Research highlights: challenges in the characterization, storage, and isolation of natural organic matter
Vivian S.
Lin
*
Institute of Biogeochemistry and Pollutant Dynamics, ETH Zurich, CH-8092, Zurich, Switzerland. E-mail: vivian.lin@usys.ethz.ch
First published on 18th November 2015
Abstract
Dissolved organic matter (DOM) is a critical component of natural waters, providing an important nutrient source for the microbial community, binding and interacting with metals and pollutants, and serving as a major reservoir of carbon in the global carbon cycle. Differences in organic material origin as well as biotic and abiotic transformation processes produce DOM of varying composition. These highly complex mixtures are difficult to characterize due to the diversity of compounds found in DOM. This Highlight examines three recent publications that study the methods used to characterize DOM and how storage and handling of DOM samples may affect its composition. Collectively, these publications underscore the challenges that researchers face when working with natural organic matter and offer important considerations for natural organic matter handling and analysis.
Introduction
Dissolved organic matter (DOM) exists in natural waters as a complex, heterogeneous mixture of carbon-containing molecules of aquatic or terrestrial origin. In the environment, DOM undergoes a multitude of biotic and abiotic transformations that contribute to the diversity of its components (Fig. 1). Microbes feed on the labile fraction of DOM, making it an important source of carbon into the food chain. Interactions with sunlight and oxidizing species can break down DOM and alter its composition. Despite ongoing efforts to characterize DOM, the exact nature of its constituents remains poorly defined. The sheer number of compounds in DOM contributes to the difficulty of DOM characterization and is proposed as a primary reason for the existence of recalcitrant DOM (DOI: 10.1126/science.1258955), which can remain intact in deep ocean waters for thousands of years (DOI: 10.1146/annurev-marine-120710-100757).To better understand DOM, researchers have investigated its physical and chemical properties using a wide array of analytical techniques, including mass spectrometry, absorbance, fluorescence, and nuclear magnetic resonance (NMR) spectroscopy (DOI: 10.1039/C4EM00062E), in conjunction with various types of separation methods (DOI: 10.1039/C5EM00223K). A fundamental consideration for all research involving DOM is how sampling, handling, and processing may alter its composition. In this Highlight, I discuss three articles that provide valuable insight into the nature of DOM and the characterization methods that have been traditionally used in the field. Specifically, the use of absorbance as a proxy for DOM quantity and quality is examined, as well as the changes observed in stored samples over time. The effects of separation methods on DOM composition are also highlighted, with attention to the fractionation of DOM by extraction methods and how this alters the proportions of molecule types that may be identified by downstream analytical techniques. Taken together, these publications highlight the complexities of DOM and emphasize the need for continued investigation of its properties and behavior in both the natural environment and the laboratory.
Complications in the association of optical properties with DOM concentration
Optical properties such as fluorescence and absorbance have been widely utilized to characterize DOM samples due to the ease and speed at which samples can be measured and also because such measurements are non-destructive. Thus, there is great interest in using UV-visible and fluorescence measurements as proxies for DOM quantity and quality. Measurements are typically taken at specific wavelengths that are thought to be characteristic of certain molecule types, with specific ultraviolet absorbance at 254 nm (SUVA254) being indicative of aromaticity and ratios of absorbance at various wavelengths correlating with the degree of humification, or extent to which the DOM is broken down. However, whether or not these associations can be generalized across samples collected from different sites remains unclear, and quantitative models that could be used for rapid predictions of DOM have not been explored in detail.In order to clarify the relationship between the absorbance and fluorescence properties of DOM and quantitative measures of its concentration and bioavailability, Baldwin and Valo systematically examined DOM samples collected from 18 diverse environmental sites (DOI: 10.1039/C4EM00473F). Initial measurements of absorbance and fluorescence intensity for certain wavelengths (“UVA-humic like” fluorophore with excitation at 311 nm, emission at 426 nm; and “tryptophan-like” fluorophore with excitation at 287 nm, emission at 345 nm) were well correlated with initial dissolved organic carbon (DOC) concentration. DOC bioavailability was measured as the change in DOC after incubating samples at 20 °C in low light for 28 days (ΔDOC). DOC bioavailability appeared to be correlated with both initial UV absorbance and initial fluorescence intensity.
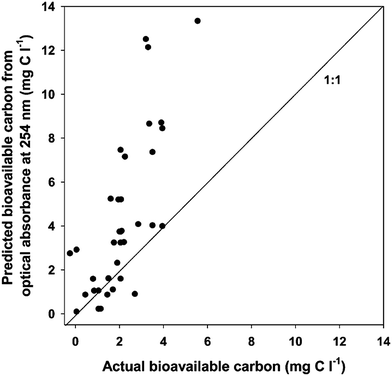
The authors then used these empirical data to establish simple linear predictive models and then evaluated the robustness of these relationships by comparison with validation samples, collected from 12 different sites and not applied to model development. The models using initial optical properties were not particularly good at predicting DOC concentration of the validation samples, with average root mean square deviations of 7.45 mg C L−1 for the model based on initial absorbance at 254 (A254) and 4.88 to 5.06 mg C L−1 for models based on fluorescence. Models for predicting DOC bioavailability based on both A254 and fluorescence intensity at Ex 311/Em 426 nm overestimated bioavailable DOC for samples above 2 mg C L−1 (Fig. 2). Furthermore, even in samples for which a large ΔDOC was measured, the overall fluorescence intensity remained relatively unchanged after incubation, suggesting fluorescence would not provide accurate predictions of DOC bioavailability. The authors concluded that, despite obtaining strong correlations between DOM optical properties and initial measurements of DOC concentration and bioavailability, the relationships appear to be co-incidental rather than causal. This work by Baldwin and Valo provides much needed insight into the limitations of models based on the optical properties of DOM. These models, while useful in certain applications, may not be generalizable and should be periodically re-validated, especially when introducing samples from sites not used in model development.
Effect of freezing and cold storage on DOM bioavailability
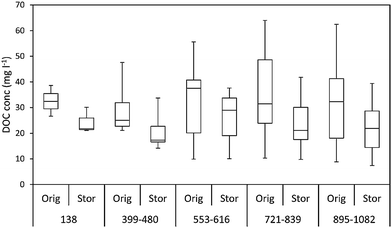
Due to time constraints and the limited portability of advanced analytical instrumentation, samples collected from the field must often be transported to the laboratory and stored prior to analysis. Freezing is frequently used as a means of preservation, to halt biological decomposition and slow chemical reactions. While previous studies have examined changes in water sample composition on the order of weeks to months at low temperatures, there is limited research on the long-term stability of DOM samples when stored cold or frozen.In their recent article, Peacock et al. critically examine how cold storage can affect DOM composition over time (DOI: 10.1039/C5EM00126A). Samples were collected from bog or fen peatland environments and initially analyzed for electrical conductivity, pH, DOC, and UV-vis absorbance. Bog peatland samples were then stored at 4 °C in the dark, to be re-analyzed 138–1082 days later, while fen peatland samples were stored for 192 days. The rate of DOC loss in the short-term was high for bog samples, with degradation rates decreasing over longer storage times (Fig. 3). Based on previous research that found 32–45% loss in DOC when stored at 20 °C for 3 years, (DOI: 10.1016/j.scitotenv.2012.06.029) Peacock et al. note that lower temperatures may better preserve DOM content, with an average 20% loss in DOC observed after cold storage at 4 °C. The authors suggest some degradation of DOC upon storage is unavoidable, with the greatest losses observed in the first few months. In terms of compositional changes after cold storage, the authors observed small differences in absorbance properties, but noted that these changes did not proportionally reflect the more dramatic changes observed in DOC concentration. Like Baldwin and Valo, (DOI: 10.1039/C4EM00473F) they propose that preferentially degraded molecules appeared to be non-aromatic based on the relatively small changes in A254 after storage.
Peacock and coworkers also investigated the effects of a freeze–thaw procedure on DOM quantity and quality. Samples for freeze–thaw analysis were collected from 6 different sites and frozen for at least 48 h, then thawed in a refrigerator and re-analyzed. Changes in DOC concentrations were generally small; approximately one-third of the samples had 2.4% median decreased DOC content after freeze–thawing, while the others increased in DOC concentration by a median of 4.4%. In contrast, the freeze–thaw procedure had a dramatic and inconsistent impact on the optical properties of the samples. Absorbance at a variety of different wavelengths increased for some samples and decreased for others, with no clear trend. Thus, freezing samples appears to be most suitable for samples in which bulk DOC measurements are needed and not recommended for experiments involving UV-visible spectroscopy.
Effect of separation methods on DOM composition
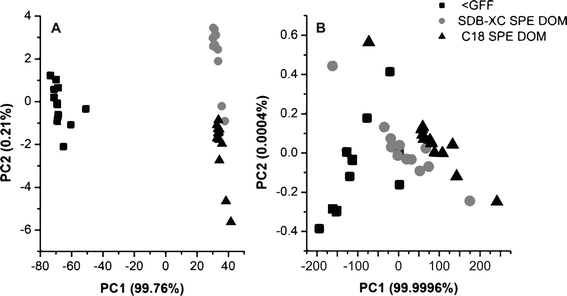
Physical and chemical handling of DOM has been extensively used to concentrate and separate DOM from other compounds often found in natural water samples, allowing for easier analysis downstream. Isolation techniques such as solid phase extraction (SPE) help remove inorganic salts that are present in marine samples and also facilitate collection of sufficient organic material for analysis. Although researchers acknowledge that selective fractionation of compounds in DOM as well as chemical transformation of DOM due to pH changes may occur during SPE, it remains a popular technique for DOM extraction due to its ease of application, especially when conducting field work in remote areas.In their recent publication, Li and Minor examine the effect of two types of SPE resins on the composition of DOM from coastal and offshore waters in Lake Superior (DOI: 10.1039/C5EM00199D). Samples were collected from 7 sites at multiple depths and measured for total organic carbon (TOC), 13C and 14C isotopic content, and UV-visible absorbance. Two types of resins in disk form, the classically used silica based octadecyl carbon (C18) and the relatively new polymeric styrene-divinylbenzene (SDB-XC), were used to extract samples. TOC analysis of blank samples showed that the disks contributed some amount of carbon contamination, indicating that blank samples should always be included in the analysis to ensure appropriate interpretation of the data. Comparison of colored or chromophoric dissolved organic matter (CDOM) before and after filtration showed little to no CDOM contamination and minor CDOM losses by irreversible sorption to the disks. Extraction recoveries were higher for SDB-XC disks compared to C18 disks for both CDOM and DOC, at 31.0 ± 3.80% and 25.8 ± 9.20%, respectively. Compositional changes were observed based on shifts in UV-visible absorbances, with both C18 and SDB-XC disks selectively isolating DOM with higher molecular weight and/or higher aromatic content. Principal Component Analysis (PCA) was conducted, using the UV-visible spectrometry data of samples before and after SPE, to identify significant factors influencing compositional variation (Fig. 4). Sample preparation, e.g. the extraction method, had the greatest impact overall on sample composition (Fig. 4A). Additionally, DOM isolated using the SDB-XC disks performed better compared to C18 disks, having greater similarity in composition to initial samples (<GFF) as shown in Fig. 4B.
Li and Minor selected electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry (ESI FT-ICR-MS) in the negative mode for investigation of molecular changes in isolate composition. FT-ICR-MS offers high mass accuracy and precision and can detect a wide range of compound types, making it an appealing technique for analysis of complex DOM samples. Blank samples contributed 11.8–15.9% of the total peaks observed in the samples. For both sites, approximately 70% of the formulae (m/z) obtained through FT-ICR-MS were common to both extraction methods, with approximately 29–31% of the peaks being attributed to unique compounds for either C18 or SDB-XC disks. Overall, SDB-XC disks outperformed C18 disks, offering better recovery of DOC and less fractionation of DOM. UV-visible absorbance and FT-ICR-MS showed that the DOM pool isolated from different resin types varied slightly in composition, indicating that comparison of extracted DOM samples should be performed with caution and account for the method used for isolation.
Concluding remarks
In our efforts to better understand the composition of DOM, the three highlighted publications serve as a reminder that the handling of samples may influence their composition and care must be taken when using generalized models to characterize DOM. The study by Baldwin and Valo showed that models using initial optical properties to predict DOM concentrations and bioavailable content may not perform well for independent samples, and researchers should regularly re-test these models to confirm their validity. Peacock and coworkers examined the effect of freezing or storing samples at low temperatures, noting that DOC loss in the short-term was relatively rapid for samples stored at 4 °C. Both DOC concentrations and optical properties were significantly and unpredictably impacted by freeze–thawing. The two aforementioned studies also observed that changes in optical properties were small relative to changes in DOC concentrations, perhaps as a result of preferential degradation of certain types of organic molecules. Lastly, the work by Li and Minor shows that two widely used types of SPE resins, C18 and SDB-XC, result in relatively good DOC recovery but also lead to some compositional changes, including enrichment of heavier molecular weight, more aromatic compounds. SDB-XC disks performed somewhat better than the C18 variety, although regardless of the resin type, the appropriate blank controls and consistent processing of all samples are necessary for valid comparison. These articles highlight the complexity of DOM, from its geographic and seasonal variability to its potential instability after sample collection, and continued research in these areas will help to identify the best practices in collecting, storing, and analyzing DOM samples.| This journal is © The Royal Society of Chemistry 2015 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: