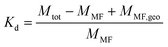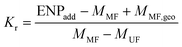Fate descriptors for engineered nanoparticles: the good, the bad, and the ugly
Geert
Cornelis
University of Gothenburg, Department of Chemistry and Molecular Biology, Kemivägen 10, 41296 Göteborg, Sweden. E-mail: geert.cornelis@chem.gu.se
First published on 29th September 2014
Abstract
Developments in hazard identification of engineered nanoparticles (ENP) have not been met with proper fate descriptors to calculate travel distances and the bioavailable concentration of ENP. Three possible fate descriptors for ENP in soils are compared – batch partitioning coefficients (Kd values), batch retention coefficients (Kr values) and column attachment efficiency – in view of both technical and practical aspects of environmental risk assessments of ENP. Kd values are deemed not appropriate fate descriptors for ENP because the equilibrium assumption is not valid. The kinetic interpretation of batch studies offered by Kr values bears a link to relevant ENP processes in the environment, but interpretation may be confounded by the conditions of high shear during batch tests complicating direct use in transport or bioavailability calculations. Column experiments are, to some extent, also operationally defined and require a more experimentally dedicated approach that does not necessarily lead to a widely carrying physical parameter. Future efforts should therefore be investigated in development of tests that strike a better balance between operational simplicity and technical accuracy.
Nano impactDevelopments of risk assessment of engineered nanoparticles (ENP) have one-sidedly occurred in hazard assessments, whereas exposure assessments are lagging behind. Quite some discussion on what proper fate descriptors should be used for environmental ENP risk assessment has therefore occurred in the regulatory arena, e.g. in OECD expert workshops. The current perspective nurtures this discussion by comparing and evaluating three ENP fate descriptors that have been suggested in the literature and in workshops. While technical arguments favor column tests, practical ones favor batch test, because any successful fate descriptor should not require many new parameters to be determined. This perspective aims to balance these two perspectives, to further possible research efforts towards fate descriptors balanced in accuracy and practical use. |
Introduction
In the past decade, knowledge and insights into the environmental hazard of engineered nanoparticles (ENPs) have markedly increased and the field has moved well beyond the reconnaissance stage, warranting research in mechanisms that lie at the basis of ENP ecotoxicity. What has been investigated much less is how interactions between ENP and the specific environmental medium affect bioavailability, transport and thus the eventual risk.1,2 One reason for this slower development may be the absence of generally agreed fate indicators for ENPs, being a preferably small set of parameter values that can be used to calculate travel distances within a particular environmental compartment and/or the bioavailable fraction of the total ENP concentration. It has become clear that the magnitude of different toxic endpoints,3–8 the travel distances of ENP in different soil types1,9,10 or sedimentation speed in different water types11,12 vary greatly with the chemical and/or physical properties of the receiving environmental compartment. Currently available hazard data of ENP and mass flux calculations are, however, based on a total mass concentration basis across all water, soil or sediment types, because there are currently no agreed fate indicators or model approaches to calculate mobile and/or bioavailable ENP concentrations, a situation that may lead to very high uncertainty. For instance, metallic silver ENPs are found to be both more hazardous and less hazardous based on total concentrations compared to their dissolved counterparts in different studies.5,13 Using a fate descriptor to estimate the bioavailable ENP fraction from the total ENP concentration could lead to more consistent trends. Likewise, basing ENP travel distances in, e.g., freshwater systems only on the concentration of ENP leads to serious overestimation of their travel distances and it is clear that interaction with the environmental matrix needs to be quantified for a more correct assessment.14 Current model approaches for ENPs are, however, very numerous and not always comparable, hampering the development of standardized tests or large data sets that could serve to discover trends in ENP behavior as a function of characteristics of the natural environment.The current perspective compares three fate indicators of ENP that have been put forward in the literature: Kd values, Kr values, both obtained in batch tests, and attachment efficiency (αatt) obtained in column tests. Praetorius et al.15 have undertaken a rigorous thermodynamic assessment in this issue and concluded that fate descriptors based on batch test are not to be used on the basis of the inapplicability of the equilibrium concept to ENP, while column tests would provide a more accurate means to assess the fate of ENPs. To the author's view, their thorough thermodynamic analysis could be broadened to better take practical arguments into account as well as a more rigorous analysis of the environmental realism of both batch and column tests. Development of appropriate fate indicators should not only build on technical arguments. The most appropriate method is most likely a compromise between technical accuracy and operational simplicity as has been the case for methods to calculate bioavailability and transport of molecular chemicals (i.e. chemicals occurring as individual molecules in the environment rather than as ENPs). Moreover, where mechanistic approaches potentially provide the most accurate results, the data requirements are also high and often intractable in highly heterogeneous systems such as natural soils or rivers so that no real increase in accuracy is effectively gained despite high costs and efforts.
Deposition and bioavailability of ENP
In the case of soils, the mobile fraction is mostly the ENP concentration that is suspended in the soil pore water.1 Deposition is, in this respect, a key process limiting the mobile fraction of ENP in soils given the large reactive immobile surface in soils to which ENP can attach in various ways.1 The term “deposition” is often only used when particle attachment to immobile surfaces is irreversible.16 The term is used more broadly here, involving also reversible attachment.17In the case of aquatic systems, the mobile fraction may be related to the ENP concentration that does not or does only slowly settle gravimetrically, thus being able to be transported further.14 The mobile ENP concentration in aquatic systems is thus, similarly to soils, the fraction that remains suspended. Recent research indicates that interactions between ENP and the much more numerous naturally occurring particles, such as clays, determine the settling and thus transport distances of ENP in aquatic systems.18 These interactions are called heteroaggregation but are termed deposition here. Deposition is often reserved for interaction of particles with immobile pore walls, but it is, in essence, the same process as heterocoagulation,19 often reserved for the interaction between mobile, unlike particles.
ENP bioavailability has yet to be defined, but it is argued here that the mobile ENP concentration may be assumed as a good estimate of the bioavailable concentration and that deposition is a key mechanism determining bioavailability. Soil pore water is the key exposure pathway to molecular contaminants for soil organisms, even for soil-ingesting invertebrates that are in direct contact with the soil matrix in the gut.20 Soil pore water is at least also an important uptake route of ENP for many soil organisms.3 Even in the case of soil-ingesting invertebrates where direct contact with the soil matrix occurs, ENP detachment from the ingested soil is required prior to uptake. Bioavailable ENPs therefore need to be suspended and mobile to be taken up, albeit in a different chemical environment in the case of invertebrate guts. Similarly, there are numerous accounts that homoaggregation of ENP reduces their toxicity in aquatic systems, probably because the rate of many biological particle uptake processes is size dependent.1 Attachment of ENP to solid particles increases their size and thus reduces their bioavailability.21 Deposition thus reduces both mobility and bioavailability. Many other processes (e.g. coatings1) are, of course, also relevant for bioavailability and the relation between mobility and bioavailability of ENP surely has not yet been fully elucidated, but the main argument here is that fate descriptors for deposition must be the first improvement in better predicting both travel distances and bioavailability of ENP relative to using total concentrations only.
K d values of molecular chemicals
K d values quantify the ratio between the concentrations of a chemical found in the solid and liquid phases (Table 1). Kd values are an equilibrium concept and are therefore termed “equilibrium partitioning coefficients” by Praetorius et al.,15 but “partitioning coefficient” is preferred here, because Kd values are often used to describe processes that are, in essence, not in equilibrium.A thorough analysis of the concept and applicability of Kd values in a soil perspective has been done by Degryse et al.22Kd values are operationally defined, i.e. the method by which they are obtained defines the values they represent and can only be seen as a way to model/estimate a real process or property, in this case the partitioning of chemicals between solid and liquid phases. Kd values are determined after shaking a suspension of soil in a liquid (usually 1 mM KNO3 or 0.1 M CaCl2) with a certain liquid-to-solid (L/S) ratio holding a certain concentration of chemical for a certain amount of time (usually 24 h) followed by a separation, usually 0.20 μm or 0.45 μm microfiltration, and measurement of the mass of the chemical in the filtrate. Variation in any of these method parameters will result in a different Kd value for the same chemical so the Kd value cannot be claimed to be equal to a real physical value such as an equilibrium constant or attachment rate constant.23 An operational definition thus presents problems if the method is not fully standardized so that Kd values cannot, in effect, often be compared to each other.22
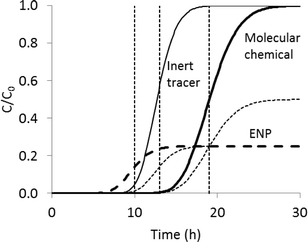
K d values are seen as a mechanistic approach to predict transport and bioavailability of chemicals in the environment, because they contrast with, e.g., empirical relations between total chemical relationships, soil properties and bioavailable concentration.24 As argued by Praetorius et al.,15Kd values are most often regarded as an estimate of true equilibrium constants. This assumption can be valid for molecular chemicals, provided that equilibrium was attained in the subjectively set time limit of the Kd determination.22Kd values can, in this case, be used in combination with the convection–dispersion equation to predict retardation during transport of molecular chemicals in soils through the so-called retardation factor (R) that expresses the ratio of the travel speed of a chemical relative to an inert tracer,22 as also schematically shown in Fig. 1:
| R = 1 + (ρb/θe)Kd | (1) |
ρ b is the soil bulk density and θe is the effective porosity. Kd values are also often combined with other mechanistic approaches such as the biotic ligand model when accurately predicting bioavailability.22
The equilibrium assumption is, however, often violated for a range of possible chemicals in soils and sediments.22,23 Many reactions of, e.g., metals in soils are slow and even irreversible, such as strong fixation of metals over time.22 Deviations between experimental and predicted transport are most often found in the case of relatively high Kd values, an observation that is explained by association with naturally occurring colloids (particles <1 μm) in soils.25 The transport of colloids occurs with entirely different mechanisms that are all essentially non-equilibrium.
Notwithstanding the many limitations of Kd values, they have been successful in providing more realistic risk assessment of many chemicals that were otherwise based on total concentrations. Determining a Kd value is, in principle, relatively straightforward and can therefore be easily standardized, resulting in precise values for a particular environmental system and a given protocol. Kd values are especially successful if the values are soil specific, if the labile fraction of the contaminant is estimated accurately (e.g. using ageing correction26 and/or using diffuse gradient thin film measurements) and if the Kd value has been calculated based on in situ concentrations.22 Because of their conceptual simplicity, Kd values have wide applicability, resulting, e.g., in relationships between Kd values and routinely measured soil properties such as texture, pH and organic matter content,22,27 relationships that allow distribution estimation in soils where a Kd measurement did not happen per se.
K d values of ENPs
Praetorius et al.15 argue that Kd values, as equilibrium concepts, cannot be used for ENPs, because deposition cannot be described as an equilibrium process. This should imply that eqn (1) cannot be used to model ENP fate in soils. The most basic and most used theory for modelling particle transport in soils, the colloid filtration theory, assumes that only irreversible attachment is relevant for particles. Fig. 1 schematically contrasts irreversible attachment, retardation and a combination of the two processes, following a step input relative to an inert (non-interacting) tracer molecule (e.g. tritiated water). If only retardation is relevant, the same concentration as the step-input feed concentration (C0) may appear at the column outflow. This behavior is often found for dissolved chemicals in soils and can be modelled purely on the basis of eqn (1). It is usually observed, however, that the concentration of ENP, like all particles, rarely reaches C0 at the outflow and arrival at the column outlet is simultaneous or even earlier (because of preferential flow1) compared to the inert tracer (e.g.ref. 28). Such a behavior owes to irreversible attachment of ENPs and cannot be modelled only based on eqn (1) that assumes that C/C0 = 1 will eventually be reached.The combination of two processes, i.e. retardation and irreversible attachment, however, occurs and is usually explained based on the existence of sites where detachment of ENPs is possible.9,29–32 Particle detachment has been studied much less compared to attachment,33 but it is a common process that explains the often high colloid concentrations found in soil pore waters34 and is necessary in the context of ENP risk assessment where the main route of exposure of soil to ENPs is through detachment from sludge applied to arable soils.35,36 The Kd value has, in this context, been seen as the approximation of the ratio between the rate constants of attachment (katt) and detachment (kdet) of ENP,29 but this is a fundamentally different definition than the conventional one for Kd values. The katt/kdet ratio is not an equilibrium constant because, as Praetorius et al.15 point out, the processes of attachment and detachment each rely on entirely different mechanisms. Moreover, a term accounting for irreversible attachment always has to be added in addition to the retardation term to the convection–dispersion equation, accounting for the loss of recovery.9,29–32 The term “pseudo-equilibrium” has been used instead to designate the steady-state colloid concentration often found in natural systems.34 Partitioning of Ag ENPs in the same natural soils was determined using a batch method37 or a column method,9 at least allowing us to investigate whether the katt/kdet ratio describing pseudo-equilibrium can be obtained in a similar manner to Kd values from batch tests based on the aforementioned batch and column tests on Ag ENP in the same set of natural soils. Kr values were calculated in the batch method study – values that are, as will be explained further, essentially obtained in the same way as Kd values.38Fig. 2a compares these values to the ratio of katt/kdet parameters that were fitted to breakthrough curves in the column study. A poor relation can be observed and Kr values are generally higher than katt/kdet ratios. Attachment rates tend to be higher during batch tests compared to column tests, because of the high shear and complex hydrodynamic conditions during batch tests.39 It can thus be concluded that the batch tests could not describe the pseudo-equilibrium that occurred in the column tests.
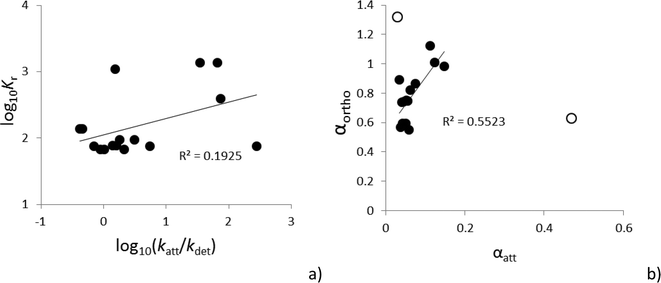 | ||
| Fig. 2 Relation between fate descriptors. a) Kr values37 compared to the ratio of fitted attachment and detachment rate constants.9 b) The orthokinetic attachment efficiency calculated from Kr values based on eqn (3) and assuming G = 100 s−1. Data points represented by open circles were not used to calculate the linear fit, and the Pearson correlation coefficients are shown. | ||
K r values
The ratio between total and aqueous ENP concentration after 24 h shaking has also been called the retention coefficient (Kr [L kg−1]) (Table 1).38 The major difference with Kd values is that no equilibrium is assumed, i.e. it is not claimed that Kr values are estimators for some equilibrium constant. The kinetics of deposition or aggregation in a batch test can be considered to discover to what physical entities Kr values can alternatively be related. A soil suspension can be conceptualized as a suspension of uniform spheres having a diameter equal to the average diameter of the soil grains (d50 [m]). Similarly, the stock ENP suspension used for spiking can be assumed insoluble for convenience and also consisting of uniform spheres having diameter dENP. Orthokinetic aggregation of ENP with soil grains will begin upon addition of ENP to the soil suspension followed by shaking, which imposes shear on both soil grains and ENPs.40 In the case of a batch system with intense shear such as during Kd or Kr determination, it can be shown that orthokinetic ENP–soil grain aggregation dominates over perikinetic, i.e. diffusion-driven, aggregation, especially if the particle size difference is large as is the case for ENPs and soil grains. The initial orthokinetic aggregation rate of ENP with soil grains can be written as40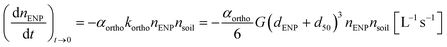 | (2) |
k ortho is the orthokinetic heteroaggregation rate constant [s−1], nENP and nsoil are the number concentrations of ENP and soil grains, respectively [L−1], G is the shear rate [s−1] and αortho is the orthokinetic collision efficiency. If α = 1, there are no repulsive barriers to be overcome and no long-range attractive forces exist. Aggregation then only depends on the shear rate, sizes and concentration of particles. The balance between repulsive barriers, e.g. similar surface charges, and attractive forces, e.g. van der Waals attraction, results in αortho ≠ 1.
Eqn (1) can only describe the very early stages of aggregation, because under conditions of high shear, large soil flocs are continuously formed and broken up. After a certain equilibration time, usually much less than 24 h,41 equilibrium average floc size deq [m] and number neq [L−1] are established, the magnitude of which depends on nsoil, G, kortho, and the particle break-up rate.42 The deq values of, e.g., clay suspensions are usually on the order of several hundreds of micrometers.41 In a realistic fate assessment, the ENP number concentration is low enough so that deposition sites on soil flocs do not become saturated, i.e. ENP can continuously aggregate with the same floc of soil granules. Moreover, the large difference between dENP and deq means that any ENP–soil grain aggregation does not significantly increase the size of soil aggregates. This means that the floc size and number is not affected by aggregation with ENP. At the same time, ENP homoaggregation is assumed unlikely during Kr determination.37,43 By assuming spherical soil granule aggregates, neq can be estimated.
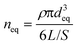 | (3) |
Moreover, dENP + deq ≈ deq in eqn (1), given that dENP ≪ deq. Kr values are ratios of mass concentrations, but number concentrations can be estimated from these in a similar way as neq was estimated from the L/S ratio (eqn (3)). Combining eqn (2) and (3), the definition of Kr values and the approximations above results in eqn (4).
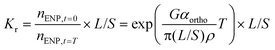 | (4) |
n ENP,t=0 and nENP,t=T [L−1] are the ENP number concentrations at the start and end (e.g. T = 24 h) of the Kr experiment. Eqn (4) shows that Kr values are dependent on the shear rate G that is currently poorly defined during most batch tests. In addition, if nENP,t=0 is increased, homoaggregation may become important, leading to higher Kr values that increase with ENP concentration.38Kr values thus suffer from a similar operational definition as do Kd values of molecular chemicals. However, if shear conditions and L/S ratio are kept constant and nENP is sufficiently low, Kr values of different ENP/soil combinations will vary with αortho, the orthokinetic collision efficiency.
Praetorius et al.15 argue that ENP fate indicators obtained from batch tests are entirely irrelevant to realistic processes in the field if starting from an equilibrium assumption. Here, it was investigated whether batch tests could be interpreted in a kinetic context, arguing that Kr values are, to some extent, related to processes such as surface potentials that determine deposition (thus also heterocoagulation). This assessment may be especially applicable if conditions are modelled where high shear predominates, e.g. wastewater treatment plants or turbulent rivers. The likelihood of deposition can thus be compared between individual ENP–soil or ENP–natural colloid combinations to some extent. However, eqn (4) assumes that αortho is independent of G, while αortho is, in fact, heavily influenced by the hydrodynamic conditions during the shaking process.39,40 It is currently unclear to what extent these hydrodynamic effects may overshadow the Kr differences between soils or natural waters that can be related to deposition efficiency, but some indications exist that Kr values may reflect relevant physicochemical variations. The relationship between Kr values and granulometric clay found, e.g., for Ag ENP37 has also been shown to exist for bioavailability.5 A similar relationship between natural colloid concentration and ENP travel distances has been concluded from the increase of ENP sedimentation rates as a function of natural colloid concentration in freshwater systems.18Fig. 2b combines αortho values calculated from Kr values for Ag ENP37 using eqn (3) assuming a relatively high G = 100 s−1 with fitted attachment efficiencies (αatt) that will be explained below and soil densities obtained during column tests with the same ENP in the same soils.9 Two outliers were found, probably owing to a poor calculation of αatt for clayey soils where breakthrough is often not detected. Omitting the two outliers from the data set leads to a significant relationship between αortho and αatt values. It is thus argued that results from batch tests can potentially be related to real properties of ENP if analyzed in a kinetic context.
ENP column deposition
Modelling of particle dynamics in porous media has been, to a large extent, built on the colloid filtration theory (CFT) (as described in ref. 40) and is also the theory of choice to model the fate of ENP in porous media.16 There are many modifications of CFT to account for all the possible interactions of particles during transport. The most basic theory conceptualizes soils as stacked uniform spheres having a diameter d50, the average diameter of soil grains. It also assumes that all successful collisions lead to irreversible attachment with a rate constant according to eqn (5).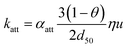 | (5) |
α att is the attachment efficiency, θ is the porosity, η is the single-collector contact efficiency and u is the pore flow velocity. η accounts for all physicochemical parameters determining the deposition efficiency under favorable conditions, i.e. in the absence of repulsive barriers. αatt quantifies the extent by which electrosteric barriers change the deposition rate relative to the favorable case.33
The environmental realism of column experiments should, however, not be overstated. Column tests can range from stacked glass beads, sand beds, and saturated stacked columns to unsaturated intact soil cores. Increases in environmental realism make the systems less well defined and thus more difficult to model, so most often relatively simple set-ups are chosen. Most column experiments study ENP transport using aqueous suspensions, of which several pore volumes are pumped through a saturated soil column. This often leads to unrealistically high ENP concentrations that deposit on soil grains, whereas in a realistic situation, low ENP concentrations first have to detach from, e.g., wastewater treatment plant sludge to be transported.35 Moreover, field soils are nearly always unsaturated, which lead to entirely different particle transport trends compared to, e.g., saturated soil columns.1
Even within the limits of a relatively simple column set-up, it is difficult to deduce an unambiguous fate descriptor. Praetorius et al.15 argue that αatt is currently the most appropriate fate indicator, while also partly acknowledging the implementation difficulties. From a practical viewpoint, eqn (5) requires many parameters that are not routinely measured for soils or for which there are no robust pedotransfer functions available. Some efforts have been invested to predict αatt based on first principles,44 but many other simultaneously occurring processes such as straining, pore wall blockage, steric repulsion, and preferential flow complicate this assessment.45–47αatt is therefore, in essence, an empirical parameter that is nearly always fitted to column outflow data using eqn (5). η can be calculated using empirical correlation equations,48,49 but these require Hamaker constants that are often available when studying homogeneous columns of, e.g., quartz, but which are much more poorly defined for the case of more realistic heterogeneous soils. Finally, αatt values vary to some extent with physical parameters such as flow rate,50 and many other mechanisms that are not taken into account in clean-bed CFT, such as straining, are also dependent on flow rate.51 Some of these effects can be taken into account by designing a more comprehensive model, but such a model would require many more parameters to be fitted or experimentally determined (e.g. blocking factors, straining coefficients, etc.) because they cannot be calculated based on first principles. αatt values are therefore, in essence, also operationally defined, meaning that it is not clear how an αatt value can be used outside of the boundaries of the test system used, at least not with a strong claim of mechanistic accuracy.
Conclusions
The best fate indicator?
Table 1 summarizes the comparison between the three fate descriptors discussed in this work. Evaluating fate indicators requires some reference point, which is ideally the situation in the field. ENP contamination has not been going on long enough to produce historically contaminated sites, and analysis of ENP in the field currently presents such technical difficulties52 that there is only one study on freshwater to the authors' knowledge.53 Comparison may occur with mesocosms and microcosms where ENP fate processes are still close to the situation in field,54,55 but the effect of the environmental matrix on any fate predictor is pronounced, making quantitative evaluation across studies using different environmental systems impossible. Column tests are intuitively seen as more environmentally realistic, but it has been argued that this realism may be overstated and column tests cannot thus serve as reference points.The current perspective investigates whether Kd-type batch methods could be used for ENP. One virtue of Kd values for molecular chemicals is that the concept is simple enough so that it can be widely applied. Similarly to Praetorius et al.,15 technical objections that showed that batch-type fate descriptors could not be related to column-type descriptors were found. Where Praetorius et al.15 focused on the lack of equilibrium, the effect of shear during batch tests was argued here to complicate comparability. However, considering previous counterarguments against the environmental realism of column tests, it is difficult to use the lack of comparability between these and batch tests to prefer the former.
Concurring with Praetorius et al.,15 deposition cannot be considered an equilibrium process and any attempt to model this process as such, e.g. using eqn (1), will lead to very large errors. Fate prediction of ENPs should be based on a kinetic assessment and fate descriptors should be developed on that basis. It remains to be investigated to what extent the results from batch tests can be used in this context to predict travel distances and bioavailability. Since it was argued that it would not be practical and not necessarily more accurate to widely apply column tests, there is still a need for a fate descriptor that is the best possible compromise between operational simplicity and technical accuracy. Alternatives to both batch and column tests could be investigated, such as, for the soil case, a centrifugal pore water extraction from soil incubated with ENP (e.g.ref. 56). Such a test could potentially eliminate effects of shear while being still operationally relatively simplistic.
References
- G. Cornelis, K. M. Hund-Rinke, T. Kuhlbusch, N. Van den Brink and C. Nickel, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2720–2764 CrossRef CAS.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Environ. Sci. Technol., 2012, 46, 6891–6899 CrossRef CAS PubMed.
- P. S. Tourinho, C. A. M. van Gestel, S. Lofts, A. Soares and S. Loureiro, Environ. Toxicol. Chem., 2013, 32, 2808–2815 CrossRef CAS PubMed.
- P. L. Waalewijn-Kool, M. D. Ortiz, S. Lofts and C. A. M. van Gestel, Environ. Toxicol. Chem., 2013, 32, 2349–2355 CrossRef CAS PubMed.
- W. A. Shoults-Wilson, B. C. Reinsch, O. V. Tsyusko, P. M. Bertsch, G. V. Lowry and J. M. Unrine, Soil Sci. Soc. Am. J., 2011, 75, 365–377 CrossRef CAS.
- S. Frenk, T. Ben-Moshe, I. Dror, B. Berkowitz and D. Minz, PLoS One, 2013, 8, 12 Search PubMed.
- I. Josko and P. Oleszczuk, Chemosphere, 2013, 92, 91–99 CrossRef CAS PubMed.
- M. Pawlett, K. Ritz, R. A. Dorey, S. Rocks, J. Ramsden and J. A. Harris, Environ. Sci. Pollut. Res., 2013, 20, 1041–1049 CrossRef CAS PubMed.
- G. Cornelis, L. Pang, C. Doolette, J. K. Kirby and M. J. McLaughlin, Sci. Total Environ., 2013, 463–464, 120–130 CrossRef CAS PubMed.
- J. Fang, X. Q. Shan, B. Wen, J. M. Lin and G. Owens, Environ. Pollut., 2009, 157, 1101–1109 CrossRef CAS PubMed.
- J. Hammes, J. A. Gallego-Urrea and M. Hassellov, Water Res., 2013, 47, 5350–5361 CrossRef CAS PubMed.
- I. Velzeboer, J. T. K. Quik, D. van de Meent and A. A. Koelmans, Environ. Toxicol. Chem., 2014, 33, 1766–1773 CrossRef CAS PubMed.
- K. Schlich, T. Klawonn, K. Terytze and K. Hund-Rinke, Environ. Sci. Eur., 2013, 25, 17 CrossRef.
- A. Praetorius, M. Scheringer and K. Hungerbühler, Environ. Sci. Technol., 2012, 46, 6705–6713 CrossRef CAS PubMed.
- A. Praetorius, N. Tufenkji, K.-U. Goss, M. Scheringer, F. Von der Kammer and M. Elimelech, Environ. Sci.: Nano, 2014, 1, 317–323, 10.1039/C4EN00043A.
- A. R. Petosa, D. P. Jaisi, I. R. Quevedo, M. Elimelech and N. Tufenkji, Environ. Sci. Technol., 2010, 44, 6532–6549 CrossRef CAS PubMed.
- Z. Adamczyk and P. Warszyński, Adv. Colloid Interface Sci., 1996, 63, 41–149 CrossRef CAS.
- J. T. K. Quik, M. A. C. Stuart, M. Wouterse, W. Peijnenburg, A. J. Hendriks and D. Van de Meent, Environ. Toxicol. Chem., 2012, 31, 1019–1022 CrossRef CAS PubMed.
- G. Trefalt, F. J. M. Ruiz-Cabello and M. Borkovec, J. Phys. Chem. B, 2014, 118, 6346–6355 CrossRef CAS PubMed.
- J. J. Scott-Fordsmand, D. Stevens and M. McLaughlin, Environ. Sci. Technol., 2004, 38, 3036–3039 CrossRef CAS.
- L. Armelao, D. Barreca, G. Bottaro, A. Gasparotto, C. Maccato, C. Maragno, E. Tondello, U. L. Stangar, M. Bergant and D. Mahne, Nanotechnology, 2007, 18, 375709 CrossRef.
- F. Degryse, E. Smolders and D. R. Parker, Eur. J. Soil Sci., 2009, 60, 590–612 CrossRef CAS PubMed.
- F. Y. Wang, P. M. Chapman and H. E. Allen, Mar. Pollut. Bull., 1999, 38, 423–425 CrossRef CAS.
- R. H. Anderson, D. B. Farrar and J. M. Zodrow, Hum. Ecol. Risk Assess., 2013, 19, 1488–1513 CrossRef CAS.
- M. Pedrot, A. Dia, M. Davranche, M. Bouhnik-Le Coz, O. Henin and G. Gruau, J. Colloid Interface Sci., 2008, 325, 187–197 CrossRef CAS PubMed.
- E. Smolders, K. Oorts, P. van Sprang, I. Schoeters, C. R. Janssen, S. P. McGrath and M. J. McLaughlin, Environ. Toxicol. Chem., 2009, 28, 1633–1642 CrossRef CAS PubMed.
- S. Sauve, W. Hendershot and H. E. Allen, Environ. Sci. Technol., 2000, 34, 1125–1131 CrossRef CAS.
- T. K. Darlington, A. M. Neigh, M. T. Spencer, O. T. Nguyen and S. J. Oldenburg, Environ. Toxicol. Chem., 2009, 28, 1191–1199 CrossRef CAS PubMed.
- F. He, M. Zhang, T. Qian and D. Zhao, J. Colloid Interface Sci., 2009, 334, 96–102 CrossRef CAS PubMed.
- Y. A. Tian, B. Gao and K. J. Ziegler, J. Hazard. Mater., 2011, 186, 1766–1772 CrossRef CAS PubMed.
- X. Liu, D. M. Carroll, E. J. Petersen, Q. Huang and C. L. Anderson, Environ. Sci. Technol., 2009, 43, 8153–8158 CrossRef CAS PubMed.
- D. Kasel, S. A. Bradford, J. Simunek, M. Heggen, H. Vereecken and E. Klumpp, Water Res., 2013, 47, 933–944 CrossRef CAS PubMed.
- M. Elimelech, J. Gregor and X. Jia, Particle Deposition and Aggregation: Measurement, Modeling, and Simulation, 1995 Search PubMed.
- C. Degueldre, P. Aeberhard, P. Kunze and K. Bessho, Colloids Surf., A, 2009, 337, 117–126 CrossRef CAS PubMed.
- D. A. Navarro, J. K. Kirby, M. J. McLaughlin, L. Waddington and R. S. Kookana, Environ. Pollut., 2014, 193, 102–110 CrossRef CAS PubMed.
- D. A. Navarro, R. S. Kookana, J. K. Kirby, S. M. Martin, A. Shareef, J. Du and M. J. McLaughlin, J. Hazard. Mater., 2013, 262, 496–503 CrossRef CAS PubMed.
- G. Cornelis, C. Doolette, M. Thomas, M. J. McLaughlin, J. K. Kirby, D. Beak and D. Chittleborough, Soil Sci. Soc. Am. J., 2012, 76, 891–902 CrossRef CAS.
- G. Cornelis, J. K. Kirby, D. Beak, D. Chittleborough and M. J. McLaughlin, Environ. Chem., 2010, 7, 298–308 CrossRef CAS.
- S. Treumann, S. Torkzaban, S. A. Bradford, R. M. Visalakshan and D. Page, J. Contam. Hydrol., 2014, 164, 219–229 CrossRef CAS PubMed.
- M. Elimelech, J. Gregory, X. Jia and R. A. Williams, Particle deposition and aggregation: measurement, modelling and simulation, Butterworth Heinemann, Woburn, USA, 1995 Search PubMed.
- F. Mietta, C. Chassagne and J. C. Winterwerp, J. Colloid Interface Sci., 2009, 336, 134–141 CrossRef CAS PubMed.
- J. C. Winterwerp, J. Hydraul. Res., 1998, 36, 309–326 CrossRef.
- G. Cornelis, B. Ryan, M. J. McLaughlin, J. K. Kirby, D. Beak and D. Chittleborough, Environ. Sci. Technol., 2011, 45, 2777–2782 CrossRef CAS PubMed.
- J. N. Ryan, M. Elimelech, R. A. Ard, R. W. Harvey and P. R. Johnson, Environ. Sci. Technol., 1999, 33, 63–73 CrossRef CAS.
- S. A. Bradford and S. Torkzaban, Vadose Zone J., 2008, 7, 667–681 CrossRef.
- N. Tufenkji and M. Elimelech, Langmuir, 2004, 20, 10818–10828 CrossRef CAS PubMed.
- N. Tufenkji and M. Elimelech, Langmuir, 2005, 21, 841–852 CrossRef CAS PubMed.
- R. Rajagopalan and C. Tien, AIChE J., 1976, 22, 523–533 CrossRef CAS.
- N. Tufenkji and M. Elimelech, Environ. Sci. Technol., 2004, 38, 529–536 CrossRef CAS.
- W. P. Johnson and M. Tong, Environ. Sci. Technol., 2006, 40, 5015–5021 CrossRef CAS.
- Y. C. Du, C. Y. Shen, H. Y. Zhang and Y. F. Huang, Transp. Porous Media, 2013, 98, 193–208 CrossRef CAS PubMed.
- F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne and J. M. Unrine, Environ. Toxicol. Chem., 2012, 31, 32–49 CrossRef CAS PubMed.
- A. P. Gondikas, F. von der Kammer, R. B. Reed, S. Wagner, J. F. Ranville and T. Hofmann, Environ. Sci. Technol., 2014, 48, 5415–5422 CrossRef CAS PubMed.
- J. M. Unrine, B. P. Colman, A. J. Bone, A. P. Gondikas and C. W. Matson, Environ. Sci. Technol., 2012, 46, 6915–6924 CrossRef CAS PubMed.
- Y. Liang, S. A. Bradford, J. Simunek, M. Heggen, H. Vereecken and E. Klumpp, Environ. Sci. Technol., 2013, 47, 12229–12237 CrossRef CAS PubMed.
- A. R. Whitley, C. Levard, E. Oostveen, P. M. Bertsch, C. J. Matocha, F. von der Kammer and J. M. Unrine, Environ. Pollut., 2013, 182, 141–149 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |