipole alignment between adjacent particles.58 A tunable LSPR is crucial for sensing applications. The overlap between laser wavelength and the LSPR peak results in high SERS enhancement factors, which will be discussed later.17 Changes in the LSPR band location can also elicit quantifiable color changes. Using 50 nm AuNPs as an example, aggregation results in the development of a new red-shifted peak at about 700 nm that falls in the red light range. Therefore, red light will be absorbed, while blue light will be scattered and the suspension color changes to blue. Because this color change is distinct and can be easily measured, it has been found to be highly useful for analyte detection.14,38,59 A broad range of analytes have been detected solely on the basis of this color change.15,59–61
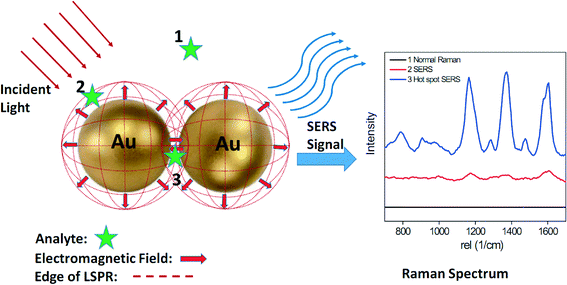
Surface-enhanced Raman scattering (SERS) is another phenomenon that arises due to LSPR. A schematic illustrating the basic working principle of SERS is shown in Fig. 2. Raman scattering is the inelastic scattering of photons by the vibrational chemical bonds of a molecule. The Raman spectrum is unique for each molecule due to the different vibrational modes present within it. Unfortunately, the Raman scattering signal is at most 10−7 of the total scattering, which makes it challenging to use Raman to detect low concentration analytes. When a molecule is adsorbed on AuNPs or AgNPs, its Raman cross section can be enhanced by several orders of magnitude due to SERS. Two primary mechanisms are responsible for SERS: electromagnetic and chemical. The former refers to the enhanced electromagnetic field near the nanoparticle surface, which is a long-range mechanism.62 Long-range enhancements occur at greater distances away from the nanoparticle surface whose edge is schematically shown by the dotted red circle in Fig. 2. As shown in Fig. 2, analyte molecules located within the dotted red circle (position 2 and 3) exhibit clear Raman spectra, while analytes located outside the dotted red circle (position 1) exhibit no detectable Raman signal. For example, the SERS signal of the CH3 group of an alkanethiol molecule decreased by a factor of 2 when its distance from a SERS enhancing silver substrate increased from 0.8 nm to 2.5 nm.63 The latter reflects charge transfer between the guest molecule and nanoparticle, which is a short-range mechanism.62 Shorter-range enhancements only occur when an analyte is absorbed to a nanoparticle surface.
Studies to understand the SERS effect have shown that the largest SERS enhancements are produced by strongly interacting metal nanoparticles.17,64 Clusters of two or more nanoparticles give rise to an extinction spectrum consisting of multiple peaks and facilitate single-molecule SERS.19 This effect can be attributed to the coupling of the intense localized electromagnetic fields on each nanoparticle produced by incident light excitation of the appropriate wavelength and polarization. The long range coupling of the electromagnetic fields, although it decays exponentially with particle distance, can extend to a distance of 2.5× the nanoparticle diameter.64,65 It is generally thought that significant Raman enhancements primarily occur within gaps smaller than 10 nm although the exact distance is still a topic of debate.66–68 These localized areas are often referred to as 'hotspots' (Fig. 2).69 As shown in Fig. 2, analyte molecules located within the hot spot (position 3) exhibit a much stronger Raman signal than those located on an AuNP monomer surface (position 2). In addition to the gap between two adjacent nanoparticles, the sharp corners and tips of anisotropic plasmonic nanoparticles such as nanorods, nanoprisms, and nanostars produce another type of SERS “hot spot”.70,71 A recent study demonstrated that isolated single gold nanorods can generate strong SERS signals that approach those obtained in the gap between spherical particles.72 Because of the importance of hot spots for SERS application, a substantial body of research has focused on the creation and maximization of the number and location of SERS hot spots.73–76
Other than SERS hot spots, several additional factors significantly influence SERS, such as nanoparticle type, shape, size, solution pH and so on.77–83 AgNPs can generate stronger SERS intensities than AuNPs because the extinction coefficient of AgNPs can be 4× larger than AuNPs of the same size and shape.84,85 Anisotropic plasmonic nanoparticles show multiple LSPR modes and are suitable for use under different laser lines.86–88 For example, gold nanostars (40 nm) show a second LSPR peak at 730 nm, while gold nanospheres (40 nm) show only one peak at 530 nm. Therefore, when excited by a 785 nm laser, the SERS intensity of gold nanostars is 2–3 orders of magnitude higher than that of gold nanospheres.89 The size of a nanoparticle affects its LSPR, which determines its SERS intensity as well. A recent study shows even under random aggregation conditions, nanoparticle size still plays an important role in the Raman signal. With 785 nm laser excitation, AuNPs with size between 46–74 nm showed the strongest Raman signal. It has been shown that for elongated shape gold nanoparticles such as rods that the aspect ratio (length/diameter) is an important factor. Results suggest that enhancement can be two orders of magnitude greater when the plasmon band of the gold nanorod overlaps with the excitation wavelength.90 These results indicate that it is necessary to carefully choose nanoparticle size according to the excitation laser wavelength.91 Solution pH influences analyte adsorption to the NP surface and can subsequently influence its SERS signal.77 For example, diclofenac sodium only exhibited a clear SERS spectrum under acidic and neutral pH conditions and not under alkaline pH conditions due to electrostatic repulsion between its carboxylic group and the citrate-coated AgNP surface.92
Organic chemical detection is comparatively easy to achieve because small molecules can readily enter SERS hot spots. Pathogens, however, such as bacteria and viruses, are too large to enter SERS hot spots thus resulting in several orders of magnitude lower Raman enhancement factors. To circumvent this problem, a SERS tag is often employed.31,93 A SERS tag includes a recognition element, Raman reporter, and a signal transducer.45 AuNPs and AgNPs are most commonly used signal transducers, while dyes with large Raman cross-sections are used as Raman reporters. Specific antibodies or aptamers against the target pathogens are used as recognition elements. Generally, a protection layer is needed for the Raman reporter modified nanoparticle to prevent the leakage of Raman reporter and improve the stability of the nanoparticle.
3. Colorimetric detection
Perhaps the most convenient mechanism for a rapid, field-deployable contaminant detection assay would be to observe color changes with our naked eye. Because the LSPRs of gold and silver colloids fall within the visible spectrum, color changes that occur due to changes in aggregation state have been exploited for colorimetric sensor fabrication. Colorimetric sensing of DNA using functionalized AuNPs was pioneered by Mirkin et al.94 In that study, two batches of 13 nm AuNPs were functionalized with two non-complementary oligonucleotides and were then combined. After the addition of a target DNA duplex with two “sticky ends” (complementary to the oligonucleotides on each type of AuNP), the suspension color changed from red to purple due to DNA hybridization induced AuNP aggregation.94 Both the oligonucleotide modification position and the AuNP size greatly influenced probe sensitivity. When the two batches of AuNPs were modified with 5′-oligonucleotide and 3′-oligonucleotide, respectively, single base imperfections could be detected.59 Importantly, larger AuNPs (50 nm, 100 nm) were found to be more sensitive than smaller AuNPs (13 nm) because of their larger extinction coefficients.95 In addition to oligonucleotide-gold nanoparticle (OGN) conjugates, oligonucleotide-silver nanoparticle (OSN) conjugates were also used as DNA probes. Because of the larger extinction coefficients of AgNPs compared with AuNPs, the detection limit for target DNA by the OSNs was 50× lower than with the OGNs.96Aggregation induced by oligonucleotide hybridization is one example of a cross-linked colorimetric sensor. Similar sensor designs have been applied for detection of a range of biomolecules, heavy metal ions, and pathogens.97 When the target directly binds to a recognition element on the nanoparticle surface, it induces aggregation and, in the case of AuNPs, a red to blue color change. Alternatively, the target can induce dissociation of nanoparticle aggregates by competitively binding to the linker between nanoparticles. Under these conditions a blue to red color change is expected. For example, an aptamer-linked gold nanoparticle aggregate was developed for adenosine detection. Aptamers are single oligonucleotide strands of DNA or RNA that can bind pathogens, molecules, or even ions with high affinity and specificity.98 Adenosine addition resulted in dissociation of the aptamer-linked aggregates due to its competitive binding to the aptamer linker between the two AuNPs. Following addition of adenosine, the suspension color changed from purple to red indicating the transformation from AuNP aggregates to monomers. This result was further indicated by the blue shift of the LSPR band in the UV–VIS spectrum from 700 to 522 nm.99 A similar protocol was successfully applied for the fabrication of a cocaine sensor with a detection limit of 50–500 μM.61 Recently this protocol was extended to development of a “smart hydrogel” sensor, where dissociation of the cross-linked hydrogel following addition of target resulted in the release of AuNPs to the solution and a change in color.100
In a non cross-linked detection protocol there is no hybridization between different gold/silver nanoparticles. In this case, aggregation/dissociation of the nanoparticles is achieved by decreasing/increasing the concentration of stabilizer on the nanoparticle surface. For example, an ultrasensitive colorimetric DNA probe (1 pM detection limit by eye) was developed by using a polyelectrolyte that forms conjugates with single stranded DNA. Following polyelectrolyte addition, AuNPs stabilized with single stranded DNA aggregated due to preferential binding between the aptamer and the polyelectrolyte, while AuNPs stabilized with target double stranded DNA remained stable.15
The detection protocols described above have been used for heavy metal detection due to their capacity to form strong complexes with chelators and other recognition agents. In this manner, a sensitive and selective probe for Hg2+ was fabricated by modifying the 13 nm AuNP surface with mercaptopropionic acid (MPA). Hg2+ forms complexes with the carboxylate groups of MPA and induces AuNP aggregation. After addition of 2,6-pyridinedicarboxylic acid (PDCA) into the probe suspension, the selectivity for Hg2+ relative to other heavy metals was significantly improved. This result was attributed to the 100× higher complexation coefficient of PDCA for Hg2+ than for other heavy metals. The combined method enabled quantitative detection of Hg2+ over a concentration range of 250–500 nM with a limit of detection of 100 nM.60 In addition to using toxic organic compounds as recognition elements, urine can also be used for Hg2+ sensing. The uric acid and creatinine in urine can synergistically bind to AuNPs as well as selectively adsorb Hg2+. In addition to the low cost sensor fabrication, a low detection limit of 50 nM was achieved in this manner.16 It has been shown that Zn2+ and Cu2+ can be detected using agglomeration and the resulting suspension color change of 20 nm chitosan-capped gold nanoparticles.101 Chitosan is a well-known chelating agent for heavy metals and the presence of Zn2+ and Cu2+ can cause colloidal instability and loose aggregation (agglomeration) of gold nanoparticles. This phenomenon causes a rapid color change that is directly related to the heavy metal concentration. Pb2+ with a tunable detection limit of 100 nM to 200 μM has been detected following an aggregation-dissociation protocol. The DNAzyme-directed assembly of gold nanoparticles cleaves in the presence of Pb2+ and results in a blue to red color change (Fig. 3A).
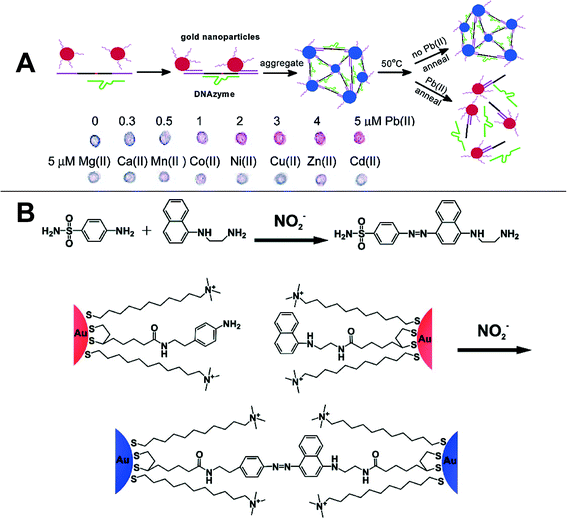 | ||
| Fig. 3 A) DNAzyme-directed assembly formation and cleavage of gold nanoparticles in a Pb+ colorimetric sensor;102 Reprinted with permission from J. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642–6643. Copyright 2014 American Chemical Society. B) Schematic of the Griess reaction and Griess reaction induced aggregation of AuNPs.14 Reprinted with permission from W. L. Daniel, M. S. Han, J. S. Lee and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 6362–6363. Copyright 2014 American Chemical Society. | ||
Nitrate and nitrite ions are two regulated contaminants in drinking water. A simple colorimetric method was developed for their detection based upon the Griess reaction (Fig. 3B). As shown in Fig. 3B, two batches of AuNPs were functionalized with 5-[1,2]dithiolan-3-yl-pentazoic acid [2-(4-amino-phenyl)ethyl]amide (DPAA) and 5-[1,2]dithiolan-3-yl-pentazoic acid [2-(naphthalene-1-ylamino)ethyl]amide, respectively. Following nitrite ion addition, the amino group and naphthalene group were linked via an azide linkage, which then resulted in AuNP aggregation and the fading of the suspension color. The color change threshold could be controlled by adjusting the incubation time and temperature to meet the EPA standard (1 ppm for nitrite ion). The same procedure was applied for nitrate detection after the nitrate ions were reduced to nitrite by nitrate reductase. The specificity of this probe is high enough that it is not affected by the presence of other inorganic ions (F−, SO42−, HCO3−, etc.) even when their concentrations are two orders of magnitude larger than that of nitrite.14
A majority of the plasmonic nanoparticle based colorimetric detection methods rely upon crosslinking. However, non-crosslinking methods are also sometimes employed. A homogeneous method for the selective detection of Hg2+ and Ag+ using Tween 20-modified AuNPs has been developed. Citrate-capped AuNPs were modified with Tween 20. In the presence of silver and mercury ions, citrate ions reduce Hg2+ and Ag+ to form Hg0 and Ag0 on the surface of the AuNPs. This phenomenon was followed by Tween 20 removal from the NP surface and aggregation of AuNPs. The detection limit can be as low as 0.1 μM in the presence of NaCl and EDTA.103 In another study, a sensor for quantitative detection and differentiation of two nitroamine explosives – hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) was developed.104 In this sensor, nitrite hydrolyzed from RDX and HMX reacted with 4-aminothiolphenol on AuNPs to form an azo dye with naphthylene diamine. Dye formation changed the LSPR of the AuNPs because of a charge-transfer interaction on the AuNP surface. The absence of a second LSPR peak indicated the color change was not due to AuNP aggregation, but instead due to dye formation. AuNPs improved the sensitivity of the probe, but the mechanism responsible for this behavior was not clearly elucidated.
Some special detection protocols have also been used for heavy metal ion detection. Cr6+ can selectively etch the tips of gold nanorods (AuNRs) due to its strong oxidation state. Shortening of the nanorod induces a blue shift in its longitudinal LSPR band and a corresponding color change. Using this approach a detection limit of 90 nM was obtained.105 This method does not require aggregation or dissociation of nanoparticles and as such it can be described as a non-aggregation method. Cu2+ can also etch the tips of AuNRs in the presence of HBr. In this case, Au0 was oxidized to Au+ and Cu2+ was reduced to Cu+, which was subsequently oxidized to Cu2+ by dissolved oxygen (Fig. 4A). The presence of cetyltrimethylammonium bromide (CTAB) was key to this redox reaction because it reduced the redox potential of Au+/Au0 from 0.93 V to less than 0.2 V. The decrease in aspect ratio due to etching resulted in a blue shift of the LSPR band and a color change from blue to red (Fig. 4A). With this method, 50 nM Cu2+ was detected by the naked eye and 0.5 nM Cu2+ was detectable by UV–VIS.106 A similar protocol was applied for Hg2+ detection. In the presence of ascorbic acid, Hg2+ was reduced to Hg0 and deposited on AuNRs, which induced a color change from purple to blue green. The detection limit of Hg2+ was 800 pM. The Hg0–AuNR can subsequently be used as a S2− sensor because S2− can exfoliate Hg0 from the AuNR surface.107
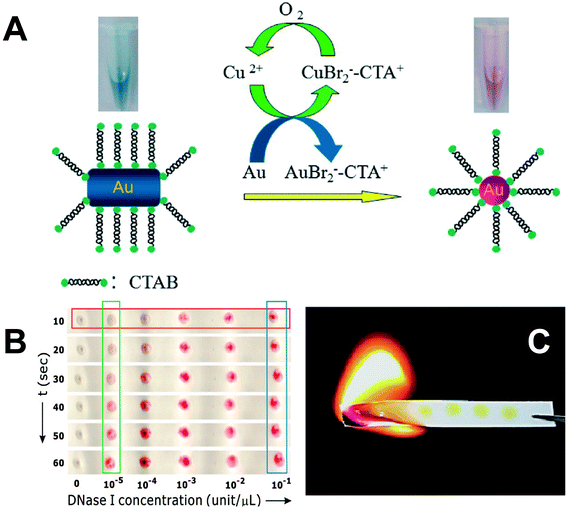 | ||
| Fig. 4 A) Schematic of colorimetric detection of Cu2+ by etching AuNR tips by Cu2+ in the presence of CTAB and HBr.106 Reprinted with permission from Z. Zhang, Z. Chen, C. Qu and L. Chen, Langmuir, 2014, 30, 3625–3630. Copyright 2014 American Chemical Society. B) DNA-hybridized AuNP aggregates on a hydrophobic paper after exposure to DNase I droplets.108 Reprinted with permission from W. Zhao, M. M. Ali, S. D. Aguirre, M. A. Brook and Y. Li, Anal. Chem., 2008, 80, 8431–8437. Copyright 2014 American Chemical Society. C) Spent paper substrates are burnt to minimize hazardous chemical handling.109 Reprinted with permission from S. C. Tseng, C. C. Yu, D. Wan, H. L. Chen, L. A. Wang, M. C. Wu, W. F. Su, H. C. Han and L. C. Chen, Anal. Chem., 2012, 84, 5140–5145. Copyright 2014 American Chemical Society. | ||
For practical field applications, paper-based colorimetric sensors may be better than suspension-based ones due to their smaller volume, longer-term stability, and convenient handling and processing. Recently it has been reported that the protocols for suspension-based colorimetric detection can also be applied on a paper substrate.108,110 For example, DNA-hybridized AuNP aggregates that were spotted on paper can be redispersed into a droplet that contains endonuclease (DNase I), which could cleave hybridized DNA. Following endonuclease addition, the blue or black spot on paper rapidly changed color to red and this color change could be discerned by the naked eye even at low nM endonuclease concentrations (Fig. 4B).108 It is notable that the paper used in these assays should be hydrophobic paper or surfactant-treated hydrophilic paper to avoid the rapid spread and drying of the droplet applied on the surface. In addition to drop-coated AuNP suspensions on paper, paper/AuNP composites can also be synthesized by a laser-induced thermal method. When 15 nm thin gold films coated on paper were exposed to KrF excimer laser irradiation, AuNPs (46 nm) formed on the paper surface with a high density of 318 μm−2. Following immersion into cysteine solution the color of the paper changed from light yellow to dark yellow. The paper could be burnt after use, which is a simple mechanism for hazardous waste disposal (Fig. 4C).109 Another paper-based analytical protocol has been reported for colorimetric sensing of Cu2+ by AgNPs functionalized with homocysteine and dithiothreitol. The LSPR peak intensity of AgNPs at 404 nm decreased while a new red-shifted band at 502 nm appeared as Cu2+ was added. Consequently, the color of the paper coated with AgNPs changed from yellow to orange or green-brown. A linear response was observed for the color intensity change as a function of Cu2+ concentration in the range of 7.8–62.8 μM.111 Based on these results, we are confident that paper-based colorimetric LSPR sensors should have applicability for detection of a broad range of environmental pollutants.
4. SERS detection
The SERS phenomenon was first observed in 1974 when the Raman signal of pyridine adsorbed on a roughened silver electrode was substantially enhanced.112 SERS was subsequently proposed as an analytical technique for many organic compounds using substrates such as roughed Ag electrodes or Ag films on nanospheres (AgFON).113,114 However, the detection limits achieved with these methods are high (above 1 μM), which limits their application. In 1997, however, single molecule detection was achieved for resonant dye molecules, such as rhodamine 6G (R6G) and crystal violet (CV) using AgNP colloids as SERS substrates.19,22 It was subsequently realized that aggregates in the colloid are responsible for the substantially enhanced Raman signal and the concept of the aforementioned SERS “hot spot”, the gap between the aggregates, was proposed.In the past decade, numerous research efforts have been devoted to create and maximize the number of “hot spots” within SERS substrates.116,118,119 Adding salts or organic electrolytes to gold or silver colloid suspensions can induce aggregation and generate SERS “hot spots”.120 However, the aggregation process is random and thus hard to replicate. Recently, methods to generate highly reproducible and controllable SERS hot spots in suspension have been reported.115,116,121,122 For example, the supermolecule cucurbit[n]uril (CB[n]) can link AuNPs with a fixed gap of 0.9 nm and this molecule can also specifically capture target analytes within the hot spot (Fig. 5A).115 DNA-mediated gold nanogap particles, which contain a 20 nm gold core and 11 nm gold shell linked by a gold nanobridge have recently been synthesized (Fig. 5B). Dyes located in the 1 nm gap were quantitatively detected over an ultra low concentration range of 10 fM to 1 pM. Raman mapping results demonstrate that 90% of these nanoparticles show SERS enhancement factors between 108 and 109 – a range that is sufficient for single molecule detection.116 Despite its excellent homogeneity, this nanoparticle is more appropriate for use as a SERS tag rather than as a SERS substrate due to the difficulty associated with getting analyte chemicals to diffuse into the nanogap.
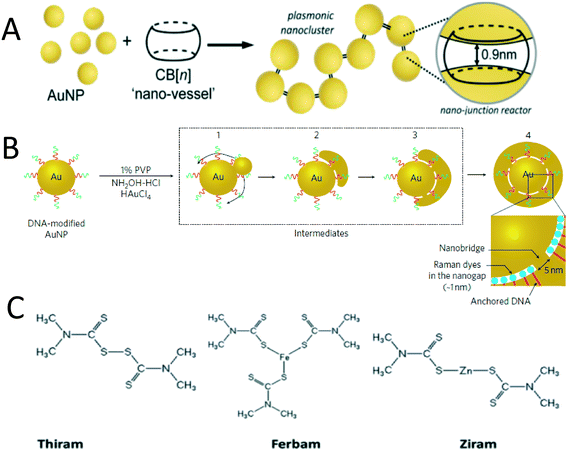 | ||
| Fig. 5 A) CN[n] induced AuNP aggregation with a fixed sub nanometer gap;115 Reprinted with permission from R. W. Taylor, R. J. Coulston, F. Biedermann, S. Mahajan, J. J. Baumberg and O. A. Scherman, Nano Lett., 2013, 13, 5985–5990. Copyright 2014 American Chemical Society. B) Formation of 1 nm gap between AuNP core and shell linked with a Au nanobridge.116 Reprinted with permission from D. K. Lim, K. S. Jeon, J. H. Hwang, H. Kim, S. Kwon, Y. D. Suh and J. M. Nam, Nat. Nanotechnol., 2011, 6, 452–460. Copyright 2014 Nature Publishing Group. C) Chemical structures of three dithiolcarbamate pesticides.117 Reproduced from B. Saute, R. Premasiri, L. Ziegler and R. Narayanan, Analyst, 2012, 137, 5082–5087. With permission from The Royal Society of Chemistry. Copyright 2014 The Royal Society of Chemistry. | ||
For real applications, solid SERS substrates are often considered superior to suspension-based SERS due to the long term stability and transport and handling convenience that the solid substrates provide. Extensive research efforts have been devoted to making homogeneous solid SERS substrates using approaches such as electron lithography, focused ion beam lithography, and nanosphere lithography.13,20,33,82,123–125 These top-down methods make highly ordered plasmonic nanostructures with tunable shape, size, and particle-to-particle gap size and have very high SERS enhancement factors.126 However, these methods, especially electron lithography, can be quite expensive and are difficult to scale up. Recently reported nanoporous gold and gold/silver nanoporous films are easy to make at large scale. After thermal treatment, the films wrinkle and create quasi-periodic nanogaps and nanotips, which act as SERS “hot spots”. With these wrinkled films, single molecule detection of R6G was achieved.20,123 Recent studies find that covering Au nano-pyramid arrays with graphene can improve the SERS signal 10× due to the enhanced charge transfer.127
In contrast to the aforementioned rigid SERS substrates, flexible substrates such as paper-based SERS substrates are cheaper, easier to make, and can be applied for curvy surfaces.128–134 A paper-based SERS swab was fabricated by simply dipping a filter paper in AuNR suspension. AuNRs were adsorbed efficiently onto the surface of filter paper due to the electrostatic attraction between the negatively charged cellulose and the positively charged CTAB-coated AuNRs. The biggest advantage of this SERS substrate is its ease of use for the collection of trace samples from a solid surface. By swabbing a glass surface contaminated with a 140 pg 1,4-benzenedithiol (1,4-BDT) residue, the chemicals were readily adsorbed on the paper surface and their Raman spectrum was easily obtained (Fig. 6A).129 Similarly, a star-shaped μPAD whose fingers were coated with polyelectrolyte was fabricated (Fig. 6B). This μPAD showed the capability to separate chemicals based upon their charge and to concentrate the chemicals into the small volume of the tips (Fig. 6B). For example, positively charged R6G readily moved to the finger tip coated with positively charged poly(allylamine hydrochloride), while it was retained at the entrance of the finger coated with negatively charged poly(sodium 4-styrenesulfonate). This μPAD exhibited a preconcentration factor of 109 for R6G and thus a super low detection limit of 100 aM was detected.133 In addition to paper, electrospun nanofiber mats have also been used as the SERS substrate scaffold.134,135 For example, an AgNP/PVA (poly(vinyl alcohol)) membrane was fabricated by electrospinning AgNPs and PVA mixture. The bulk material and nanofibers coated with AgNPs are shown in Fig. 6C and D, respectively. 4-mercaptobenzoic acid (4-MBA) at a concentration of 10−6 M was detected using this SERS substrate.135
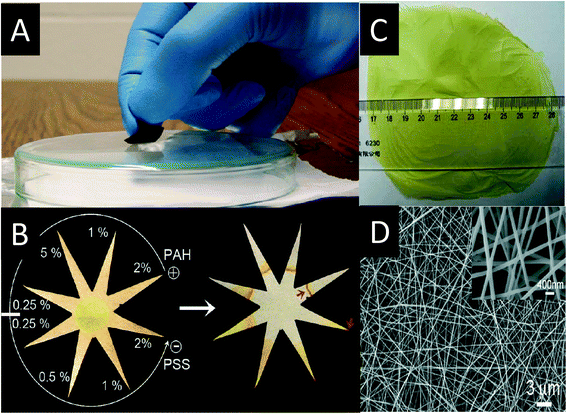 | ||
| Fig. 6 A) A glass with 1,4-BDT residue is swabbed by the paper-based SERS substrate;129 Reprinted with permission from C. H. Lee, L. Tian and S. Singamaneni, ACS Appl. Mater. Interfaces, 2010, 2, 3429–3435. Copyright 2014 American Chemical Society. B) A star-shape paper with eight fingers were coated by polyelectrolyte, which could separate and preconcentrate chemicals efficiently;133 Reprinted with permission from A. Abbas, A. Brimer, J. M. Slocik, L. Tian, R. R. Naik and S. Singamaneni, Anal. Chem., 2013, 85, 3977–3983. Copyright 2014 American Chemical Society. C) The photo and D) SEM image of AgNP/PVA membrane fabricated by electrospinning.135 Reprinted with permission from D. He, B. Hu, Q. F. Yao, K. Wang and S. H. Yu, ACS Nano, 2009, 3, 3993–4002. Copyright 2014 American Chemical Society. | ||
Although significantly improved average enhancement factors (EF) have been achieved (generally greater than 109) for Raman active dyes and other test materials, the application of such SERS substrates for ultrasensitive detection of organic pollutants are few.117,136–140 The reason for this is that many organic pollutants are non-resonant under the laser excitation wavelengths (>514 nm) typically used for Raman spectroscopy. Accordingly, their Raman cross-sections are generally several orders of magnitude lower than those for the resonant dyes most commonly used for SERS substrate development.
SERS detection of pesticides with high affinity to AuNPs has been reported.117,138 Dithiolcarbamate pesticides – thiram, ferbam, and ziram were detected and differentiated by SERS using a gold nanorod suspension as the SERS substrate. The structures of these three chemically similar pesticides are shown in Fig. 5C. Each pesticide contains sulfur groups that can form covalent Au–S bonds with the AuNP surface. To obtain high SERS intensity, gold nanorods whose longitudinal LSPR was well coupled with the laser wavelength were used as the SERS substrate. The detection limits of these three pesticides are 34 nM, 26 nM, and 13 nM, respectively, well below the EPA standards (17 μM, 10 μM, 23 μM).117 These results indicate that for organic pollutants showing high affinity with gold or silver nanoparticles, SERS detection is feasible if the LSPR of the SERS substrate matches the excitation laser wavelength. An organophosphorus pesticide – paraoxon at a concentration of 10 nM was detected using a self-assembled gold nanoparticle film. The film is made by casting methoxy-mercapto-poly(ethylene glycol) (mPEG-SH) functionalized AuNP suspension onto a solid substrate. The AuNPs were closely packed on the substrate with 5 nm gaps. Self-assembly induced by mPEG-SH modification significantly improved the SERS intensity and homogeneity of the film.138 This is a simple and cost-efficient method for SERS substrate fabrication. However, the author did not explain how the mPEG-SH-AuNP suspension and the analyte solution overcame the “coffee ring effect” when cast on a solid substrate.
A significant challenge that has limited SERS detection of organic pollutants is not only their generally small Raman cross sections, but also their low affinity to the NP surface. Therefore, methods to enhance the affinity between pollutants and the gold/silver NP surface have been pursued to solve this problem.141–146 One way to achieve this goal is through addition of a molecular trap on the gold/silver nanoparticle surface to specifically capture organic molecules. The thermally sensitive polymer poly-(N-isopropylacrylamide) (pNIPAM) was recently used as the trap for 1-naphthol (1-NOH). At a temperature of 277 K, pNIPAM exists in a swollen state, such that 1-NOH trapped within the polymer is far away from the AuNP surface, which then results in a weak SERS signal. In contrast, at a temperature of 333 K, pNIPAM shrinks to half of its swollen volume, thus bringing 1-NOH closer to the AuNP surface resulting in a substantial increase in the SERS signal.143 This method enabled acquisition of the SERS spectrum of 1-NOH for the first time. However, the limit of detection for 1-NOH is high (10 μM). TNT was trapped on a cysteine-functionalized AuNP surface by the formation of a Meisenheimer complex with cysteine (Fig. 7A). Electrostatic attraction between Meisenheimer complex-bound AuNPs and cysteine-bound AuNPs subsequently resulted in AuNP aggregation and the generation of a number of SERS hot spots. With this method, 2 pM TNT was detected in aqueous solution.141 TNT has also been adsorbed onto the AuNR surface by a peptide linker containing a TNT-binding tail, a cysteine terminal, and a glycine spacer. The peptide-functionalized AuNRs were embedded in a filter paper and tested with both liquid phase and vapor phase TNT. Notably, this material could detect 10 μM TNT in a shampoo solution thus indicating its high selectivity for TNT.147 Dithiolcarbamate calix[4]arene was also used as a linker between AgNPs and polycyclic aromatic hydrocarbons (PAHs). The cup shape calix[4]arene is able to host hydrophobic PAHs and the dithiolcarbamate on the linker increases the affinity between the linker and the nanoparticle (Fig. 7B). This novel SERS substrate can achieve a limit of detection for four PAHs (pyrene, benzo[c]phenanthrene, triphenylene, and coronene) in the range between 10 nM to 100 pM.142 Calixarene-functionalized AgNP embedded in silica film was applied in a flow cell designed for in situ monitoring of PAHs in seawater.148–150 Limits of detection of 100 pM and 310 pM for pyrene and anthracene were achieved when artificial sea water spiked with PAHs traveled through the flow cell.148 A field study using this SERS substrate was conducted in the Gulf of Gdańsk (Baltic Sea). The limit of detection for 12 different PAHs was 150 ng L−1, which is comparable to the results obtained via GC/MS, thus indicating the SERS technique has potential for monitoring pollution events in situ.150 Viologens have also been used as a PAH linker. Because of their high affinity to both AgNPs and guest PAHs, viologens could induce the aggregation of AgNPs and thus further increase the SERS intensity. With this method, a detection limit of 80 pyrene molecules was obtained – this is the lowest limit of detection for pyrene ever reported.144 The drawback of this method is the high background signal from the linker, which makes spectrum analysis challenging.
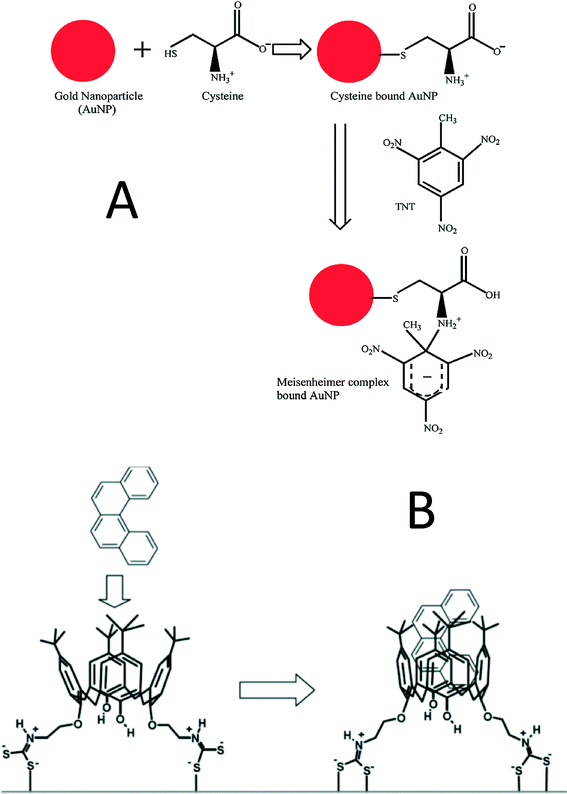 | ||
| Fig. 7 A) Trinitrotoluene (TNT) is captured by cysteine-functionalized AuNPs by formation of a Meisenheimer complex;141 Reprinted with permission from S. S. Dasary, A. K. Singh, D. Senapati, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2009, 131, 13806–13812. Copyright 2014 American Chemical Society. B) calix[4]arene links PAHs and AgNPs.142 L. Guerrini, J. V. Garcia-Ramos, C. Domingo and S. Sanchez-Cortes, Anal. Chem., 2009, 81, 953–960. Copyright 2014 American Chemical Society. | ||
The SERS spectrum of the dioxin 2-benzoyldibenzo-p-dioxin (BDPD), a highly toxic compound, was first reported in 2009 using AgNPs loaded in poly(diallyldimethylammonium chloride) (PDDA) and poly-(acrylic acid) (PAA) film. This film was fabricated using a layer-by-layer method and subsequently impregnated with AgNPs. After drying in air, this SERS substrate showed a 5× higher Raman signal for 1-naphthalenethiol (1-NAT) than an AgNP suspension due to hot spot formation in the 3D structure. More importantly, the SERS dioxin spectrum at 10 nM was observed on this substrate although the signal was very weak.140 This substrate works for dioxin partly because the PDDA–PAA can trap dioxin in the film thus creating the opportunity for dioxin contact with the AgNP surface. Recently, a detection limit down to three molecules was reported for atrazine detection via SERS.151 This detection limit was achieved by directly adding a specific volume of 100 μM atrazine to an AgNP colloid suspension. This result demonstrates that SERS achieved similar detection limit (ppt) to sophisticated liquid chromatography-tandem mass spectroscopy (LC-MS/MS) and outperformed it due to its facile operation and fast measurement. However, this paper did not report a detailed characterization of the SERS substrate, the Raman measurement conditions, or the reproducibility of the data. The reason why the authors were able to achieve such a low detection limit is probably the addition of high concentrations of atrazine (100 μM) that induced AgNP aggregation. More research efforts are required in this field to discuss if SERS can be used for single or few molecule detection of organic pollutants in environmentally relevant samples.
To facilitate on site pollutant detection, a portable Raman instrument integrating a SERS sensor is highly desired.152,153 Recently such an instrument containing a silver dendrite SERS substrate was developed for pesticide detection. The large laser spot of 2 mm minimizes SERS intensity variation among parallel samples. The pesticide ferbam with concentrations of 0 ppm, 4 ppm, 7 ppm, and 14 ppm was used as reference materials indicating no risk, low risk, risk, and high risk, respectively. The self checking tests for the four references all passed, indicating this instrument shows potential for on site pesticide detection.152 Combining microfluidic chips and SERS substrates in the portable Raman instrument is promising for real-time on site pollutant detection. With a micropillar array PDMS chip integrated in the instrument, complete mixing of the two confluents – AgNPs and pollutants (dipicolinic acid and malachite green) is achieved. Dipicolinic acid and malachite green were quantitatively detected with limits of detection of 200 ppb and 500 ppb, respectively.153
For larger targets, such as biomolecules, viruses, cancer cells, bacteria and protozoa, it is very difficult to directly acquire their SERS spectra by adding them to SERS substrates because they are too big to fit into the hot spots due to their large size.155,156 Instead, a SERS tag is used to specifically bind the targets and the SERS spectrum of a Raman reporter functionalized on the SERS tag is then monitored.93,157 Raman reporter is usually a dye having a large Raman cross section. Ideal SERS tags are able to generate strong enough signals for single target detection. Interested readers are referred to a very good review for additional details on SERS tags.31 Yang et al. fabricated a nanopillar-based SERS substrate to detect the macromolecule vasopressin, which was labeled by a Raman reporter 5-carboxytetramethylrhodamine. The nanopillar is made by depositing gold vapor onto etched silicon wafer. The coated gold film on the tip of silicon wire formed a pillar, which was functionalized with a vasopressin-specific aptamer. After exposure to vasopressin and subsequent drying, the intensified SERS signal of TAMRA was acquired due to the capillary force-driven aggregation of the nanopillars. The detection limit of vasopressin was reported to be 1 pM.119 Recently, graphene oxide (GO) was used for SERS tag fabrication because of its capacity to significantly enhance the SERS signal.154,158 The schematic of this SERS tag synthesis is shown in Fig. 8. Different from the traditional SERS tag fabrication, the Raman reporter – tris(2,2′-bipyridyl)ruthenium(II) chloride (Rubpy) was first adsorbed on GO and subsequently AuNPs formed by in situ reduction of HAuCl4 on GO/Rubpy. GO was able to not only enhance the SERS signal by two fold but also improve the colloid stability by wrapping around the small nanoparticle aggregates. AuNP/GO/Rubpy was subsequently functionalized with positively charged poly(allylamine hydrochloride) (PAH), which provided amine groups to link to the recognition element glutaraldehyde (GA). GA can bind to both gram-positive and gram-negative bacteria by crosslinking with the peptidoglycan layer on their surfaces. In addition to its single cell identification capability, this SERS tag can also be used for photothermal ablation of bacteria when exposed to a 400 mW 785 nm laser. The decrease in the SERS signal can be used to monitor the bacterial ablation process (Fig. 8).154
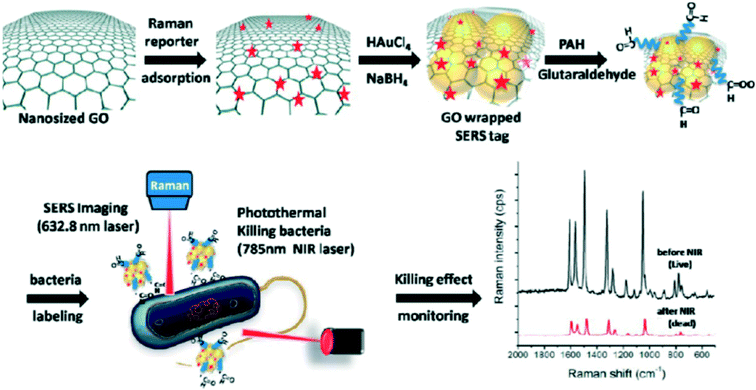 | ||
| Fig. 8 Schematic for AuNP/GO/Rubpy/GA SERS tag synthesis and its application for monitoring the photothermal ablation of bacteria.154 Reprinted with permission from D. Lin, T. Qin, Y. Wang, X. Sun and L. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 1320–1329. Copyright 2014 American Chemical Society. | ||
Although detecting large targets using SERS tags can achieve very high sensitivity, it is complex and costly to fabricate these tags. Most recently, the SERS spectrum of virus on SERS substrates without a SERS tag has been reported. This is called label-free SERS detection of virus.159 Progress made in this promising area of research was recently summarized elsewhere.159 Briefly, a highly sensitive and reproducible SERS substrate was fabricated by oblique angle deposition. The obtained SERS substrate contains tilted silver nanowire arrays. Virus was directly added to the SERS substrate and its SERS spectrum was readily acquired. Using this technique, three viruses – adenovirus, rhinovirus, and HIV were distinguished and even different strains of respiratory syncytial virus (RSV) could be differentiated. This approach was also applied to measure the SERS spectrum of RSV in its infected cell lysate although the background interference is strong. These results indicate that label-free detection of virus is feasible if SERS substrates are well designed. However, the weak signal, strong background disturbance, and subtle change of spectrum between different viruses make the data analysis challenging. Principle component analysis (PCA) and other chemometic approaches are often required to differentiate the viruses from the background and from one another.
5. Challenges
Although the rapid development of nanotechnology has facilitated substantial progress towards improved colorimetric and SERS detection, the high costs of sensor fabrication still impede their practical environmental applications. Development of low-cost and scalable detection platforms remains a big challenge. It is thus desirable to incorporate detection components within paper or other sustainable materials without using costly lithography techniques. Paper-based colorimetric sensors can be used at home to monitor drinking water quality by simply dipping test strips into water. However, the sensitivity and resistance of these test strips to potential interferents such as drinking water disinfectants should be improved to make such a sensor truly useful. SERS sensors have the capacity to replace the complex lab assays currently used in water and wastewater treatment plants because of their simple sample preparation and rapid detection process. Suspension-based sensors may not be appropriate for use in real water samples since the colloids may not be stable in complex water chemistries and the challenges associated with long-term storage. As noted, paper-based SERS substrates have potential application. However, their SERS hot spot densities and affinities for specific organic pollutants currently do not meet real world application requirements. It is a considerable challenge to develop universal SERS substrates that have broad applicability to all of the organic chemicals of interest because the size, polarity, and isoelectric point of the chemicals determine their capacity to enter the hot spots on the SERS substrate. For on-site detection, portable SERS instrumentation is required and those systems currently rely only on near infrared lasers because of their ease of miniaturization. Accordingly, the SERS substrate must be optimized for application with near infrared lasers. Unfortunately, most organic pollutants are non-resonant at this laser wavelength, which makes their detection more challenging. Moreover, if we want to achieve real-time detection, the laser integration time must be very short, which further increases the difficulty. In addition to organic pollutant detection, SERS sensors also show potential for label-free pathogen detection. Since pathogens are generally too large to readily enter hot spots, the SERS substrate must have extremely high enhancement factor to make the pathogen spectrum visible. The reproducibility of SERS pathogen detection is also challenged because the contact between pathogens and Au or AgNPs may vary with time. The steps required for development of low-cost and efficient SERS substrates for pathogen detection are an ongoing area of research focus.Acknowledgements
Funding for this study was provided by the US National Science Foundation (NSF; CBET 1236005 and 1133736) and the Virginia Tech Institute for Critical Technology and Applied Science. Support for HW was provided by the Virginia Tech Graduate School through the Sustainable Nanotechnology Interdisciplinary Graduate Education Program (VTSuN IGEP). Additional funding was provided by NSF and the Environmental Protection Agency under NSF Cooperative Agreement EF-0830093, Center for the Environmental Implications of NanoTechnology (CEINT). Any opinions, findings, conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NSF or the EPA. This work has not been subjected to EPA review and no official endorsement should be inferred.References
- J. Manuel, Environ. Health Perspect., 2014, 122, A214–A219 CrossRef PubMed
.
-
C. Fitzpatrick, Water21 - Magazine of The International Water Association, 2014 Search PubMed
.
- W. R. Mac Kenzie, N. J. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fox and J. B. Rose, N. Engl. J. Med., 1994, 331, 161–167 CrossRef CAS PubMed
.
- M. Widerström, C. Schönning, M. Lilja, M. Lebbad, T. Ljung, G. Allestam, M. Ferm, B. Björkholm, A. Hansen and J. Hiltula, Emerging Infect. Dis., 2014, 20, 581–589 CrossRef PubMed
.
- L. B. van Alphen, F. Dorléans, A. C. Schultz, J. Fonager, S. Ethelberg, C. Dalgaard, M. Adelhardt, J. H. Engberg, T. K. Fischer and S. G. Lassen, PLoS One, 2014, 9, e105053 Search PubMed
.
- A. Abbott and H. Pearson, Nature, 2004, 427, 472–473 CrossRef CAS PubMed
.
- F. Karasek and O. Hutzinger, Anal. Chem., 1986, 58, 633A–642A CrossRef CAS PubMed
.
- A. J. Haes, C. L. Haynes, A. D. McFarland, G. C. Schatz, R. P. Van Duyne and S. Zou, MRS Bull., 2005, 30, 368–375 CrossRef CAS
.
- A. J. Haes and R. P. Van Duyne, Anal. Bioanal. Chem., 2004, 379, 920–930 CrossRef CAS PubMed
.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410–8426 CrossRef CAS
.
- B. Sepúlveda, P. C. Angelomé, L. M. Lechuga and L. M. Liz-Marzán, Nano Today, 2009, 4, 244–251 CrossRef
.
- J. Mock, M. Barbic, D. Smith, D. Schultz and S. Schultz, J. Chem. Phys., 2002, 116, 6755–6759 CrossRef CAS
.
- C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2001, 105, 5599–5611 CrossRef CAS
.
- W. L. Daniel, M. S. Han, J.-S. Lee and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 6362–6363 CrossRef CAS PubMed
.
- F. Xia, X. Zuo, R. Yang, Y. Xiao, D. Kang, A. Vallée-Bélisle, X. Gong, J. D. Yuen, B. B. Hsu and A. J. Heeger, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10837–10841 CrossRef CAS PubMed
.
- J. Du, B. Zhu and X. Chen, Small, 2013, 9, 4104–4111 CrossRef CAS PubMed
.
-
G. C. Schatz, M. A. Young and R. P. Van Duyne, Surface-enhanced Raman Scattering, Springer, 2006, pp. 19–45 Search PubMed
.
- C. L. Haynes, A. D. McFarland and R. P. V. Duyne, Anal. Chem., 2005, 77, 338 A–346 A CrossRef CAS
.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed
.
- H. Liu, L. Zhang, X. Lang, Y. Yamaguchi, H. Iwasaki, Y. Inouye, Q. Xue and M. Chen, Sci. Rep., 2011, 1, 1–5 Search PubMed
.
- J. Kneipp, H. Kneipp and K. Kneipp, Chem. Soc. Rev., 2008, 37, 1052–1060 RSC
.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R.
R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667 CrossRef CAS
.
- E. Podstawka, Y. Ozaki and L. M. Proniewicz, Appl. Spectrosc., 2005, 59, 1516–1526 CrossRef CAS PubMed
.
- J. C. Costa, R. A. Ando, P. H. Camargo and P. Corio, J. Phys. Chem. C, 2011, 115, 4184–4190 CAS
.
- N. A. Abu Hatab, J. M. Oran and M. J. Sepaniak, ACS Nano, 2008, 2, 377–385 CrossRef CAS PubMed
.
- R. Tan, A. Agarwal, N. Balasubramanian, D. Kwong, Y. Jiang, E. Widjaja and M. Garland, Sens. Actuators, A, 2007, 139, 36–41 CrossRef CAS
.
- W. J. Cho, Y. Kim and J. K. Kim, ACS Nano, 2011, 6, 249–255 CrossRef PubMed
.
- A. Campion and P. Kambhampati, Chem. Soc. Rev., 1998, 27, 241–250 RSC
.
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, Chem. Rev., 1999, 99, 2957–2976 CrossRef CAS PubMed
.
- M. D. Porter, R. J. Lipert, L. M. Siperko, G. Wang and R. Narayanan, Chem. Soc. Rev., 2008, 37, 1001–1011 RSC
.
- Y. Wang, B. Yan and L. Chen, Chem. Rev., 2012, 113, 1391–1428 CrossRef PubMed
.
- M. Vendrell, K. K. Maiti, K. Dhaliwal and Y. T. Chang, Trends Biotechnol., 2013, 31, 249–257 CrossRef CAS PubMed
.
- S. C. Luo, K. Sivashanmugan, J. D. Liao, C. K. Yao and H. C. Peng, Biosens. Bioelectron., 2014, 61, 232–240 CrossRef CAS PubMed
.
- R. A. Halvorson and P. J. Vikesland, Environ. Sci. Technol., 2010, 44, 7749–7755 CrossRef CAS PubMed
.
- B. Sharma, R. R. Frontiera, A. I. Henry, E. Ringe and R. P. Van Duyne, Mater. Today, 2012, 15, 16–25 CrossRef CAS
.
- S. Schlücker, Angew. Chem., Int. Ed., 2014, 53, 4756–4795 CrossRef PubMed
.
- D. Liu, Z. Wang and X. Jiang, Nanoscale, 2011, 3, 1421–1433 RSC
.
- W. Zhao, M. A. Brook and Y. Li, ChemBioChem, 2008, 9, 2363–2371 CrossRef CAS PubMed
.
- D. Vilela, M. C. González and A. Escarpa, Anal. Chim. Acta, 2012, 751, 24–43 CrossRef CAS PubMed
.
- S. Su, W. Wu, J. Gao, J. Lu and C. Fan, J. Mater. Chem., 2012, 22, 18101–18110 RSC
.
- R. Alvarez-Puebla and L. Liz-Marzan, Energy Environ. Sci., 2010, 3, 1011–1017 CAS
.
- T. Masciangioli and W. X. Zhang, Environ. Sci. Technol., 2003, 37, 102A–108A CrossRef CAS PubMed
.
- C. Wang and C. Yu, Rev. Anal. Chem., 2013, 32, 1–14 CrossRef
.
- L. Wang, W. Ma, L. Xu, W. Chen, Y. Zhu, C. Xu and N. A. Kotov, Mater. Sci. Eng., R, 2010, 70, 265–274 CrossRef
.
- P. J. Vikesland and K. R. Wigginton, Environ. Sci. Technol., 2010, 44, 3656–3669 CrossRef CAS PubMed
.
- S. Szunerits and R. Boukherroub, Chem. Commun., 2012, 48, 8999–9010 RSC
.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442–453 CrossRef CAS PubMed
.
- J. Homola, Chem. Rev., 2008, 108, 462–493 CrossRef CAS PubMed
.
- R. A. Alvarez-Puebla and L. M. Liz-Marzán, Angew. Chem., Int. Ed., 2012, 51, 11214–11223 CrossRef CAS PubMed
.
- Y. Sun and Y. Xia, Analyst, 2003, 128, 686–691 RSC
.
- P. Mulvaney, Langmuir, 1996, 12, 788–800 CrossRef CAS
.
- A. Zielińska, E. Skwarek, A. Zaleska, M. Gazda and J. Hupka, Procedia Chem., 2009, 1, 1560–1566 CrossRef
.
- S. Link and M. A. El-Sayed, Annu. Rev. Phys. Chem., 2003, 54, 331–366 CrossRef CAS PubMed
.
- C. J. Orendorff, T. K. Sau and C. J. Murphy, Small, 2006, 2, 636–639 CrossRef CAS PubMed
.
- C. L. Nehl and J. H. Hafner, J. Mater. Chem., 2008, 18, 2415–2419 RSC
.
- L. Zhong, X. Zhou, S. Bao, Y. Shi, Y. Wang, S. Hong, Y. Huang, X. Wang, Z. Xie and Q. Zhang, J. Mater. Chem., 2011, 21, 14448–14455 RSC
.
- I. Blakey, Z. Merican and K. J. Thurecht, Langmuir, 2013, 29, 8266–8274 CrossRef CAS PubMed
.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed
.
- J. J. Storhoff, R. Elghanian, R. C. Mucic, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 1998, 120, 1959–1964 CrossRef CAS
.
- C. C. Huang and H. T. Chang, Chem. Commun., 2007, 1215–1217 RSC
.
- J. Liu and Y. Lu, Angew. Chem., 2006, 118, 96–100 CrossRef
.
- I. Mrozek and A. Otto, J. Electron Spectrosc. Relat. Phenom., 1990, 54, 895–911 CrossRef
.
- G. Compagnini, C. Galati and S. Pignataro, Phys. Chem. Chem. Phys., 1999, 1, 2351–2353 RSC
.
- K. H. Su, Q. H. Wei, X. Zhang, J. Mock, D. R. Smith and S. Schultz, Nano Lett., 2003, 3, 1087–1090 CrossRef CAS
.
- X. Qian, X. Zhou and S. Nie, J. Am. Chem. Soc., 2008, 130, 14934–14935 CrossRef PubMed
.
- L. Qin, S. Zou, C. Xue, A. Atkinson, G. C. Schatz and C. A. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13300–13303 CrossRef CAS PubMed
.
- H. Im, K. C. Bantz, N. C. Lindquist, C. L. Haynes and S. H. Oh, Nano Lett., 2010, 10, 2231–2236 CrossRef CAS PubMed
.
- H. Wang, C. S. Levin and N. J. Halas, J. Am. Chem. Soc., 2005, 127, 14992–14993 CrossRef CAS PubMed
.
- K. L. Wustholz, A.-I. Henry, J. M. McMahon, R. G. Freeman, N. Valley, M. E. Piotti, M. J. Natan, G. C. Schatz and R. P. V. Duyne, J. Am. Chem. Soc., 2010, 132, 10903–10910 CrossRef CAS PubMed
.
- L. Fabris, A. S. D. Indrasekara, S. Meyers, S. Shubeita, L. C. Feldman and T. Gustafsson, Nanoscale, 2014 Search PubMed
.
- M. Potara, A. M. Gabudean and S. Astilean, J. Mater. Chem., 2011, 21, 3625–3633 RSC
.
- A. McLintock, C. A. Cunha-Matos, M. Zagnoni, O. R. Millington and A. W. Wark, ACS Nano, 2014, 8, 8600–8609 CrossRef CAS PubMed
.
- G. Braun, I. Pavel, A. R. Morrill, D. S. Seferos, G. C. Bazan, N. O. Reich and M. Moskovits, J. Am. Chem. Soc., 2007, 129, 7760–7761 CrossRef CAS PubMed
.
- S. L. Kleinman, R. R. Frontiera, A.-I. Henry, J. A. Dieringer and R. P. Van Duyne, Phys. Chem. Chem. Phys., 2013, 15, 21–36 RSC
.
- A. Shiohara, Y. Wang and L. M. Liz-Marzán, J. Photochem. Photobiol., C, 2014, 21, 2–25 CrossRef CAS
.
- N. H. Kim, S. J. Lee and M. Moskovits, Adv. Mater., 2011, 23, 4152–4156 CrossRef CAS PubMed
.
- R. A. Alvarez-Puebla, E. Arceo, P. J. Goulet, J. J. Garrido and R. F. Aroca, J. Phys. Chem. B, 2005, 109, 3787–3792 CrossRef CAS PubMed
.
- L. S. Lawson, J. W. Chan and T. Huser, Nanoscale, 2014, 6, 7971–7980 RSC
.
- K. V. Kong, U. Dinish, W. K. O. Lau and M. Olivo, Biosens. Bioelectron., 2014, 54, 135–140 CrossRef CAS PubMed
.
- W. Ji, N. Spegazzini, Y. Kitahama, Y. Chen, B. Zhao and Y. Ozaki, J. Phys. Chem. Lett., 2012, 3, 3204–3209 CrossRef CAS PubMed
.
- W. Hill and B. Wehling, J. Phys. Chem., 1993, 97, 9451–9455 CrossRef CAS
.
- R. Alvarez-Puebla, B. Cui, J. P. Bravo-Vasquez, T. Veres and H. Fenniri, J. Phys. Chem. C, 2007, 111, 6720–6723 CAS
.
- U. K. Sarkar, Chem. Phys. Lett., 2003, 374, 341–347 CrossRef CAS
.
- H. Yuan, A. M. Fales, C. G. Khoury, J. Liu and T. Vo-Dinh, J. Raman Spectrosc., 2013, 44, 234–239 CrossRef CAS PubMed
.
- D. K. Lim, I. J. Kim and J. M. Nam, Chem. Commun., 2008, 5312–5314 RSC
.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS PubMed
.
- L. J. Sherry, R. Jin, C. A. Mirkin, G. C. Schatz and R. P. Van Duyne, Nano Lett., 2006, 6, 2060–2065 CrossRef CAS PubMed
.
- J. E. Millstone, S. Park, K. L. Shuford, L. Qin, G. C. Schatz and C. A. Mirkin, J. Am. Chem. Soc., 2005, 127, 5312–5313 CrossRef CAS PubMed
.
- L. Rodríguez-Lorenzo, R. A. Alvarez-Puebla, I. Pastoriza-Santos, S. Mazzucco, O. Stéphan, M. Kociak, L. M. Liz-Marzán and F. J. García de Abajo, J. Am. Chem. Soc., 2009, 131, 4616–4618 CrossRef PubMed
.
- C. J. Orendorff, L. Gearheart, N. R. Jana and C. J. Murphy, Phys. Chem. Chem. Phys., 2006, 8, 165–170 RSC
.
- S. E. Bell and M. R. McCourt, Phys. Chem. Chem. Phys., 2009, 11, 7455–7462 RSC
.
- T. Iliescu, M. Baia and W. Kiefer, Chem. Phys., 2004, 298, 167–174 CrossRef CAS
.
- H. Y. Lin, C. H. Huang, W. H. Hsieh, L. H. Liu, Y. C. Lin, C. C. Chu, S. T. Wang, I. Kuo, L. K. Chau and C. Y. Yang, Small, 2014, 10, 4700–4710 CrossRef CAS PubMed
.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed
.
- R. A. Reynolds, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 2000, 122, 3795–3796 CrossRef CAS
.
- D. G. Thompson, A. Enright, K. Faulds, W. E. Smith and D. Graham, Anal. Chem., 2008, 80, 2805–2810 CrossRef CAS PubMed
.
- L. Liu, S. Li, L. Liu, D. Deng and N. Xia, Analyst, 2012, 137, 3794–3799 RSC
.
- D. E. Huizenga and J. W. Szostak, Biochemistry, 1995, 34, 656–665 CrossRef CAS PubMed
.
- J. Liu and Y. Lu, Nat. Protoc., 2006, 1, 246–252 CrossRef CAS PubMed
.
- Z. Zhu, C. Wu, H. Liu, Y. Zou, X. Zhang, H. Kang, C. J. Yang and W. Tan, Angew. Chem., 2010, 122, 1070–1074 CrossRef
.
- A. Sugunan, C. Thanachayanont, J. Dutta and J. Hilborn, Sci. Technol. Adv. Mater., 2005, 6, 335–340 CrossRef CAS
.
- J. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642–6643 CrossRef CAS PubMed
.
- C. Y. Lin, C. J. Yu, Y. H. Lin and W. L. Tseng, Anal. Chem., 2010, 82, 6830–6837 CrossRef CAS PubMed
.
- A. E. Üzer, Z. Can, I. l. Akın, E. Erçağ and R. A. Apak, Anal. Chem., 2013, 86, 351–356 CrossRef PubMed
.
- F. M. Li, J. M. Liu, X. X. Wang, L. P. Lin, W. L. Cai, X. Lin, Y. N. Zeng, Z. M. Li and S. Q. Lin, Sens. Actuators, B, 2011, 155, 817–822 CrossRef CAS
.
- Z. Zhang, Z. Chen, C. Qu and L. Chen, Langmuir, 2014, 30, 3625–3630 CrossRef CAS PubMed
.
- G. Wang, Z. Chen, W. Wang, B. Yan and L. Chen, Analyst, 2011, 136, 174–178 RSC
.
- W. Zhao, M. M. Ali, S. D. Aguirre, M. A. Brook and Y. Li, Anal. Chem., 2008, 80, 8431–8437 CrossRef CAS PubMed
.
- S. C. Tseng, C. C. Yu, D. Wan, H. L. Chen, L. A. Wang, M. C. Wu, W. F. Su, H. C. Han and L. C. Chen, Anal. Chem., 2012, 84, 5140–5145 CrossRef CAS PubMed
.
- D. Mazumdar, J. Liu, G. Lu, J. Zhou and Y. Lu, Chem. Commun., 2010, 46, 1416–1418 RSC
.
- N. Ratnarathorn, O. Chailapakul, C. S. Henry and W. Dungchai, Talanta, 2012, 99, 552–557 CrossRef CAS PubMed
.
- M. Fleischmann, P. Hendra and A. McQuillan, Chem. Phys. Lett., 1974, 26, 163–166 CrossRef CAS
.
- T. Vo-Dinh, M. Hiromoto, G. Begun and R. Moody, Anal. Chem., 1984, 56, 1667–1670 CrossRef CAS
.
- M. M. Carrabba, R. B. Edmonds and R. D. Rauh, Anal. Chem., 1987, 59, 2559–2563 CrossRef CAS PubMed
.
- R. W. Taylor, R. J. Coulston, F. Biedermann, S. Mahajan, J. J. Baumberg and O. A. Scherman, Nano Lett., 2013, 13, 5985–5990 CrossRef CAS PubMed
.
- D. K. Lim, K. S. Jeon, J. H. Hwang, H. Kim, S. Kwon, Y. D. Suh and J. M. Nam, Nat. Nanotechnol., 2011, 6, 452–460 CrossRef CAS PubMed
.
- B. Saute, R. Premasiri, L. Ziegler and R. Narayanan, Analyst, 2012, 137, 5082–5087 RSC
.
- D. K. Lim, K. S. Jeon, H. M. Kim, J. M. Nam and Y. D. Suh, Nat. Mater., 2009, 9, 60–67 CrossRef PubMed
.
- J. Yang, M. Palla, F. G. Bosco, T. Rindzevicius, T. S. Alstrøm, M. S. Schmidt, A. Boisen, J. Ju and Q. Lin, ACS Nano, 2013, 7, 5350–5359 CrossRef CAS PubMed
.
- W. Leng and P. J. Vikesland, Langmuir, 2014, 30, 8342–8349 CrossRef CAS PubMed
.
- S. Basu, S. Pande, S. Jana, S. Bolisetty and T. Pal, Langmuir, 2008, 24, 5562–5568 CrossRef CAS PubMed
.
- R. W. Taylor, T. C. Lee, O. A. Scherman, R. Esteban, J. Aizpurua, F. M. Huang, J. J. Baumberg and S. Mahajan, ACS Nano, 2011, 5, 3878–3887 CrossRef CAS PubMed
.
- L. Zhang, X. Lang, A. Hirata and M. Chen, ACS Nano, 2011, 5, 4407–4413 CrossRef CAS PubMed
.
- A. Tao, F. Kim, C. Hess, J. Goldberger, R. He, Y. Sun, Y. Xia and P. Yang, Nano Lett., 2003, 3, 1229–1233 CrossRef CAS
.
- T. H. Reilly, J. D. Corbman and K. L. Rowlen, Anal. Chem., 2007, 79, 5078–5081 CrossRef CAS PubMed
.
- Y. Jin, Adv. Mater., 2012, 24, 5153–5165 CrossRef CAS PubMed
.
- P. Wang, O. Liang, W. Zhang, T. Schroeder and Y. H. Xie, Adv. Mater., 2013, 25, 4918–4924 CrossRef CAS PubMed
.
- R. Zhang, B. B. Xu, X. Q. Liu, Y. L. Zhang, Y. Xu, Q. D. Chen and H. B. Sun, Chem. Commun., 2012, 48, 5913–5915 RSC
.
- C. H. Lee, L. Tian and S. Singamaneni, ACS Appl. Mater. Interfaces, 2010, 2, 3429–3435 CAS
.
- W. Zhang, B. Li, L. Chen, Y. Wang, D. Gao, X. Ma and A. Wu, Anal. Methods, 2014, 6, 2066–2071 RSC
.
- Y. H. Ngo, D. Li, G. P. Simon and G. Garnier, Langmuir, 2012, 28, 8782–8790 CrossRef CAS PubMed
.
- L. Polavarapu and L. M. Liz-Marzán, Phys. Chem. Chem. Phys., 2013, 15, 5288–5300 RSC
.
- A. Abbas, A. Brimer, J. M. Slocik, L. Tian, R. R. Naik and S. Singamaneni, Anal. Chem., 2013, 85, 3977–3983 CrossRef CAS PubMed
.
- L. Zhang, X. Gong, Y. Bao, Y. Zhao, M. Xi, C. Jiang and H. Fong, Langmuir, 2012, 28, 14433–14440 CrossRef CAS PubMed
.
- D. He, B. Hu, Q. F. Yao, K. Wang and S. H. Yu, ACS Nano, 2009, 3, 3993–4002 CrossRef CAS PubMed
.
- H. Ko, S. Chang and V. V. Tsukruk, ACS Nano, 2008, 3, 181–188 CrossRef PubMed
.
- P. Aldeanueva-Potel, E. Faoucher, R. N. A. Alvarez-Puebla, L. M. Liz-Marzán and M. Brust, Anal. Chem., 2009, 81, 9233–9238 CrossRef CAS PubMed
.
- X. Zhou, F. Zhou, H. Liu, L. Yang and J. Liu, Analyst, 2013, 138, 5832–5838 RSC
.
- B. Saute and R. Narayanan, Analyst, 2011, 136, 527–532 RSC
.
- S. Abalde-Cela, S. Ho, B. Rodríguez-González, M. A. Correa-Duarte, R. A. Álvarez-Puebla, L. M. Liz-Marzán and N. A. Kotov, Angew. Chem., 2009, 121, 5430–5433 CrossRef
.
- S. S. Dasary, A. K. Singh, D. Senapati, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2009, 131, 13806–13812 CrossRef CAS PubMed
.
- L. Guerrini, J. V. Garcia-Ramos, C. Domingo and S. Sanchez-Cortes, Anal. Chem., 2009, 81, 953–960 CrossRef CAS PubMed
.
- R. A. Álvarez-Puebla, R. Contreras-Cáceres, I. Pastoriza-Santos, J. Pérez-Juste and L. M. Liz-Marzán, Angew. Chem., Int. Ed., 2009, 48, 138–143 CrossRef PubMed
.
- L. Guerrini, J. V. Garcia-Ramos, C. Domingo and S. Sanchez-Cortes, Anal. Chem., 2009, 81, 1418–1425 CrossRef CAS PubMed
.
- P. Leyton, C. Domingo, S. Sanchez-Cortes, M. Campos-Vallette and J. Garcia-Ramos, Langmuir, 2005, 21, 11814–11820 CrossRef CAS PubMed
.
- L. Guerrini, J. V. Garcia-Ramos, C. Domingo and S. Sanchez-Cortes, Langmuir, 2006, 22, 10924–10926 CrossRef CAS PubMed
.
- S. Z. Nergiz, N. Gandra, M. E. Farrell, L. Tian, P. M. Pellegrino and S. Singamaneni, J. Mater. Chem. A, 2013, 1, 6543–6549 CAS
.
- A. Kolomijeca, Y. Kwon, K. Sowoidnich, R. Prien, D. Schulz-Bull and H. Kronfeldt, Proc. 21th, 2011, 859–862 Search PubMed.
- H. Schmidt, N. Bich Ha, J. Pfannkuche, H. Amann, H.-D. Kronfeldt and G. Kowalewska, Mar. Pollut. Bull., 2004, 49, 229–234 CrossRef CAS PubMed
.
- J. Pfannkuche, L. Lubecki, H. Schmidt, G. Kowalewska and H.-D. Kronfeldt, Mar. Pollut. Bull., 2012, 64, 614–626 CrossRef CAS PubMed
.
- R. J. Rubira, S. A. Camacho, P. H. Aoki, M. D. Maximino, P. Alessio, C. S. Martin, O. N. Oliveira Jr, F. M. Fatore, F. V. Paulovich and C. J. Constantino, Colloid Polym. Sci., 2014, 1–10 Search PubMed
.
- J. Zheng, S. Pang, T. P. Labuza and L. He, Analyst, 2013, 138, 7075–7078 RSC
.
- L. XuanáQuang, G. HunáSeong and K. JunáDo, Lab Chip, 2008, 8, 2214–2219 RSC
.
- D. Lin, T. Qin, Y. Wang, X. Sun and L. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 1320–1329 CAS
.
- K. L. Rule and P. J. Vikesland, Environ. Sci. Technol., 2009, 43, 1147–1152 CrossRef CAS PubMed
.
- K. K. Maiti, U. Dinish, C. Y. Fu, J. J. Lee, K. S. Soh, S. W. Yun, R. Bhuvaneswari, M. Olivo and Y. T. Chang, Biosens. Bioelectron., 2010, 26, 398–403 CrossRef CAS PubMed
.
- B. Guven, N. Basaran-Akgul, E. Temur, U. Tamer and İ. H. Boyacı, Analyst, 2011, 136, 740–748 RSC
.
- S. Deng, W. Xu, J. Wang, X. Ling, J. Wu, L. Xie, J. Kong, M. S. Dresselhaus and J. Zhang, Nano Res., 2014, 7, 1271–1279 CrossRef CAS
.
- R. A. Tripp, R. A. Dluhy and Y. Zhao, Nano Today, 2008, 3, 31–37 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |