Open Access Article
Open Access ArticleHierarchical tubular structures constructed from ultrathin TiO2(B) nanosheets for highly reversible lithium storage†
Han
Hu
a,
Le
Yu
a,
Xuehui
Gao
b,
Zhan
Lin
*b and
Xiong Wen (David)
Lou
*a
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459. E-mail: xwlou@ntu.edu.sg; davidlou88@gmail.com; Web: http://www.ntu.edu.sg/home/xwlou/
bInstitute of Chemical Engineering, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, Zhejiang 310027, China. E-mail: zhanlin@zju.edu.cn
First published on 20th March 2015
Abstract
Hierarchical tubular structures constructed from TiO2(B) nanosheets were fabricated by an efficient Cu nanowire templated solvothermal method. A dense layer of TiO2(B) nanosheets was grown on the Cu nanowires during the initial stage of the reaction. The Cu nanowires were then gradually dissolved during the later stage of the prolonged reaction. The as-obtained TiO2(B) hierarchical tubes showed a large surface area and good shell permeability. When evaluated for electrochemical lithium storage properties, these TiO2(B) hierarchical tubular structures showed a high specific capacity, excellent rate performance and long-term cycling stability.
Broader contextLithium ion batteries (LIBs) have become the dominant power source for portable electronics and have great potential to power electrical vehicles in the near future. To meet the requirements for use in high-power applications, battery electrodes that can deliver capacitor-like rate-performances are in high demand. TiO2(B) with favorable open channels allows lithiation/delithiation in an interesting pseudocapacitive manner. The electrochemical performance of TiO2(B) electrodes can be effectively enhanced by engineering nanostructures. However, TiO2(B) is a less stable phase and it is highly challenging to synthesize TiO2(B) electrode materials with desired nanostructures. In this work, we report the synthesis of hierarchical tubular structures constructed from TiO2(B) nanosheets through an efficient Cu nanowire templated solvothermal method. Benefiting from unique structural features, the as-obtained TiO2(B) hierarchical tubular structures show excellent electrochemical lithium storage properties with a high capacity, excellent rate capability and cycling stability. |
Owing to the fabulous architectures and tunable properties, hollow micro-/nanostructures have been demonstrated to have great potential in a myriad of applications, such as drug delivery, nanoreactors and energy storage and conversion devices.1–9 Over the past few decades, numerous projects have been dedicated to developing reliable methods for the controllable synthesis of hollow structures with remarkable progress achieved.2,3,5 In a normal templating method, removal of the templates by methods like etching in harsh solutions, calcination at high temperature or dissolution in organic solvents is simple in concept, however, practically it requires special care as deformation and collapse of the shells will otherwise occur.3,10,11 In this regard, efficient syntheses of hollow structures with alternative templates that impose less influence on the shell materials when removed are highly desirable. For example, anatase TiO2 nanocages have been synthesized by templating against Cu2O polyhedral particles, which are conveniently eliminated by mild HCl etching while the shells remain intact.12
Titanium dioxide (TiO2) has been intensively studied as one of the most prominent anode materials for lithium-ion batteries (LIBs) because of the greatly improved safety and high rate capability.13–16 Among all the polymorphs, TiO2(B) possesses favorable open channels, which facilitate lithiation/delithiation in an interesting pseudocapacitive manner, rather than the solid-state diffusion process observed in anatase TiO2.17–21 As such, the theoretical capacity of TiO2(B) (335 mA h g−1) is much higher than that of anatase TiO2 (∼170 mA h g−1), and comparable to the value of commercial graphite anodes.22–24 Besides, theoretical and experimental studies have revealed a morphological dependence of the lithium insertion mechanism where the electrochemical lithium storage performance of TiO2(B) nanosheets growing along the ab plane is superior to that of other nanostructures such as nanowires, nanoparticles and nanobelts.25 Motivated by the improved lithium storage capability of hollow structures with metal oxide nanosheets as building blocks,26,27 hollow structures constructed from TiO2(B) nanosheets with a large surface area and good shell permeability are highly anticipated to exhibit enhanced lithium storage properties. However, TiO2(B) is a less stable phase of TiO2 that will easily undergo irreversible phase transitions, which makes it difficult to construct TiO2(B) hollow structures.28 Thus, successful examples of hierarchical hollow structures constructed from TiO2(B) nanosheets have not been reported previously to the best of our knowledge.
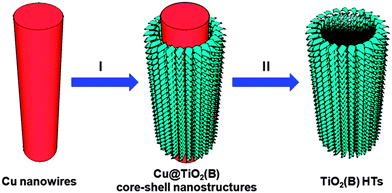
Herein, we report a template-engaged synthesis of hierarchical tubular structures constructed from TiO2(B) nanosheets (denoted as TiO2(B) HTs) through an efficient Cu nanowire templated solvothermal method. After initial deposition of the TiO2(B) shells against the Cu nanowires, the Cu nanowires were simultaneously removed during the later stage of the solvothermal reaction. Thus, the influence on the shells during the template removing process has been minimized. When evaluated for lithium storage properties, these TiO2(B) HTs showed a greatly enhanced electrochemical performance.
The synthesis strategy is schematically depicted in Fig. 1. In this method, highly uniform Cu nanowires with smooth surfaces (Fig. S1, see ESI†) synthesized by a hydrothermal process29 were employed as templates. The Cu nanowires were first uniformly coated with a layer of TiO2(B) through a solvothermal process with ethylene glycol as the solvent and TiCl3 as the precursor.30 By precisely controlling the reaction time, well-defined TiO2(B) nanosheets were formed, giving rise to Cu@TiO2(B) core–shell structures. Owing to their high reactivity, the Cu nanowires were gradually oxidized and removed in the acidic solution during the prolonged solvothermal treatment. During the whole process, the robust shells remained largely intact. As such, an interesting evolution from core–shell structures to hierarchical tubes could be observed with different solvothermal durations. The as-obtained TiO2(B) HTs were further annealed in air to remove the organic species adsorbed on the nanosheets (Fig. S2, see ESI†) and also to improve the crystallinity at the same time.
The crystal structure of the as-prepared TiO2(B) HTs was determined by X-ray diffraction (XRD). As shown in Fig. S3a (see ESI†), the peaks for (001), (110), (002) and (020) can be indexed to the TiO2(B) phase (JCPDS card No. 46-1237).31 On the other hand, the peaks associated with Cu (Fig. S1a, see ESI†) were totally absent, indicating the complete removal of the templates, which was also confirmed by X-ray photoelectron spectroscopy (XPS) measurements (Fig. S4, see ESI†). The phase purity of the TiO2(B) HTs was further supported by Raman spectrum (Fig. S3b, see ESI†) in which all the vibrational modes are in good agreement with previous studies on TiO2(B).28 After calcination, the organic species were fully removed (Fig. S5a, see ESI†) without deteriorating the phase purity, as confirmed by the XRD pattern shown in Fig. 2a. Panoramic field-emission scanning electron microscope (FESEM) images (Fig. 2b and Fig. S5b, see ESI†) show that the annealed product consisted entirely of uniform one-dimensional (1D) nanostructures. Compared to the original Cu nanowires (Fig. S1, see ESI†), both the surface roughness and diameter of these 1D nanostructures were increased significantly, indicative of the successful deposition of TiO2(B) shells. The enlarged FESEM image (Fig. 2c) reveals that these 1D structures were constructed from randomly oriented nanosheets. Some broken shells in the FESEM images (Fig. 2c and Fig. S5c, see ESI†) reveal the hollow interior. The hollow interior was further examined by TEM. From the TEM images (Fig. 2d and e and Fig. S5d, see ESI†), the thickness of the shell was estimated to be around 50 nm. From the high-resolution TEM image in Fig. 2f, it can be clearly observed that the shell was constructed from nanosheets with a thickness of only a few nanometers. The d-spacing for the lattice fringe perpendicular to the sheet plane was determined to be about 0.36 nm, corresponding to the (110) lattice plane.22,23 This observation indicates that the nanosheets grew along the ab plane as the growth along the c direction was hindered by the selectively adsorbed organic species.22,23,28
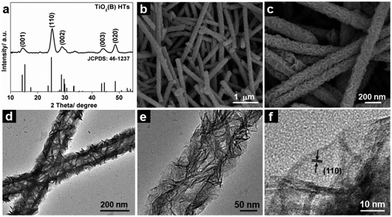 | ||
| Fig. 2 XRD pattern (a), FESEM images (b, c), and TEM images (d–f) of the TiO2(B) HTs after annealing at 350 °C for 2 h. | ||
Time dependent experiments were conducted to investigate the structural evolution from Cu@TiO2(B) core–shell structures to TiO2(B) HTs. FESEM and TEM were used to characterize the products with different solvothermal durations. A uniform TiO2(B) layer was formed after solvothermal treatment for 6 h (Fig. S6, see ESI†). However, well-defined nanosheets could only be observed with a duration longer than 9 h. As shown in Fig. 3a and Fig. S7 (see ESI†), a layer of TiO2(B) nanosheets was uniformly coated on the Cu nanowires with a solvothermal duration of 9 h, producing core–shell structures. After that, there were little morphological changes observed in the TiO2(B) shells from FESEM observations with solvothermal treatments for up to 24 h (Fig. S7–S9, see ESI†). Nevertheless, TEM images reveal an interesting hollowing process where the interior cavities started to appear after reaction for 12 h (Fig. 3b) and the structure became fully hollow after reaction for 24 h (Fig. 3c), giving rise to the TiO2(B) HTs. The sequential processes of TiO2 shell formation and selective Cu core removal may be associated with the different redox potentials of the Ti4+/Ti3+ pair (−0.67 V vs. standard hydrogen electrode (SHE)) and Cu+/Cu pair (0.52 V vs. SHE) or Cu2+/Cu pair (0.337 V vs. SHE).32,33 The Ti3+ species is more prone to oxidization, leading to the fast formation of TiO2(B) shells. On the other hand, the existence of Ti3+ may also protect the Cu core from being oxidized, which allows the Cu@TiO2 core–shell structures to be preserved before the complete oxidation of Ti3+. Afterwards, the Cu cores can be gradually oxidized by dissolved oxygen and the oxidized Cu will be quickly etched away in the acidic solution. It is interesting to note that the Cu nanowires were etched into discontinuous particles during the initial hollowing process (Fig. 3b). Finally, highly porous TiO2(B) HTs were formed, which exhibit a high surface area of 141 m2 g−1 and porosity of 0.746 cm3 g−1 (Fig. S10, see ESI†).
 | ||
| Fig. 3 TEM images of the products with different solvothermal durations: (a) 9 h, (b) 12 h, (c) 24 h. | ||
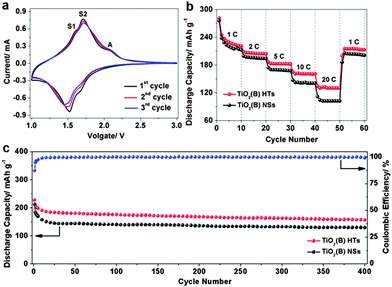
To evaluate the lithium storage performance of the annealed TiO2(B) HTs, various electrochemical measurements were carried out. Fig. 4a shows the cyclic voltammetry (CV) curves at a scan rate of 1.0 mV s−1. The S peaks associated with pseudocapacitive lithium storage behaviour are quite distinctive in the potential range of 1.5–1.7 V, while the A peaks assigned to the solid-state lithium diffusion in anatase at 1.7/2.0 V are less pronounced. This observation indicates the high purity of the TiO2(B) phase and that the lithiation/delithiation processes mainly proceed in a pseudocapacitive manner.22,23,34,35 Typical discharge–charge profiles of the TiO2(B) HTs at a current rate of 1 C (1 C = 335 mA g−1) over a voltage window of 1–3 V are displayed in Fig. S11 (see ESI†). The initial discharge and charge capacities were 290 and 240 mA h g−1, respectively. The unique architecture and high phase-purity endow the TiO2(B) HTs with a fast response to continuously varying current rates. As shown in Fig. 4b, high capacities of 216, 202, 182, 160 and 130 mA h g−1 can be delivered at current rates of 1, 2, 5, 10 and 20 C, respectively. After cycling at high rates, a capacity of 210 mA h g−1 can be restored when reducing the current rate back to 1 C (Fig. 4b and Fig. S12a, see ESI†). The electrochemical lithium storage performance of TiO2(B) nanospheres (NSs) synthesized under identical conditions except for the addition of Cu nanowires28,30 (Fig. S13, see ESI†) was also evaluated. The capacities of these TiO2(B) NSs at low current rates were close to those of the TiO2(B) HTs, but much lower at high current rates (Fig. 4b and Fig. S12b, see ESI†). Moreover, the rate performance of the TiO2(B) HTs is superior to that of many previously reported TiO2(B) nanostructures (Fig. S14, see ESI†).20,34,36,37 The cycling performance was tested for hundreds of cycles. Despite the decay in the initial few cycles, the capacity of the TiO2(B) HTs became stable and remained at 160 mA h g−1 after 400 cycles at a current rate of 5 C. This is superior to that of the TiO2(B) NSs (Fig. 4c and Fig. S15, see ESI†). A similar cycling performance was also demonstrated at a current rate of 10 C (Fig. S16, see ESI†). Electrochemical impedance spectroscopy (EIS) studies (Fig. S17, see ESI†) of the cells based on these two electrodes revealed that the TiO2(B) HTs showed a reduced diameter of the semicircle in the high-frequency region, indicative of lower resistance. Evidently, the superior electrochemical performance of the TiO2(B) HTs is related to the unique structural features. On the one hand, the porous shells constructed from randomly oriented nanosheets enlarge the electrolyte–electrode interface. The greatly reduced thickness of the nanosheets shortens the diffusion length for Li+ ions, which allows faster lithiation/delithiation.25,31 Furthermore, the interior cavities can efficiently accommodate the volume variation during the repeated lithium intake–extract processes. A post-mortem study revealed that the structure of these TiO2(B) HTs was largely retained after being discharged–charged over 400 cycles (Fig. S18, see ESI†). As such, these TiO2(B) HTs deliver excellent electrochemical lithium storage properties in terms of high capacity, good rate capacity and stable cycling performance.
In summary, an efficient template-assisted solvothermal method has been developed to synthesize TiO2(B) hierarchical tubular structures. The uniform deposition of TiO2(B) shells against Cu nanowires and the subsequent gradual dissolution of the Cu cores can be sequentially observed with different solvothermal durations. The as-prepared TiO2(B) hierarchical tubes were constructed from ultrathin nanosheets with a high surface area. Because of the unique structural features, these TiO2(B) hierarchical tubular structures deliver a high lithium storage capacity, remarkable rate performance and long-term cycling stability when evaluated as anodes for LIBs.
Notes and references
- M. H. Oh, T. Yu, S. H. Yu, B. Lim, K. T. Ko, M. G. Willinger, D. H. Seo, B. H. Kim, M. G. Cho, J. H. Park, K. Kang, Y. E. Sung, N. Pinna and T. Hyeon, Science, 2013, 340, 964 CrossRef CAS PubMed.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604 CAS.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- J. Liu, H. Q. Yang, F. Kleitz, Z. G. Chen, T. Y. Yang, E. Strounina, G. Q. Lu and S. Z. Qiao, Adv. Funct. Mater., 2012, 22, 591 CrossRef CAS.
- J. Hu, M. Chen, X. S. Fang and L. W. Wu, Chem. Soc. Rev., 2011, 40, 5472 RSC.
- Z. H. Dong, X. Y. Lai, J. E. Halpert, N. L. Yang, L. X. Yi, J. Zhai, D. Wang, Z. Y. Tang and L. Jiang, Adv. Mater., 2012, 24, 1046 CrossRef CAS PubMed.
- J. W. Nai, Y. Tian, X. Guan and L. Guo, J. Am. Chem. Soc., 2013, 135, 16082 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. Lou, X. R. Xing and G. Q. Lu, Chem. Commun., 2011, 47, 12578 RSC.
- J. B. Joo, M. Dahl, N. Li, F. Zaera and Y. D. Yin, Energy Environ. Sci., 2013, 6, 2082 CAS.
- Z. Z. Yang, Z. W. Niu, Y. F. Lu, Z. B. Hu and C. C. Han, Angew. Chem., Int. Ed., 2003, 42, 1943 CrossRef CAS PubMed.
- S. S. Feng, W. Li, Q. Shi, Y. H. Li, J. C. Chen, Y. Ling, A. M. Asiri and D. Y. Zhao, Chem. Commun., 2014, 50, 329 RSC.
- Z. Y. Wang and X. W. Lou, Adv. Mater., 2012, 24, 4124 CrossRef CAS PubMed.
- D. Deng, M. G. Kim, J. Y. Lee and J. Cho, Energy Environ. Sci., 2009, 2, 818 CAS.
- X. F. Li and C. L. Wang, J. Mater. Chem. A, 2013, 1, 165 CAS.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Y. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124 CrossRef CAS PubMed.
- Y. Zhao, X. F. Li, B. Yan, D. J. Li, S. Lawes and X. L. Sun, J. Power Sources, 2015, 274, 869 CrossRef CAS PubMed.
- A. G. Dylla, G. Henkelman and K. J. Stevenson, Acc. Chem. Res., 2013, 46, 1104 CrossRef CAS PubMed.
- Y. Ren, Z. Liu, F. Pourpoint, A. R. Armstrong, C. P. Grey and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 2164 CrossRef CAS PubMed.
- H. S. Liu, Z. H. Bi, X. G. Sun, R. R. Unocic, M. P. Paranthaman, S. Dai and G. M. Brown, Adv. Mater., 2011, 23, 3450 CrossRef CAS PubMed.
- A. R. Armstrong, G. Armstrong, J. Canales, R. Garcia and P. G. Bruce, Adv. Mater., 2005, 17, 862 CrossRef CAS.
- A. R. Armstrong, G. Armstrong, J. Canales and P. G. Bruce, Angew. Chem., Int. Ed., 2004, 43, 2286 CrossRef CAS PubMed.
- S. H. Liu, H. P. Jia, L. Han, J. L. Wang, P. F. Gao, D. D. Xu, J. Yang and S. N. Che, Adv. Mater., 2012, 24, 3201 CrossRef CAS PubMed.
- S. H. Liu, Z. Y. Wang, C. Yu, H. B. Wu, G. Wang, Q. Dong, J. S. Qiu, A. Eychmuller and X. W. Lou, Adv. Mater., 2013, 25, 3462 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- A. G. Dylla, P. H. Xiao, G. Henkelman and K. J. Stevenson, J. Phys. Chem. Lett., 2012, 3, 2015 CrossRef CAS.
- L. Zhang, G. Q. Zhang, H. B. Wu, L. Yu and X. W. Lou, Adv. Mater., 2013, 25, 2589 CrossRef CAS PubMed.
- J. X. Zhu, Z. Y. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Y. Yan, Energy Environ. Sci., 2013, 6, 987 CAS.
- G. L. Xiang, Y. G. Wang, J. Li, J. Zhuang and X. Wang, Sci. Rep., 2013, 3, 1411 Search PubMed.
- M. Mohl, P. Pusztai, A. Kukovecz, Z. Konya, J. Kukkola, K. Kordas, R. Vajtai and P. M. Ajayan, Langmuir, 2010, 26, 16496 CrossRef CAS PubMed.
- G. L. Xiang, T. Y. Li, J. Zhuang and X. Wang, Chem. Commun., 2010, 46, 6801 RSC.
- V. Etacheri, J. E. Yourey and B. M. Bartlett, ACS Nano, 2014, 8, 1491 CrossRef CAS PubMed.
- Z. Y. Wang, D. Y. Luan, S. Madhavi, C. M. Li and X. W. Lou, Chem. Commun., 2011, 47, 8061 RSC.
- R. Nakamura, A. Okamoto, H. Osawa, H. Irie and K. Hashimoto, J. Am. Chem. Soc., 2007, 129, 9596 CrossRef CAS PubMed.
- C. Wessel, L. A. Zhao, S. Urban, R. Ostermann, I. Djerdj, B. M. Smarsly, L. Q. Chen, Y. S. Hu and S. Sallard, Chem. – Eur. J., 2011, 17, 775 CrossRef CAS PubMed.
- J. M. Li, W. Wan, H. H. Zhou, J. J. Li and D. S. Xu, Chem. Commun., 2011, 47, 3439 RSC.
- J. Qu, J. E. Cloud, Y. A. Yang, J. N. Ding and N. Y. Yuan, ACS Appl. Mater. Interfaces, 2014, 6, 22199 CAS.
- H. Huang, J. W. Fang, Y. Xia, X. Y. Tao, Y. P. Gan, J. Du, W. J. Zhu and W. K. Zhang, J. Mater. Chem. A, 2013, 1, 2495 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, additional FESEM images, TEM images, EDX spectra, TG curves, XRD patterns, Raman and XPS spectra, N2 adsorption–desorption isotherm, charge–discharge voltage curves, EIS patterns, CV curves and cycling performances of the TiO2(B) HTs and TiO2(B) NSs. See DOI: 10.1039/c5ee00101c |
| This journal is © The Royal Society of Chemistry 2015 |