Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Annual air pollution caused by the Hungry Ghost Festival†
B.
Khezri
ab,
Y. Y.
Chan
ac,
L. Y. D.
Tiong
a and
R. D.
Webster
*abc
aDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore. E-mail: webster@ntu.edu.sg; Fax: +65-6791-1961
bCambridge Centre for Carbon Reduction in Chemical Technology, CARESCAM.CREATE, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459, Singapore
cNEWRI-ECMG, Nanyang Environment and Water Research Institute (NEWRI), 1 Cleantech Loop, Clean Tech One, #06-08, Singapore 637141, Singapore
First published on 16th July 2015
Abstract
Burning of joss paper and incense is still a very common traditional custom in countries with a majority Chinese population. The Hungry Ghost Festival which is celebrated in the 7 month of the Chinese calendar is one of the events where joss paper and incense are burned as offerings. This study investigates the impact of the Ghost Month Festival (open burning event) on air quality by analysis of the chemical composition of particulate matter (PM) and rainwater samples collected during this event, compared with data collected throughout the year, as well as bottom ash samples from burning the original joss paper and incense. The results showed that the change in the chemical composition of the rainwater and PM2.5 (PM ≤ 2.5 μm) atmospheric samples could be correlated directly with burning events during this festival, with many elements increasing between 18% and 60% during August and September compared to the yearly mean concentrations. The order of percentage increase in elemental composition (in rain water and PM2.5) during the Hungry Ghost Festival is as follows: Zn > Ca > K > Mg > Fe > Al > Na ∼ Mn ∼ Ti ∼ V > Cu > As > Ni > Co > Cd > Cr > Pb. The chemical composition of the original source materials (joss paper and incense for combustion) and their associated bottom ash were analysed to explain the impact of burning on air quality.
Environmental impactUncontrolled outdoor burning of paper objects and incense is commonly conducted in countries with Chinese populations as part of religious ceremonies. In this study, we have identified a significant source of atmospheric pollution above normal background levels that occurs in August/September each year, due to the Hungry Ghost Festival. The pollution takes the form of an increase in the metallic composition of particulate matter ≤ 2.5 μm (PM2.5) released into the atmosphere as well as a measurable contamination of rainwater. The compositions of the PM2.5 and rain water were compared with the chemical composition of a range of common materials that are burnt as offerings (Joss paper, incense and papier-mâché) for source apportionment. |
1 Introduction
Knowledge of the concentrations of trace elements in particulate matter is an essential primary step in identifying the source characteristics of air pollution for the development of air quality control strategies and evaluating possible implications for public health. Several epidemiological studies have identified a correlation between the exposure to airborne particles and increased health risk such as mortality, respiratory or cardiovascular diseases, and respiratory symptoms.1Rain plays a role in the removal of gases and particulate matter from the atmosphere, therefore, the study of the chemical composition of rainwater can also aid in understanding the contribution of the different sources of atmospheric pollutants.2 The rainwater composition is often influenced by anthropogenic activities releasing acidic (such as SOx and NOx) or basic (such as NH3) gases into the atmosphere. Acidic rainwater aids the dissolution of many trace metals, which enhances their bioavailability, thus, the study of trace metals in rainwater has increased because of their adverse environmental and human health effects.
An ongoing study was conducted commencing in 2009 to monitor rainwater and airborne particulate matter in a sampling site in western Singapore. One of the interesting features uncovered in this dataset is a local event (common in all countries with a Chinese population), called the Ghost Month, that surprisingly substantially changed the chemical composition of rainwater and particulate matter by increasing the concentrations of a large number of the elements. The Hungry Ghost Month is a traditional festival celebrated by the Chinese in the seventh month of the lunar calendar. They believe that the gates of Hell are opened to allow ghosts and spirits access to the world of the living on the first day of the month. The spirits visit their families, feast and look for victims during this month. The fifteenth day of the seventh month in the lunar calendar is called the Hungry Ghost Festival (Ghost Day/Lu Yan). Ghosts are in a heightened state and it is important to hold a large feast to please them and bring luck to the family. In the last day of this month the ghosts and spirits leave the earth and the gates of Hell finally close. These three important days are celebrated and family members pray to God and for their ancestors, make offerings of food and drink and burn a significant amount of joss paper, hell bank notes and incense throughout Singapore (and other countries with a Chinese population). Fig. 1 illustrates the large scale burning of joss paper in the afternoon of the 15th day of the Ghost Month. Fig. S1 (Groups A, B and C) and Video S1 in the ESI† provide more details about Ghost Month celebrations. In recent years, new “luxury goods” made from joss paper, cardboard or papier-mâché have been introduced into the market for burning, with the items ranging from representations of clothing, computers, cars, houses, jewelry, watches, beer cans and even models of servants.
Despite the high number of burning events, relatively few studies have been performed regarding pollutants released from joss paper and incense which are known to generate large quantities of particulates.3 The burning process has also been shown to release polychlorinated dibenzo-p-dioxin/dibenzofuran (PCDD/F), polycyclic aromatic hydrocarbons (PAH)4 and metals.5
Large scale burning of joss paper and incense often occurs during important outside festivals, although previous studies on joss paper and incense burning have concentrated mainly on assessing the air quality of the indoor/outdoor environments of temples.4a,e,5c,d,6 To the best of the author's knowledge, only one study has been performed on characterizing the ambient air PAH concentrations emitted during a massive joss paper open-burning event held at a suburban site,4c while the elemental compositions emitted from open air burning of joss paper have never been investigated. Consequently, this study was aimed at investigating the impact of outdoor burning of joss paper and incense during the Hungry Ghost Festival on air quality in urban areas. In order to differentiate between the background air pollution of Singapore and the concurrency of the Ghost month with haze events brought about by transboundary air pollution (due to biomass burning in Indonesia), the Ghost Month data were compared with long-term environmental sampling collected over a 5 year period at a site at Nanyang Technological University (NTU). The average PM10 value in Singapore is 40 μg m−3. PM10 values above 60 μg m−3 are typically associated with the transboundary air pollution from biomass burning events (“haze”) from nearby Indonesia. In order to remove uncertainty in the pollution originating from the Ghost festival with coincident pollution from transboundary biomass burning, data from days with PM10 > 60 values were excluded from the comparisons.
The atmospheric PM detected due to the Ghost Month burning events will naturally increase as the sampling device is placed closer to the point of burning. In this study, the sampling site was located away from residential areas so that there was no burning associated with the Hungry Ghost Festival within a 2 km radius (the sampling site location and the closest burning site locations are provided in Fig. S2(b) in the ESI†). We were interested in determining how the Hungry Ghost Festival affected the background pollution levels in airborne particulate matter and rainwater, which required a comparison with data collected from a high volume PM2.5 sampler and rain collection system over a long period. Since no long-term background data are available at the sites used for burning, the NTU site provided the best measure of how background levels varied due to the Hungry Ghost Festival. The increase in elements in the PM2.5 and rainwater observed over the Hungry Ghost Month indicated that the burnt material was dispersed readily into the atmosphere. The environmental data were compared with data collected from unburnt and burnt (ash) samples of commercial joss paper, incense and papier-mâché offerings.
2 Materials and methods
2.1 Sampling
Rainwater (using an autosampler, New Star Environmental LCC, US) and particulate matter (using Ecotech air sampler HiVol-3000, Australia) samples were collected within the campus of Nanyang Technological University, in Singapore, from August 2009 onward. Airborne particulate matter samples were collected on Emfab filters (Borosilicate glass microfibers reinforced with woven glass cloth and bonded with PTFE, size: 8 × 10 inch, Pallflex, Emfab TX40HI20-WW, Pal, USA). This location encompasses different potential sources of anthropogenic activities as it is located approximately 8 km north of the Tuas and Jurong Island districts which are the most industrialized areas of the country, and also lies within 300 m of the Pan Island Expressway (PIE), a major motorway with heavy truck traffic (Fig. S2(c) in the ESI†). The air pollutants encountered in the region are expected to be of both particulate and gaseous in nature, coming from vehicle emissions and industrial activities. Bottom ash samples from burning joss paper and incense were collected immediately after each event in different residential locations in 2012 and 2013 during the Ghost month.2.2 Sample preparation
2.3 Analysis
3 Results and discussion
3.1 Phase I
Phase I of this study involved the continuous collection of rainwater and atmospheric PM2.5 samples between August 2009 and December 2013 at the NTU sampling site (551 PM2.5 and 574 rainwater samples).| Year | Hungry Ghost Festival | Ghost Day |
|---|---|---|
| 2009 | 20 Aug–18 Sep | 2 Sep |
| 2010 | 10 Aug–7 Sep | 24 Aug |
| 2011 | 31 July–28 Aug | 14 Aug |
| 2012 | 17 Aug–15 Sep | 30 Aug |
| 2013 | 7 Aug–4 Sep | 20 Aug |
For PM2.5, the major elements (Na, Ca, Zn, K, Al, and Mg) appeared in concentrations ranging from 5 ng m−3 to a maximum of approximately 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng m−3 (50 μg m−3). The concentrations of trace elements were found to vary from values less than the detection limit to a maximum of 1 μg m−3. Concentration time series graphs were examined to find out the impact of different industrial and local events on the composition of airborne particulate matter at the NTU sampling site. It was notable that the percentage concentrations of a large number of the elements (listed in Table S3 in the ESI†) increased during August and September and this finding was in agreement with rainwater results (see discussion below).
000 ng m−3 (50 μg m−3). The concentrations of trace elements were found to vary from values less than the detection limit to a maximum of 1 μg m−3. Concentration time series graphs were examined to find out the impact of different industrial and local events on the composition of airborne particulate matter at the NTU sampling site. It was notable that the percentage concentrations of a large number of the elements (listed in Table S3 in the ESI†) increased during August and September and this finding was in agreement with rainwater results (see discussion below).
The PM2.5 elemental composition data indicated that the average concentration of many elements during the Ghost Month was higher than their monthly and annual averages. Table S4 in the ESI† contains a comparison of the absolute chemical composition of PM2.5 (μg m−3) during August and September (Ghost Month Festival) and the yearly monthly average.
Fig. 2 presents data for 9 metals that are listed among the 188 hazardous air pollutant substances (“HAPS” or “air toxics”) defined under the Clean Air Act Amendments of 1990 (ref. 7) (Fig. S3 and Tables S3 and S4 in the ESI† include the full list of the analyzed elements). Fig. 2 shows that there is a significant increase during the Ghost Month from 2010 to 2013 (data for 2009 have not been included since the yearly average is not available). It can be seen in Fig. 2 that the majority of the increase in the elemental composition occurred in August, except for the year 2012 where the commencement of the Ghost month was late. September 2011 experienced the lowest impact since the ghost month ended in August for this year.
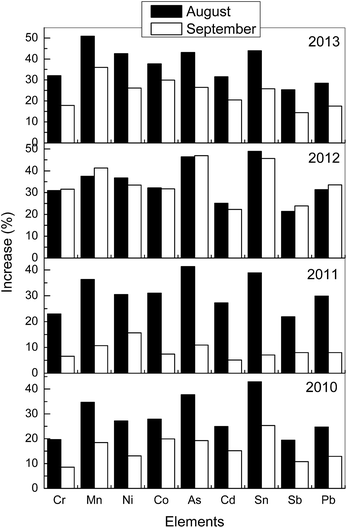 | ||
| Fig. 2 Percentage increase in the elemental chemical composition of PM during August and September (Ghost month festival). | ||
In general, the results indicated that Zn, Ca, K, Mg, Fe and Al followed by Zr > Ba > Sn > V > Na ∼ Ti ∼ Mn ∼ Cu showed the greatest percentage increase in their concentrations (34–60%) while As > W > Sr > Ni > Co > Rb > Cd > Cr > Pb > Mo > Ce > Sb varied between 18 and 40%. The major health related effects of inhalation of many of the listed elements (Na, Al, Pb, Cd, Ni and Cr) to the lungs, kidney and heart are well known and potentially of concern.8
According to the few studies that have been performed regarding the elemental and organic constituents of incense smoke produced through burning, it was found that the major components are inorganic compounds with some carcinogenic elements.5c,9 The Lau and Luk study in 2001 was the only literature source that showed joss paper burning released a significant source of elements such as Fe, Cu, Zn and Pb.10
The results indicated that substantial increase in the concentration of ions and elements in rainwater samples was observed in the beginning, mid and at the end of Ghost month for all years which can be assigned as due to burning large amount of joss paper and incense throughout Singapore (a strong fragrant smoke smell pervades during these days).
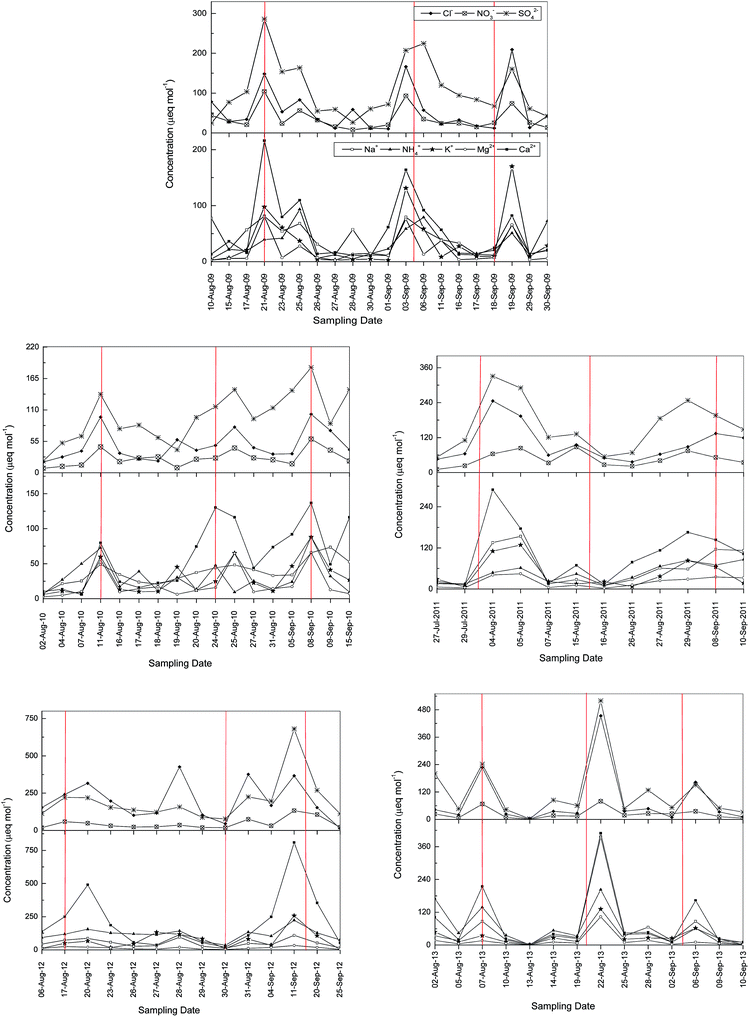
Fig. 3 displays the fluctuation of concentration of the major ionic components of rainwater samples collected during the Ghost month. The red vertical lines in Fig. 3 correspond to the 1st, 15th and final day of the Ghost month for each year as indicated in Table 1. The highest concentration was observed in rain events after the 1st, 15th and end day of the Ghost month due to massive burning of joss paper and incense in those special days. However, the relationship between precipitation events and the Hungry Ghost Festival is complicated because, unlike the PM2.5 data which are collected continuously, the rain frequency does not necessarily perfectly match the days with the highest burning. Nevertheless, the high frequency of rain events in Singapore in the August–September period over the 5 year period did indicate that there is a notable correlation between an increase in the ionic concentration of the precipitation and the major burning events (Fig. 3).
The concentrations of cations and anions both fell significantly after the first or second rain event. The highest ionic concentrations were measured during the Ghost month in 2012 because of the coincidence of the Ghost month and the transboundary haze period. Na+ and Ca2+ are present in much higher concentrations in the cation concentration profile, while K+ shows a higher concentration as an index of biomass burning in year 2012. Mg2+ also shows a high concentration during these events.
It can be seen from the plots in Fig. 3 that there were differences in the absolute concentrations of ions detected in the rainwater from year to year, which is likely to occur due to two major reasons. Firstly, the background levels in the rain samples can vary due to local industrial events unrelated to the Hungry Ghost Festival as well as from transboundary pollution from Indonesia or Malaysia. Secondly, the quantities of materials released into the atmosphere each year due to the festival burning are not the same and will vary depending on the number of fires, the quantities of materials burnt, the exact locations of the fire pits, the period of time that ash is left before clean-up, and the exact meteorological conditions. Therefore, the absolute concentrations are less significant than the percentage increase observed during the times of burning for both the PM2.5 as well as the rain samples.
3.2 Phase II
The second phase of this project was commenced in January 2011 to determine the elemental chemical composition of the unburnt and burnt (bottom ash) samples from joss paper and incense collected during the Ghost month in order to further investigate the link with the annual spikes in pollution in the August/September period (compared to the yearly averages).Hsueh et al.13 studied the composition (Na, Ca, Mg, Al, K, Fe, Cu, Pb, Sr, Mn and Zn) and the distribution of the emitted particles (aerodynamic diameter 0.01–100 μm) during the burning process of joss paper. Their results showed that elements were partly released in emitted particles mostly in the form of PM2.5 (>70% for Na, Al, Pb, and Cu) and smaller particles. Fang et al.3c also demonstrated that the finer particulates (PM2.5) make up the majority of emitted particulates in temples.
According to these findings and the list of the elements presented in this study, the composition of the particulate matter generated from joss paper and incense burning is alarming. In addition, it has been reported that the presence of transition metals (Cu, Fe and Zn) which might act as catalysts increase the formation of some organic chemicals such as polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs).14
It can be seen in Table S5(b)† that there are some differences in the transition metal content (such as Sn, Pb, Cr, Ni and W) between the different joss paper bottom ash samples. This composition difference is likely related to the varying textures and the paint that are used in the different types of the joss papers. Lau and Luk concluded that the pollution composition varied depending on the exact type of the joss paper.10
Analysis of bottom ash data from incenses indicated that incense No. 3 showed higher concentrations of Ba, Cu, Sr, Ga, Zn, Mn and W than the other samples. From Table S5(b) in the ESI,† it can also be concluded that the incense bottom ash has lower concentrations of Cu, Zn, Pb, Cr and Co than the bottom ash from joss papers, while Mn, Ni and As were present in higher concentrations than the bottom ash from joss papers.
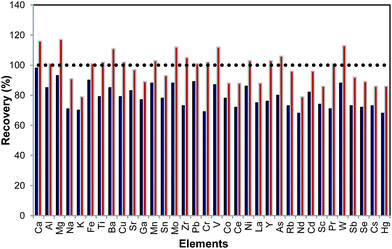
Since there is not any Certified Reference Material (CRM) available for paper/cardboard, recovery tests were performed for both bottom ash and unburnt joss paper and incense to validate the method. An identical trend was observed for various types of joss paper and incense. Table S6 in the ESI† shows the recovery for different types of joss paper and incense that varies from 68 to 98% and from 79 to 123% for bottom ash and unburnt joss paper and incense, respectively. Fig. 4 shows a representative example for bottom ash of joss paper (JP7) which shows that the recovery is often less than 90%, with Na, K and Cr having the lowest recovery. Hsueh et al.13 suggested that bottom ash digestion is more difficult than digesting the unburnt material because of the strong post burning binding energy.
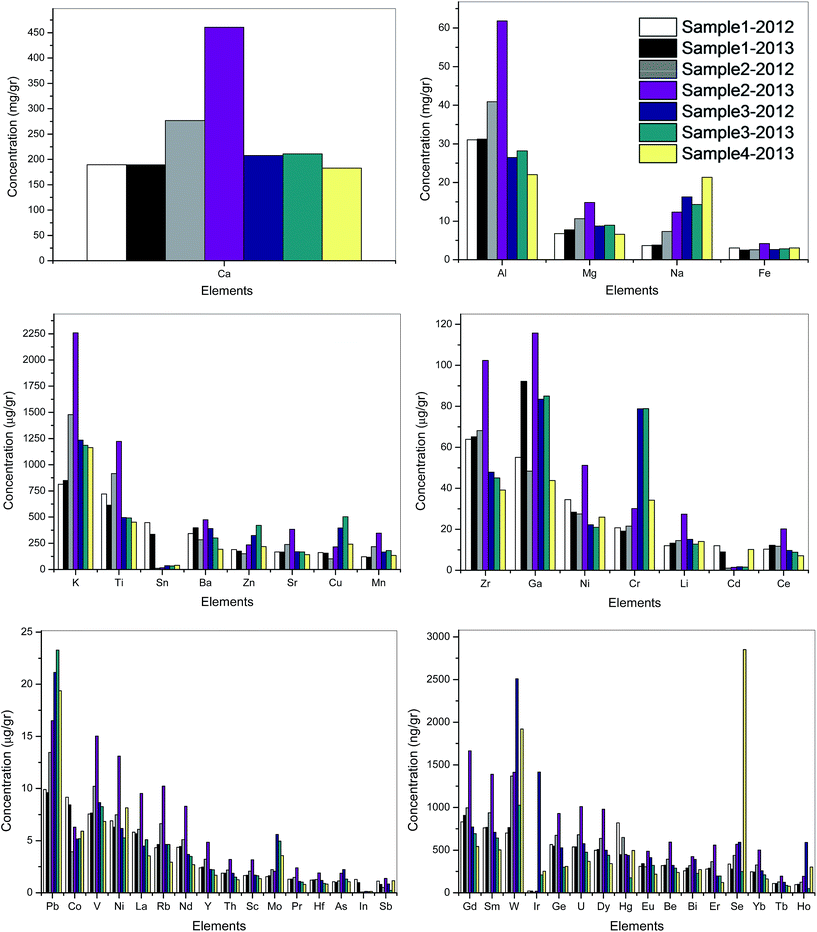 | ||
| Fig. 5 Concentration profiles of bottom ash samples collected during the Hungry Ghost Festival in years 2012 and 2013 in different parts of Singapore. | ||
(a) Ca was present in the highest amount (high mg g−1).
(b) Al, Mg, Na and Fe were present in the next highest concentrations (mg g−1).
(c) K and Ti were the major elements in the third group followed by Ba, Zn, Sr, Cu and Mn. Surprisingly Sn showed very high concentrations (∼400 μg g−1) for the first two samples while for the rest of the samples the Sn concentration varied from 11 to 40 μg g−1, which likely relates to different types of joss paper and incense that were used for burning.
(d) The concentrations of Pb, Co, V, Ni, La, Rb, Nd, Ba, Y, Th, Sc, Mo, Pr, Hf, As, In and Sb were less than 25 μg g−1 and are considered as the moderate concentration.
(e) The last group has been listed as low concentration elements (ng g−1) including Gd, Sm, W, Ir, Ge, U, Dy, Hg, Eu, Be, Bi, Er, Se, Yb, Tb and Ho.
As part of an effort to preserve racial and religious harmony in multicultural Singapore, the National Environmental Agency (NEA) provides burning pits and containers to encourage people to burn joss papers and incense sticks responsibly during festivals. Frequently, the designated containers provided are not sufficient and people resort to burning joss papers and incense sticks on the pavements, grass fields or common corridors at the ground level near their residential high rise buildings.
However cleaners are also deployed to clear up the residual ashes in the neighbourhood on a daily basis; although in some areas the ashes are left inside the containers for the whole month and are blown by the wind. These ashes are treated as normal waste and are disposed in either in incinerators or in an offshore sanitary landfill where the components can penetrate into the groundwater.15 Unfortunately, some people believe that drinking a slurry of burned joss paper mixed with water (water is added to bottom ash) can be used to cure diseases, protect themselves, rejuvenate their relationships and other remedies.16
3.3 Ti Kong Dan Day and Ching Ming Festival
An inspection of the dataset showed that a similar pattern of increase in the metallic composition in both particulate matter and rain water samples was observed for Ti Kong Dan Day (9th day of Chinese New Year, birthday of the Jade Emperor) and Ching Ming Festival (on the 104th day after the winter solstice or the 15th day from the Spring Equinox, usually occurring around April 4th or 5th of the Gregorian calendar) since residents follow the same custom of making burning offerings. However, during these two events the burning mainly takes place in furnaces of the temples. While a large amount of combustion occurs, the impact is much lower than the Hungry Ghost Festival because the burning occurs in closed furnaces equipped with filtration systems.4 Conclusions
A five year continual investigation of rainwater and atmospheric PM2.5 indicated consistently higher concentrations for many elements during August and September, which can be linked to the extensive outdoor burning events that occur during the Hungry Ghost Festival. The individual elements detected in the PM2.5 over the Hungry Ghost period were 18–60% higher than those of the average background.In general, Ca is present in the highest concentration in the unburnt joss paper followed by Al. For incense, Ca is also present in the highest concentration followed by K. The concentration of Cu, Zn, Mn, Mo, Pb, Cr, V, Co, Ni and Cd fluctuated in the different types of joss paper and incense, presumably according to the raw material and production process. The chemical composition and the order of concentration of the elements detected in the bottom ash of the burnt joss paper and incense are similar to those observed for the unburnt joss paper and incense.
A major conclusion of this study is that the pollution generated by open burning of joss paper and incense requires more attention. There is currently a lack of corroborating information about the impact on air quality and air pollutants emitted from open burning of joss paper and incense burning. Furthermore, there are limited literature reports of evidence for epidemiological links between respiratory disease and skin allergies and burning of incense and joss paper in an indoor or outdoor environment. However, unofficially it has been reported that a greater number of patients during this festival period seek treatment for ailments such as asthma, eye irritation, and nasal and skin allergies.17
In order to respect religious practices and not to disturb devotees (pilgrims) bottom ash samples were collected after the festivals. However, it is believed that in order to better investigate the health effects caused by inhalation of particulate matter emitted from burning; the sampling would preferably be carried out in the festival venues at the same time.
Burning joss paper and incense has been part of religious ceremonies for thousands of years but some regulatory monitoring may be required to control air and water pollution and reduce the health effects for the greater good of the entire population. This issue needs government agencies, temple boards, religious leaders, research scientists and private firms working together to reduce the pollution as much as possible. With the utmost respect to this religious practice the concern is about the control of the pollution and care of devotee's health.
Possible solutions have been proposed, including the following:
(a) Increased public education on air quality and the composition of bottom ash (especially if ingested as a slurry).
(b) Providing closed furnace with a filtering system in convenient areas.
(c) Using eco-friendly materials for production of joss paper and incense and control of the production process.
Acknowledgements
This work was supported by the Singapore Government Ministry of Education AcRF Tier 1 research grant (RG 61/11), the Singapore Ministry of Defence-NTU Joint programme (MINDEF-NTU/JPP/12/02/04) grant and the National Research Foundation of Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.References
- (a) J. J. Schauer, M. P. Fraser, G. R. Cass and B. R. T. Simoneit, Environ. Sci. Technol., 2002, 36, 3806–3814 CrossRef CAS; (b) M. Zheng, R. G. Cass, J. J. Schauer and E. S. Edgerton, Environ. Sci. Technol., 2002, 36, 2361–2371 CrossRef CAS; (c) A. Cincinelli, S. Mandorlo, R. M. Dickhut and L. Lepri, Atmos. Environ., 2003, 37, 3125–3133 CrossRef CAS; (d) C. A. Pope, Aerosol Sci. Technol., 2000, 32, 4–14 CrossRef CAS PubMed; (e) C. I. Davidson, R. F. Phalen and P. A. Solomon, Aerosol Sci. Technol., 2005, 39, 737–749 CrossRef CAS PubMed.
- (a) J. N. Galloway, G. E. Likens and M. E. Hawley, Science, 1984, 226, 829–831 CAS; (b) J. L. Moody, A. A. P. Pszenny, A. Gaudry, W. C. Keene, J. N. Galloway and G. Polian, J. Geophys. Res., 1991, 96, 769–786 CrossRef; (c) F. L. T. Gonçalves, M. F. Andrade, M. C. Forti, R. Astolfo, M. A. Ramos, O. Massambani and A. J. Melfi, Environ. Pollut., 2003, 121, 63–73 CrossRef; (d) P. R. Salve, A. Maurya, R. Sinha, A. G. Gawane and S. R. Wate, Bull. Environ. Contam. Toxicol., 2006, 77, 305–311 CrossRef CAS PubMed; (e) A. Malik, V. K. Singh and K. P. Singh, Bull. Environ. Contam. Toxicol., 2007, 79, 639–645 CrossRef CAS PubMed; (f) Y. W. F. Tham and H. Sakugawa, Bull. Environ. Contam. Toxicol., 2007, 79, 670–673 CrossRef CAS PubMed.
- (a) C. S. Li and Y. S. Ro, Atmos. Environ., 2000, 34, 611–620 CrossRef CAS; (b) C. W. Fan and J. J. Zhang, Atmos. Environ., 2001, 35, 1281–1290 CrossRef CAS; (c) G. C. Fang, C. N. Chang, Y. S. Wu, C. J. Yang, S. C. Chang and I. L. Yang, Sci. Total Environ., 2002, 299, 79–87 CrossRef CAS.
- (a) H. H. Yang, R. C. Jung, Y. F. Wang and L. T. Hsieh, Atmos. Environ., 2005, 39, 3305–3312 CrossRef CAS PubMed; (b) C. C. Lin, S. J. Chen, K. L. Huang, W. J. Lee, W. Y. Lin, J. H. Tsai and H. C. Chaung, Environ. Sci. Technol., 2008, 42, 4229–4235 CrossRef CAS; (c) M. D. Lin, J. Y. Rau, H. H. Tseng, M. Y. Wey, C. W. Chu, Y. H. Lin, M. C. Wei and C. H. Lee, J. Hazard. Mater., 2008, 156, 223–229 CrossRef CAS PubMed; (d) S. C. Lung and S. C. Hu, Chemosphere, 2003, 50, 673–679 CrossRef CAS; (e) T. C. Lin, F. H. Chang, J. H. Hsieh, H. R. Chao and M. R. Chao, J. Hazard. Mater., 2002, 95, 1–12 CrossRef CAS; (f) G. C. Fang, Y. S. Wu, M. H. Chen, T. T. Ho and J. Y. Rau, Atmos. Environ., 2004, 38, 3385–3391 CrossRef CAS PubMed.
- (a) R. Caggiano, M. D'Emilio, M. Macchiato and M. Ragosta, Environ. Monit. Assess., 2005, 102, 67–84 CrossRef CAS; (b) F. Eldabbagh, A. Ramesh, J. Hawari, W. Hutny and J. A. Kozinski, Combust. Flame, 2005, 142, 249–257 CrossRef CAS PubMed; (c) G. C. Fang, C. N. Chang, C. C. Chu, Y. S. Wu, P. P. C. Fu, S. C. Chang and I. L. Yang, Chemosphere, 2003, 51, 983–991 CrossRef CAS; (d) O. W. Lau and S. F. Luk, Atmos. Environ., 2001, 35, 3113–3120 CrossRef CAS.
- B. Wang, S. C. Lee, K. F. Ho and Y. M. Kang, Sci. Total Environ., 2007, 377, 52–60 CrossRef CAS PubMed.
- http://www.epa.gov/airtoxics/orig189.html .
- (a) M. Costa, Human Exposures and Their Health Effects, ed. M. Lippmam, John Wiley & Sons, Inc, Hoboken, NJ, USA, 2nd edn, 2000, pp. 811–850 Search PubMed; (b) S. G. Donkin, D. L. Ohlson, C. M. Teaf, Properties and effects of metals, Principles of toxicology: environmental and industrial applications, ed. P. L. Williams, R. C. James and S. M. Roberts, John Wiley & Sons, Inc, Hoboken, NJ, USA, 2nd edn, 2000, pp. 325–344 Search PubMed; (c) M. E. Gerlofs-Nijland, M. Rummelhard, A. J. F. Boere, D. L. Leseman, R. Duffin, R. P. F. Schins, P. J. A. Borm, M. Sillanpaa, R. O. Salonen and F. R. Cassee, Environ. Sci. Technol., 2009, 43, 4729–4736 CrossRef CAS; (d) S. K. Park, M. S. ONeill, P. S. Vokonas, D. Sparrow, R. O. Wright, B. Coull, H. Nie, H. Hu and J. Schwartz, Epidemiology, 2008, 19, 111–120 CrossRef PubMed.
- (a) R. E. Rasmussen, Bull. Environ. Contam. Toxicol., 1987, 38, 827–833 CrossRef CAS; (b) C. Y. Mimi, D. H. Garabran, T. B. Huan and B. E. Henderson, Int. J. Cancer, 1990, 45, 1033–1039 CrossRef PubMed.
- O. W. Lau and S. F. Luk, Atmos. Environ., 2001, 35, 3113–3120 CrossRef CAS.
- (a) C. Tortajada, Int. J. Water Resour. Environ. Eng., 2006, 22, 227–240 Search PubMed; (b) I. O. B. Luan, Int. J. Water Resour. Environ. Eng., 2010, 26, 65–80 Search PubMed.
- B. Khezri, H. Mo, Z. Yan, S.-L. Chong, A. K. Heng and R. D. Webster, Atmos. Environ., 2013, 80, 352–360 CrossRef CAS PubMed.
- H. T. Hsueh, T. H. Ko, W. C. Chou, W. C. Hung and H. Chu, Environ. Chem. Lett., 2012, 10, 79–87 CrossRef CAS.
- M. T. Hu, S. J. Chen, Y. C. Lai, K. L. Huang, G. P. Chang-Chien and J. H. Tsai, Aerosol Air Qual. Res., 2009, 9, 369–377 CAS.
- (a) J. Robson, Hist. Religions, 2008, 48, 130–169 CrossRef PubMed; (b) Taoism, ed. Z. Mou, (Trans: J. Pan, S. Normand), Leiden [The Netherlands], Boston, Brill, 2012, pp. 293–308 Search PubMed.
- (a) http://app2.nea.gov.sg/energy-waste/waste-management/overview ; (b) http://app2.nea.gov.sg/energy-waste/waste-management/solid-waste-management-infrastructure .
- http://www.healthxchange.com.sg/News/Pages/More-ailments-during-Hungry-Ghost-Festival.aspx .
Footnote |
| † Electronic supplementary information (ESI) available: Full tables of elemental data, maps of sampling locations, instrumental parameters used for analysis and photographs of materials for burning. See DOI: 10.1039/c5em00312a |
| This journal is © The Royal Society of Chemistry 2015 |