Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the role of the sampler housing in limiting uptake of semivolatile organic compounds in passive air samplers†
Xianming
Zhang
,
Michelle
Hoang
,
Ying D.
Lei
and
Frank
Wania
*
Department of Physical and Environmental Sciences, University of Toronto Scarborough, 1265 Military Trail, Toronto, Ontario M1C 1A4, Canada. E-mail: frank.wania@utoronto.ca; Tel: +1-416-287-7225
First published on 18th November 2015
Abstract
Passive air samplers (PASs) are simple, versatile devices that are increasingly used to determine the concentrations of semivolatile organic compounds (SVOCs) in the atmosphere. Using PAS and interpreting PAS-derived data with confidence requires a detailed understanding of the factors that control the uptake kinetics. A number of experiments were aimed at clarifying the role that the housing has in limiting the uptake of SVOCs in a PAS. Specifically, we quantified the gradient in the amount of polychlorinated biphenyls (PCBs) accumulated in XAD-filled mesh cylinders with increasing distance from the PAS housing's opening. That gradient was non-existent in an artificially ventilated housing (i.e. different segments of a cylinder contained the same amount of PCBs), minor during outdoor deployments (i.e. the bottom third of the cylinder sampled approximately 20% more PCBs than the top third), and strong during indoor deployments (i.e. the bottom third of the cylinder sampled twice the amount sampled by the top third). This is consistent with the thickness of the air boundary layer surrounding the XAD-resin increasing with increasing distance from the housing's opening and decreasing with increasing air turbulence. An experiment with housings absorbing different amounts of sunlight revealed that heat-induced convection has a minor effect on the gradient within the mesh cylinder and on the total amount of accumulated PCB. Similarly, this gradient and the total amount sorbed was also not influenced by the number of XAD-filled mesh cylinders placed within a housing as long as they were deployed outdoors. However, if four mesh cylinders were placed in one housing in a calm indoor setting, the top third of the mesh cylinders was notably starved of PCBs, suggestive of an air concentration gradient within the sampler housing.
Environmental impactThe results of experiments described here provide practical information for planning passive air sampling campaigns. Differences in sun exposure of different samplers are unlikely to cause strong differences in the sampling rate. During outdoor deployments, two mesh cylinders can be safely placed in a single housing without affecting their sampling rate. This will often reduce the number of housings, and therefore the cost, required for a campaign. Data from long and short versions of the XAD-based passive sampler can be compared by normalization to the surface area of the mesh cylinder. |
Introduction
Passive air samplers (PASs) are increasingly used for sampling semivolatile organic compounds (SVOCs) at ambient atmospheric concentrations. While several types of PASs using different passive sampling media (PSMs) have been introduced and their performance characteristics determined, the most prominent PASs in use today are semipermeable membranes, polyurethane form (PUF) disks, and styrene-divinylbenzene copolymer (XAD) resin-filled mesh cylinders.1–3 They are linear uptake samplers, in which the sampled chemicals should not approach equilibrium between gas phase and PSM. A volumetric air concentration CA (ng m−3) can be derived from the amount of chemical accumulated in the PSM (m, ng) during the deployment time t (day) using a sampling rate R (m3 day−1): CA = m/(Rt). The sampling rate R is the product of a chemical mass transfer coefficient from air to PSM and an interfacial area.Even though a widely applied model of the uptake of SVOCs in PAS relies on the assumption of uniform uptake within the PSM, this assumption has rarely been tested experimentally by analyzing separately various sections of a PSM.4 Some experimental results, however, hinted that this assumption may not be valid. For example, May et al.5 noted that the wind speed in the widely-used double bowl PAS is different above and below the PUF disk, and Chaemfa et al.6 observed that the number and size distribution of particles trapped in the PUF varied between different locations within the disk. And indeed, Zhang et al.7 observed a highly non-uniform distribution of polychlorinated biphenyls (PCBs) within the PSMs of both the XAD-resin based PAS and PUF-disk PAS. Specifically, they reported a strong radial concentration gradient within cylindrical-shaped PSMs: the outer XAD and PUF layers collected much higher levels of PCBs than the inner layers.7 They identified the underlying cause for this phenomenon to be the resistance on the PSM side to the uptake of SVOCs, which previously had been largely assumed to be negligible and therefore ignored.
However, there is likely also another source of non-uniformity in the uptake of SVOCs in the PSM of PAS that is not related to the resistance on the PSM side, but rather to variations in the air-side resistance within the PAS housing. May et al.'s5 observations revealed large differences in the wind speed within the double bowl housing. As the thickness of the air boundary layer is inversely correlated to wind speed we may presume that this thickness varies within a PAS housing: it will be thicker in the more sheltered parts of the housing compared to parts closer to the housing's opening that are more exposed to ambient wind.8 As the boundary air layer gets thicker, the sampling rate decreases because the thicker boundary air layer poses a resistance to the transfer of gas molecules to the PSM.4 We thus hypothesize that the more exposed parts of the PSM accumulate greater amounts of SVOCs than the more sheltered parts.
Incidentally, the same non-uniform distribution would presumably be observed if the transport of the SVOCs within the sampler housing is a rate-limiting step, i.e. if there is an air concentration gradient with levels deep within the shelter being lower than close to the opening. We refer to this as a “starvation effect”: if the rate of chemical transfer from the bottom to the top of the housing is slower than the chemical transfer across the boundary layer to the sampling medium, the concentrations of the chemicals in the air further removed from the opening of the sampler housing would be lower than those closer to it. Whether the origin of non-uniform uptake in the PSM with varying distance from the sampler housing opening is a variable boundary layer thickness or the existence of an uptake resistance within the sampler housing, we would expect the effect to be most pronounced under wind still conditions, especially during indoor deployments.
While we know of no previous studies that analyzed separately parts of PSMs that are exposed to a different extent within a PAS housing, there are some studies that showed that the uptake rate depends on the placement of the PSM within a PAS housing. Tao et al.9 noted that uptake rates of PAHs in a PUF disk placed at the top of an inverted cylindrical can were more than an order of magnitude lower than those reported for PUF disks in double bowl housings, which they attributed to limited air circulation in the sampling shelter. Similarly, Abdallah and Harrad10 noted a slight decrease in the uptake rates in PUF disks if they were placed at the top of the double bowl housing rather than the middle. Similar effects were noted for the PAS relying on a resin-filled mesh cylinder: the sampling rate decreased with increasing distance of the PSM from the opening of the cylindrical housing.11
The objective of this study was three-fold. First, we sought to confirm experimentally, whether there is a gradient in the amount of SVOC taken up in a PAS's PSM with variable distance from the shelter opening. Secondly, we investigated how that gradient may be affected by the presence of turbulence within the PAS housing. The latter was created either by blowing wind directly into the sampler housing or by varying the insolation of the housing, which may create heat induced convection. Thirdly, by varying the number of PSM-filled containers in a housing, we explored to what extent the gradient is affected by the amount of PSM in a sampler housing. If the gradient increases with the amount of PSM, we may infer that there is a starvation effect, i.e. the axial concentration gradient is not restricted to the PSM, but also occurs in the air inside the housing. We relied upon the XAD-resin based PAS for this study, because its design and length allows for relatively large differences in the distance of the PSM from the shelter opening, because it has been used extensively for a number of field studies worldwide and also because the factors controlling its uptake have been more fully characterized than for other PASs.3,7,11–18
Materials and methods
Experimental setup
Sample preparation and extraction
Upon retrieval, the XAD-filled mesh cylinders were stored in air-tight metal tubes and placed in a −20 °C freezer until extraction. Segmented mesh cylinders were disassembled, and stored and analyzed individually. Before extraction, each sample was spiked with 100 μL of a solution with 0.2 ng μL−1 13C12-labeled polychlorinated biphenyl congeners PCB-77, -101, -141 and -178 (Cambridge Isotope Labs) as surrogate standards. All samples, with the exception of the samples from the experiments that tested the axial distribution of PCBs in the PAS with multiple PSM containers, were Soxhlet extracted for 24 h with ∼500 mL dichloromethane. Samples from the indoor and outdoor experiments that tested the axial distribution of PCBs in the PAS with multiple PSM containers were extracted using pressurized liquid extraction using an Accelerated Solvent Extractor (ASE®) 350 (Dionex, Sunnyvale, CA, USA) with 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (%) acetone
50 (%) acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane at 75 °C for approximately 30 minutes.19 All extracts were roto-evaporated to ∼2 mL and eluted through dehydrated sodium sulphate packed in a disposable pasteur pipet to remove moisture. The eluent was blown down with high purity (5.0) nitrogen, solvent exchanged to iso-octane and reduced to ∼0.5 mL in a vial, to which 100 ng mirex was added as internal standard for quantification.
hexane at 75 °C for approximately 30 minutes.19 All extracts were roto-evaporated to ∼2 mL and eluted through dehydrated sodium sulphate packed in a disposable pasteur pipet to remove moisture. The eluent was blown down with high purity (5.0) nitrogen, solvent exchanged to iso-octane and reduced to ∼0.5 mL in a vial, to which 100 ng mirex was added as internal standard for quantification.
Chemical analysis
PCBs, whose partition properties overlap with many SVOCs of environmental interest, were selected as the target chemicals. An Agilent 6890 gas chromatograph coupled with an Agilent 7683 auto-sampler and an Agilent 5973 mass spectrometric detector were used for the analysis. PCBs in 1.0 μL of extract were injected in splitless mode (injector temperature 250 °C) and separated using a DB5-MS capillary column (60 m length × 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific) with helium (1.4 mL min−1) as carrier gas. The chromatograph's oven temperature was programmed as 80 °C for 1 min, to 160 °C at 10 °C min−1, to 280 °C at 3 °C min−1, and held for 6 min. Temperatures for the ion source and quadrupole of the mass spectrometer were 230 °C and 150 °C. The mass spectrometer was operated in electron impact ionization (70 eV) and selective ion monitoring mode. The quantitative and qualitative ions monitored are listed in Table S1.† A description of the analytical method used for the samples from Hawaii for different SVOCs is provided in the SI.QA/QC
Recoveries of the PCBs as indicated by the four surrogate standards ranged 73–144% (interquartile range < 15%). One field blank was included at each of the sampling locations for the axial distribution experiment. A solvent blank was included in every batch (every 5 samples) of Soxhlet extractions. No PCBs were detected in the solvent blanks. Field blanks contained less than 5% of the PCB amounts in the samples. Because the uncertainty introduced by recoveries and blank levels was smaller than the variability of trace organic contaminants analysis, the reported values were not recovery or blank corrected. At site L4, interference appeared to affect the analysis of PCB-31/28, -49, -44 because the abundance ratios between the qualifying and quantifying ions peaks deviated by more than 30% from the theoretical values, while for samples from other locations, the differences were <15%. As such, PCB-31/28, -49, -44 in samples from L4 were not included in the data analysis.Excluding the two indoor PASs with blowing fans, the relative difference between duplicate PASs with axially segmented cylinders was 17% ± 14% for the targeted PCB congeners. The amounts of PCBs sampled by the two indoor PASs with blowing fans had a relative difference of 58% ± 7% (discussed below).
Results and discussion
Axial distributions in PAS deployed indoors
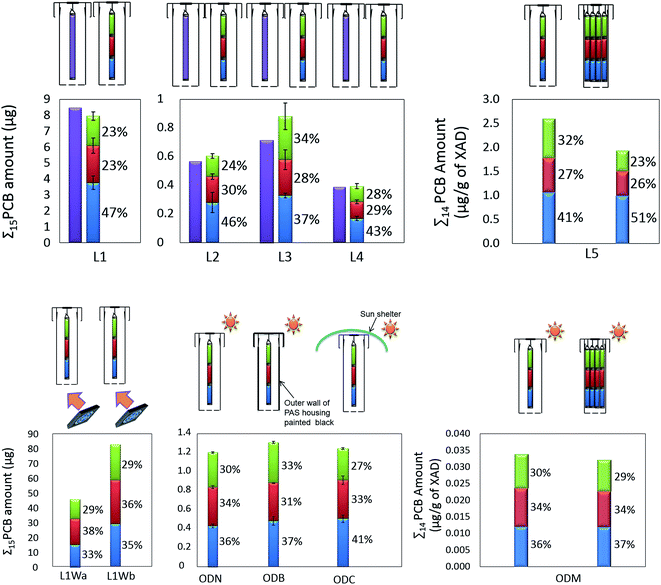
The distribution of PCBs in the PSM was investigated by separately analyzing each segment of axially segmented XAD-filled mesh cylinders. In all four indoor locations (L1–L4) the sum of the amounts of a single PCB congener accumulated in the three segments was not significantly different (relative difference 11% ± 8%, p = 0.53, Wilcoxon signed-rank test) from the amount in the non-segmented mesh cylinder deployed at the same location (Fig. 1 and S2†), indicating that segmentation did not change uptake characteristics.However, the amount of PCBs that accumulated in the three segments was different (Fig. S2†). Analysis of variance (ANOVA) and multiple comparisons on log-transformed data (Table S2†) indicated that at L1, L2 and L4, the amount of PCBs in the bottom segments was significantly higher (p < 0.05) than that in the middle and top segments. This agrees with a previous study showing a higher sampling rate of water when a silica gel filled mesh cylinder was positioned closer to the opening of the sampler housing.11 L1, L2, and L4 are offices or storage rooms with little activities and thus, little air turbulence. In contrast, the differences between the amounts of PCBs that accumulated in the three segments of the PASs deployed at L3 were not significant (Table S2†). L3 is an underground cargo loading area with truck traffic and other activities; thus air turbulence is expected to be stronger here than the other indoor locations. Stronger air turbulence may expose the upper segments of the mesh cylinder to wind to a similar extent as the bottom segments, resulting in similar uptake rates for the three segments.
Axial distributions in PAS deployed outdoors
Separate analysis of the three segments of XAD-filled mesh cylinders deployed in normal housings outdoors showed non-uniform axial distributions of PCBs in the XAD mesh cylinder (Fig. S3†), with segments closer to the opening of the PAS housing generally accumulating more PCBs (ODN in Fig. 1). However, the gradient was not as strong as observed for deployments at wind still indoor conditions at L1 and L2, where the PCB amounts in the top segments were only about half (51 ± 2%) of that in the bottom segments. Outdoors, the top segment contained 83 ± 2% of the amount accumulated in the bottom segment. This suggests that limited air turbulence is responsible for the strong axial distributional gradient of PCBs in a PAS deployed indoors.PCBs accumulating in PASs deployed within and on top of the same building had similar congener profiles (Fig. S2†). Even though sampling rates tend to be higher outdoors than indoors, the amounts of PCBs accumulated in the PASs deployed at L1 were ∼10 times higher than in the outdoor PASs. This suggests that the building was the source of the PCBs measured on the building rooftop. This is consistent with previous studies, which suggested that PCBs are still continuously emitted from indoor sources.11
Axial distributions in artificially ventilated PAS deployed indoors
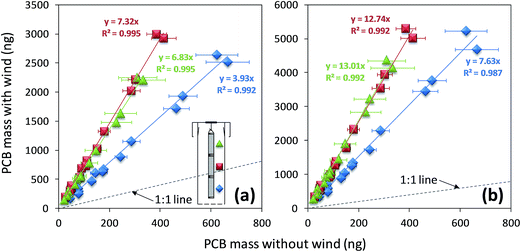
To test whether air turbulence is the contributing factor to the axial distribution of chemicals in the XAD-filled mesh cylinder, we set up two electric fans blowing at 45° angle towards the openings of two PASs with segmented cylinders (Fig. S1†). While the PCB congener profile under these conditions was similar to that under wind still condition (Fig. S2†), wind exposure greatly increased the amounts of PCBs accumulated in the PASs (by ∼8 times). The amounts of PCBs accumulated in the duplicates varied much more than in the other deployments (over 50%). Such large variation is probably caused by the difficulty to precisely replicate wind patterns; both speed and angle of incidence of the wind are potential factors varying the uptake rate.20 Despite of the variations between duplicates in the absolute PCB amounts, the relative distribution of PCBs among the three segments was quite consistent (p = 0.9) (Fig. S4†) in that no statistically differences in the distributions of PCBs among the three segments was observed, i.e. no concentration gradient in the axial direction was established (Fig. S5 and Table S2†).Comparing the amounts of PCBs accumulated in the segmented cylinders of PASs deployed in L1 under wind still and windy conditions (Fig. 2), we note that wind increased the uptake rates for all three segments of the mesh cylinder (all the points in Fig. 2 fall on the upper left side of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 line). In each segment of the XAD mesh cylinder no statistical difference in the extent of increase (relative to wind still condition) was found among different PCB congeners (the points representing different PCB congeners in a segment fall on a line through the origin in Fig. 2). Wind increased the uptake rate of the top and middle segments to the same extent (no significant difference was found between the slopes of the corresponding red and green regression lines in Fig. 2; p > 0.4 for the interaction factor in the analysis of covariance; more detail on the statistical test of the slopes are presented in SI†). In both duplicates, the increase in uptake rate (the slopes of the lines in Fig. 2a and b) for the top and middle segments was ∼1.7 times higher than the increase of the uptake in the bottom segments. The smaller increase of uptake rate for the bottom segments is likely due to them being already influenced by the air movement in a normally ventilated indoor environment with limited activities.
1 line). In each segment of the XAD mesh cylinder no statistical difference in the extent of increase (relative to wind still condition) was found among different PCB congeners (the points representing different PCB congeners in a segment fall on a line through the origin in Fig. 2). Wind increased the uptake rate of the top and middle segments to the same extent (no significant difference was found between the slopes of the corresponding red and green regression lines in Fig. 2; p > 0.4 for the interaction factor in the analysis of covariance; more detail on the statistical test of the slopes are presented in SI†). In both duplicates, the increase in uptake rate (the slopes of the lines in Fig. 2a and b) for the top and middle segments was ∼1.7 times higher than the increase of the uptake in the bottom segments. The smaller increase of uptake rate for the bottom segments is likely due to them being already influenced by the air movement in a normally ventilated indoor environment with limited activities.
One explanation for the lower uptake of PCBs in the upper cylinder segments is an air boundary layer surrounding the XAD-filled mesh cylinder, whose thickness increases with increasing distance from the opening of the PAS housing. Thus uptake in the bottom segment of the cylinder is faster than in the middle and top segments. Another process might contribute to a lower uptake in the top segment: if the rate of chemical transfer from the bottom to the top of the housing is slower than the rate of chemical transfer across the boundary layer surrounding the sampling medium, the concentrations of the chemicals in the air surrounding the middle and top segments could be lower than that in the air surrounding the bottom segment. In other words, the air in the top of the sampler housing would be starved of chemical. Whether the latter process contributes to the gradient in the PSM is explored further below.
A study using PUF as the sampling medium observed a decreased sampling rate when the PUF was moved further from the opening of the sampler housing.16 The decreased sampling rate was partially attributed to less particles being trapped by a PUF placed further from the opening.16 In the present study, the congeners with less than 5 chlorines that are predominantly (>95%) in the gas phase also showed decreased uptake in the middle and top cylinder segments, indicating that differences in particle uptake cannot explain the observations.
Effect of heat convection on axial distributions
Passive air samplers with axially segmented XAD-filled mesh cylinders in normal housings, black painted housings and housings shaded from the sunlight were deployed outdoors to test whether solar radiation would affect chemical uptake and axial distribution in PASs (Fig. S1†). The total PCB amounts (i.e. sum of amounts in three segments) taken up by the PAS placed in black housings were no different (Scheffe multiple comparison p = 0.17) from those in shaded housings, but higher (p < 0.001) than those in normal housings. The shaded housing had more PCBs (41 ± 1)% in the bottom segments than the normal and black housing, which had (36 ± 1)% and (37 ± 2)% of PCBs accumulated in the bottom segments.Because all PASs were deployed at the same location, they were exposed to the same wind. The smaller amounts of PCBs accumulating in the middle and top segments of the cylinder in the housings shaded from sunlight could be caused by less heat convection. As expected, the records of the temperature loggers (Fig. S6 and Table S3†) showed that the black housing experienced the highest maximum temperatures, followed by the normal housing and the shaded housing. However, differences were mostly less than 2 °C and occurred only during the day when the samplers were exposed to direct sunshine. For most of the sampling period, there was no difference in the temperatures measured in different housings and thus the temperature differences averaged over the whole sampling period were small (<1 °C). No temperature gradients were observed within any of the sampler housings (Fig. S7†). We conclude that any variation in the sampling rates potentially caused by heat convection is likely so small to be dwarfed by other factors with greater influence on the kinetics of uptake.
Effect of the amount of XAD on axial distribution
In order test to what extent the gradient in the amount of accumulated PCBs is affected by the amount of PSM in a sampler housing, two shelters were set up in both an outdoor (ODM) and indoor setting (L5): one containing a single axially segmented XAD-filled mesh cylinder, and another containing four axially segmented XAD-filled mesh cylinders. The assumption was that the boundary layer thickness around the cylinders is largely unaffected by the number of cylinders within a housing, which is supported by the very minor effect that mesh cylinder diameter has on uptake rate.11In the PASs deployed outdoors, the amount of PCBs that accumulated in the bottom and middle segments of the PSM was identical irrespective of the number of cylinders in the housing. The amount of PCBs in the top segments was somewhat lower (Randomized Block ANOVA with Scheffe's multiple comparisons, Table S4†) when four cylinders shared a single housing. In general, however, the total accumulated amount and the axial distribution of PCBs was hardly affected by the amount of PSM in a housing deployed outdoors (Fig. 1, ODM). This trend was not observed with the PASs deployed indoors, as the amount of PCB accumulated per sampler was significantly lower (Randomized Block ANOVA with Scheffe's multiple comparisons, Table S4†) when four mesh cylinders shared one housing (Fig. 1, L5). Furthermore, the axial PCB concentration gradient was steeper in the four cylinders sharing a housing (51% in the bottom segment) than in the lone mesh cylinder (41% in bottom segment) (Fig. 1, L5). This suggest that in this rather extreme case (very large amount of PSM in a housing placed in a wind still setting) the axial concentration gradient in the XAD was not only a result of lower air turbulence (and therefore a thicker boundary layer) at the top of the housing, but that the top of the sampler housing was indeed “starved” for PCBs, i.e. there was also an axial concentration gradient in the air within the sampler housing.
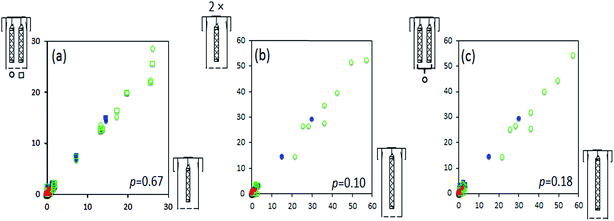
Effect of the amount of XAD on uptake kinetics during field deployments
The sampling campaign in Hawaii served to test whether a starvation effect may limit the uptake of SVOCs in a sampler housing containing multiple cylinders under realistic field deployment conditions. The amount of pesticides, polycyclic aromatic hydrocarbons, and polybrominated diphenyl ethers in each of the two short XAD-filled mesh cylinder sharing a housing was not statistically different (Fig. 3a, p = 0.17) from the amount in the short mesh cylinder that had been placed in a housing by itself. Similarly, the sum of the amounts in the two short mesh cylinders sharing one housing was the same as that in one long mesh cylinder in a single PAS housing (Fig. 3c, p = 0.51). Obviously, this also implies that the amounts in the long mesh cylinder in a single PAS housing were twice as high as those in the short mesh cylinder in a single housing (Fig. 3b). This was expected as the interfacial transfer area between the PSM and the surrounding air, which ultimately determines the sampling rate of the sampler configuration, are identical.11This suggest that under these conditions (twice the amount of PSM in a housing placed outdoors) the starvation effect is negligible, as the existence of such an effect would have resulted in a smaller amounts of SVOCs accumulated in each of the two short XAD filled mesh cylinders that share a single housing. Because the starvation effect is clearly negligible under outdoor sampling condition, it is possible to deploy multiple XAD-filled mesh cylinders in a single PAS housing without compromising the uptake kinetics. This can be beneficial because it may often reduce the number of housings that need to be manufactured and shipped.11
Conclusions
The axial gradient measured within the PSM of the PAS, i.e. the difference in the PCBs amount in the three segments of a XAD-filled mesh cylinder, was variable and decreased in the following sequence: four cylinders in one housing deployed indoors ≥ single cylinder in housing deployed indoors > single cylinder in shaded housing deployed outdoors ≥ single or four cylinders in one housing deployed outdoors > single cylinder in artificially ventilated housing. This means that the gradient depended on the extent of air turbulence at the deployment site (outdoor vs. indoor, ventilated vs. not ventilated) and, if the samplers were deployed indoors, also on the number of mesh cylinders within a housing. The sampler housing reduces the uptake rate of a PAS by reducing air turbulence and thus increasing the thickness of the air boundary layer surrounding the PSM. This effect is not uniform within the sampler housing, but increases with increasing distance from the housing's opening. Only under rather extreme circumstances, namely if a large amount of PSM is placed within a housing in a calm setting, may the housing further cause a “starvation” effect, whereby movement of chemical from the housing's opening to the sheltered parts of the housing becomes rate-limiting and an axial air concentration gradient develops within the sampler housing. However, under regular outdoor conditions, it is possible to place multiple mesh cylinders in a single PAS housing without compromising the amount of SVOC taken up, thus making PAS an even more cost-efficient tool to monitor SVOCs in the ambient atmosphere on a local, regional, and global scale.Acknowledgements
We acknowledge funding from the Natural Sciences and Engineering Research Council of Canada, including an undergraduate summer research fellowship to MH. The Hawaiian fieldwork was supported through a grant to XM by the Centre for Global Change Science of the University of Toronto.References
- J. D. Petty, J. N. Huckins and J. L. Zajicek, Chemosphere, 1993, 27, 1609–1624 CrossRef CAS.
- M. Shoeib and T. Harner, Environ. Sci. Technol., 2002, 36, 4142–4151 CrossRef CAS PubMed.
- F. Wania, L. Shen, Y. D. Lei, C. Teixeira and D. C. G. Muir, Environ. Sci. Technol., 2003, 37, 1352–1359 CrossRef CAS.
- M. Bartkow, K. Booij, K. Kennedy, J. Müller and D. Hawker, Chemosphere, 2005, 60, 170–176 CrossRef CAS PubMed.
- A. A. May, P. Ashman, J. Huang, S. Dhaniyala and T. M. Holsen, Atmos. Environ., 2011, 45, 4354–4359 CrossRef CAS.
- C. Chaemfa, E. Wild, B. Davison, J. L. Barber and K. C. Jones, J. Environ. Monit., 2009, 11, 1135–1139 RSC.
- X. Zhang, M. Tsurukawa, T. Nakano, Y. D. Lei and F. Wania, Environ. Sci. Technol., 2011, 45, 10509–10515 CrossRef CAS PubMed.
- P. Nobel, Plant Physiol., 1974, 54, 177–181 CrossRef CAS.
- S. Tao, Y. Liu, W. Xu, C. Lang, S. Liu, H. Dou and W. Liu, Environ. Sci. Technol., 2007, 41, 568–573 CrossRef CAS PubMed.
- M. A. Abdallah and S. Harrad, Environ. Sci. Technol., 2010, 44, 3059–3065 CrossRef CAS PubMed.
- X. Zhang, C. Wong, Y. D. Lei and F. Wania, Environ. Sci. Technol., 2012, 46, 397–403 CrossRef CAS PubMed.
- C. Schummer, L. Tuduri, O. Briand, B. M. Appenzeller and M. Millet, Environ. Pollut., 2012, 170, 88–94 CrossRef CAS PubMed.
- X. Wang, P. Gong, T. Yao and K. C. Jones, Environ. Sci. Technol., 2010, 44, 2988–2993 CrossRef CAS PubMed.
- S. Baek, S. Choi and Y. Chang, Environ. Sci. Technol., 2011, 45, 4475–4482 CrossRef CAS PubMed.
- Y. M. Li, D. W. Geng, Y. B. Hu, P. Wang, Q. H. Zhang and G. B. Jiang, Chin. Sci. Bull., 2012, 57, 1499–1503 CrossRef CAS.
- C. Shunthirasingham, R. Barra, G. Mendoza, M. Montory, C. E. Oyiliagu, Y. D. Lei and F. Wania, Atmos. Environ., 2011, 45, 303–309 CrossRef CAS.
- X. Y. Zheng, D. Z. Chen, X. D. Liu, Q. F. Zhou, Y. Liu, W. Yang and G. B. Jiang, Chemosphere, 2010, 78, 92–98 CrossRef CAS.
- X. Zhang and F. Wania, Environ. Sci. Technol., 2012, 46, 9563–9570 CrossRef CAS PubMed.
- Dionex, Application Note 347, 2002.
- X. Zhang, T. N. Brown, A. Ansari, B. Yeun, K. Kitaoka, A. Kondo, Y. D. Lei and F. Wania, Environ. Sci. Technol., 2013, 47, 7868–7875 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Figures of axially segmented PCBs concentration in each experimental set-up and further analytical details including method detection limit values, list of target ions monitored and surrogate standards used are included in the ESI. See DOI: 10.1039/c5em00447k |
| This journal is © The Royal Society of Chemistry 2015 |