Can a pentamethylcyclopentadienyl ligand act as a proton-relay in f-element chemistry? Insights from a joint experimental/theoretical study†
Abstract
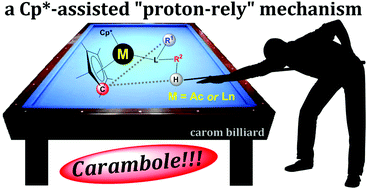
Isomerisation of buta-1,2-diene to but-2-yne by (Me5C5)2Yb is a thermodynamically favourable reaction, with the ΔrG° estimated from experimental data at 298 K to be −3.0 kcal mol−1. It proceeds in hydrocarbon solvents with a pseudo first-order rate constant of 6.4 × 10−6 s−1 and 7.4 × 10−5 s−1 in C6D12 and C6D6, respectively, at 20 °C. This 1,3-hydrogen shift is formally forbidden by symmetry and has to occur by an alternative pathway. The proposed mechanism for buta-1,2-diene to but-2-yne isomerisation by (Me5C5)2Yb involves coordination of methylallene (buta-1,2-diene) to (Me5C5)2Yb, and deprotonation of methylallene by one of the Me5C5 ligands followed by protonation of the terminal methylallenyl carbon to yield the known coordination compound (Me5C5)2Yb(η2-MeC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe). Computationally, this mechanism is not initiated by a single electron transfer step, and the ytterbium retains its oxidation state (II) throughout the reactivity. Experimentally, the influence of the metal centre is discussed by comparison with the reaction of (Me5C5)2Ca towards buta-1,2-diene, and (Me5C5)2Yb with ethylene. The mechanism by which the Me5C5 acts as a proton-relay within the coordination sphere of a metal also rationalises the reactivity of (i) (Me5C5)2Eu(OEt2) with phenylacetylene, (ii) (Me5C5)2Yb(OEt2) with phenylphosphine and (iii) (Me5C5)2U(NPh)2 with H2 to yield (Me5C5)2U(HNPh)2. In the latter case, the computed mechanism is the heterolytic activation of H2 by (Me5C5)2U(NPh)2 to yield (Me5C5)2U(H)(HNPh)(NPh), followed by a hydrogen transfer from uranium back to the imido nitrogen atom using one Me5C5 ligand as a proton-relay. The overall mechanism by which hydrogen shifts using a pentamethylcyclopentadienyl ligand as a proton-relay is named Carambole in reference to carom billiards.
CMe). Computationally, this mechanism is not initiated by a single electron transfer step, and the ytterbium retains its oxidation state (II) throughout the reactivity. Experimentally, the influence of the metal centre is discussed by comparison with the reaction of (Me5C5)2Ca towards buta-1,2-diene, and (Me5C5)2Yb with ethylene. The mechanism by which the Me5C5 acts as a proton-relay within the coordination sphere of a metal also rationalises the reactivity of (i) (Me5C5)2Eu(OEt2) with phenylacetylene, (ii) (Me5C5)2Yb(OEt2) with phenylphosphine and (iii) (Me5C5)2U(NPh)2 with H2 to yield (Me5C5)2U(HNPh)2. In the latter case, the computed mechanism is the heterolytic activation of H2 by (Me5C5)2U(NPh)2 to yield (Me5C5)2U(H)(HNPh)(NPh), followed by a hydrogen transfer from uranium back to the imido nitrogen atom using one Me5C5 ligand as a proton-relay. The overall mechanism by which hydrogen shifts using a pentamethylcyclopentadienyl ligand as a proton-relay is named Carambole in reference to carom billiards.
- This article is part of the themed collection: Dalton Discussion 14: Advancing the chemistry of the f-elements

 Please wait while we load your content...
Please wait while we load your content...