Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot reductive amination of aldehydes with nitroarenes over an Au/Al2O3 catalyst in a continuous flow reactor†
E. A.
Artiukha
ab,
A. L.
Nuzhdin
*ab,
G. A.
Bukhtiyarova
a,
S. Yu.
Zaytsev
bc,
P. E.
Plyusnin
bc,
Yu. V.
Shubin
bc and
V. I.
Bukhtiyarov
ab
aBoreskov Institute of Catalysis, SB RAS, Novosibirsk, 630090, Russia. E-mail: anuzhdin@catalysis.ru
bResearch and Educational Center for Energy Efficient Catalysis, Novosibirsk National Research University, Novosibirsk, 630090, Russia
cNikolaev Institute of Inorganic Chemistry, SB RAS, Novosibirsk, 630090, Russia
First published on 21st July 2015
Abstract
One-pot reductive amination of aromatic and aliphatic aldehydes with nitroarenes over an Au/Al2O3 catalyst in a continuous flow reactor using molecular hydrogen as a reducing agent was performed. Various secondary aromatic amines were obtained in good to excellent yields.
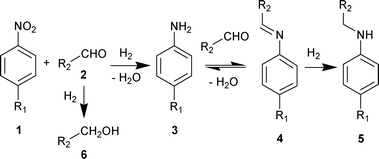
Secondary amines are important intermediates in the synthesis of agrochemicals, pharmaceuticals and other chemical products.1 Primary amines are usually employed as starting materials for the synthesis of secondary amines through reactions with alkyl halides,1b,2a alcohols2 and carbonyl compounds.3 The growing interest in these products induces continuous efforts to develop more efficient and environmentally friendly methods for the synthesis of secondary amines with high selectivity. Recently, one-pot reductive amination of aldehydes directly with nitroarenes has been described,4–7 where several steps are carried out using the same reaction vessel and catalyst under hydrogenation conditions. This process does not require preliminary reduction of nitroarenes, avoiding intermediate separation and purification steps, and minimizes waste production due to the use of molecular hydrogen as a reducing agent. In general, reductive amination of aldehydes with nitroarenes proceeds through three consecutive reactions: hydrogenation of nitroarene into primary aromatic amine, condensation of amine with aldehyde to imine and hydrogenation of imine to corresponding secondary amine.4a,f,5–7
Syntheses of secondary amines through reductive amination of carbonyl compounds with nitroarenes have been examined over Pd–Ag bimetallic nanoparticles,4a Pt nanowires,4b MOF-supported Pt, Pd and Ir nanoparticles,5 gold catalysts6 and Pd nanoparticles supported on different materials, such as carbon,4c,d polymers,4e gum,4f,g SiO2,4h and Fe3O4.4i,j Recently, reductive amination of aldehydes with nitroarenes over carbon-supported iron and cobalt oxide catalysts has been described, but the proceeding of the reactions at high temperature and hydrogen pressure for 24 hours provided a secondary amine yield not higher than 80%.7
Different carbonyl compounds and nitrobenzene derivatives containing alkyl, hydroxy, alkoxy, halogen, carbonyl and carboxylate groups have been examined in the above mentioned examples4–7 but the selective synthesis of secondary amines with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group has received little attention.6b Supported gold nanocatalysts could provide the one-pot synthesis of more complicated secondary aromatic amines due to their ability to hydrogenate selectively the nitro-groups without hydrogenating the other functionalities.8 Indeed, the Au/TiO2 catalyst demonstrated high selectivity in the one-pot synthesis of N-(3-vinylbenzyl)-aniline from nitrobenzene and 3-vinylbenzaldehyde.6b
C group has received little attention.6b Supported gold nanocatalysts could provide the one-pot synthesis of more complicated secondary aromatic amines due to their ability to hydrogenate selectively the nitro-groups without hydrogenating the other functionalities.8 Indeed, the Au/TiO2 catalyst demonstrated high selectivity in the one-pot synthesis of N-(3-vinylbenzyl)-aniline from nitrobenzene and 3-vinylbenzaldehyde.6b
One-pot reductive amination of aldehydes with nitroarenes has been investigated in batch reactors under hydrogen atmosphere (H2 pressure of 1 bar)4 or under higher pressure.5–7 In most cases, the reaction should be carried out for a long time or by using several stages with different conditions to achieve a high yield of secondary amine.4b–e,5,7 The analysis of the catalyst's behavior in continuous flow mode is missing, in spite of the growing interest in the development and application of the flow-based approach for organic synthesis in academic and industrial areas. In general, continuous flow processes are more efficient than standard batch protocols and make organic synthesis safer and more environmentally friendly.9 However, only a few reports devoted to the synthesis of secondary amines in flow mode have been found.10 Benzylpiperazine derivatives have been prepared directly from the corresponding benzaldehyde and piperazine in continuous flow over Pd/C, Pt/C and Pd(OH)2/C catalysts.10a Aliphatic and aromatic secondary amines have been synthesized selectively by one-pot reductive amination of nitriles with primary amines using a Pt/C catalyst in a continuous flow multichannel microreactor.10b The Au/TiO2-catalyzed alkylation of amines with alcohols has been performed in a flow reactor at 180–200 °C and a rather high pressure (100 bar of hydrogen), but the reactor should be used in recycle mode for several hours to achieve high conversion.10c
In the present study, we report the one-pot reductive amination of aldehydes with nitroarenes using hydrogen as the reducing agent in a packed bed one-through flow reactor over an Au/Al2O3 catalyst. To the best of our knowledge, this is the first realization of such a type of reaction in continuous flow mode.
An Au/Al2O3 catalyst containing 2.5 wt% Au with the mean Au particle diameter equal to 3.4 nm (ESI†) has been used for the reductive amination of aldehydes with nitroarenes in a flow reactor.‡ The reaction has been examined using several different nitroarenes and aldehydes in toluene and the results of the experiments have been summarized in Table 1. The reductive amination of aldehyde 2 with nitroarene 1 starts from the hydrogenation of nitroarene 1 to the corresponding aniline 3, which reacts with aldehyde 2 to form imine 4 and further hydrogenation of 4 gives secondary amine 5 (Scheme 1). Simultaneously, aldehydes 2 could produce the corresponding alcohols 6 in a side reaction. The catalytic activity of the Au/Al2O3 catalyst was initially evaluated for the reaction of nitrobenzene and benzaldehyde using different ratios of the reactants and different solvents (Table 1, entries 1–4). It should be noted that the full conversion of nitrobenzene, as well as the other examined nitroarenes, was observed in all the experiments. The formation of any previously reported8 by-products of nitrobenzene reduction to aniline such as nitrosobenzene, benzylhydroxylamine, azoxybenzene, azobenzene and hydrazobenzene was not detected. The prevailing products of the nitrobenzene conversion were secondary amine 5 and aniline 3, when the reaction was carried out using equimolar ratio of nitrobenzene and benzaldehyde in toluene (Table 1, entries 1 and 2). The utilization of methanol instead of toluene led to a drastic decrease in N-benzylaniline yield and an increase in aniline 3 and imine 4 formation (Table 1, entry 3). Simultaneously, high conversion of benzaldehyde to benzyl alcohol was observed. This reaction is competitive with the imine formation which results in the decrease of secondary amine yield. The slight excess of aldehyde 2 at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 equal to 1
2 equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 resulted in the increase of N-benzylaniline yield from 57 to 84%. A favorable effect of the aldehyde excess on the secondary amine yield was also confirmed by the enhancement of N-benzyl-4-methylaniline yield from 68 to 91% after the decrease of 1
1.5 resulted in the increase of N-benzylaniline yield from 57 to 84%. A favorable effect of the aldehyde excess on the secondary amine yield was also confirmed by the enhancement of N-benzyl-4-methylaniline yield from 68 to 91% after the decrease of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios from 1
2 ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (Table 1, entries 5 and 6). Therefore, all of the following experiments were performed using the ratios of nitroarenes and aldehydes equal to 1
1.5 (Table 1, entries 5 and 6). Therefore, all of the following experiments were performed using the ratios of nitroarenes and aldehydes equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. Under these optimized reaction conditions, the scope of the reaction was explored with structurally different aldehydes.
1.5. Under these optimized reaction conditions, the scope of the reaction was explored with structurally different aldehydes.
| Entry | Nitroarene, 1 | Aldehyde, 2 | Product, 5 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
T, °C | Conversion of 2, % | Yield, %b | ||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | |||||||
| a Catalyst (Au/Al2O3) = 200 mg, concentration of 1 = 0.025 mol L−1, hydrogen flow rate = 60 mL min−1, pressure = 50 bar, solvent = toluene, flow rate of liquid = 0.5 mL min−1. b GC yield. The values in parentheses are the yields of the isolated products. c Reaction in methanol. d n.d. = not detected. | |||||||||
| 1 |
|
|
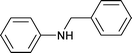
|
1 | 80 | 98 | 42 | 1 | 57 |
| 2 | 1 | 90 | 99.6 | 39.9 | 0.3 | 59.8 | |||
| 3c | 1 | 90 | 82 | 79 | 13 | 8 | |||
| 4 | 1.5 | 80 | 85 | 14 | 2 | 84 | |||
| 5 |
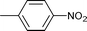
|
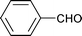
|
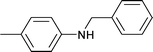
|
1 | 80 | 99 | 31.9 | 0.2 | 67.9 |
| 6 | 1.5 | 80 | 65 | 6 | 3 | 91 | |||
| 7 |
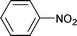
|

|
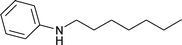
|
1.5 | 70 | 87 | 5 | 1 | 94 |
| 8 | 1.5 | 80 | 93 | 5.7 | 0.6 | 93.7 (94) | |||
| 9 | 1.5 | 90 | 98 | 7.5 | 0.4 | 92.1 | |||
| 10 |
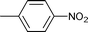
|

|
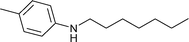
|
1.5 | 70 | 86 | 1.5 | 0.6 | 97.9 |
| 11 | 1.5 | 80 | 93 | 0.6 | 0.1 | 99.3 (>99) | |||
| 12 | 1.5 | 90 | 97 | 0.7 | n.d.d | 99.3 | |||
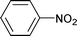
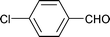
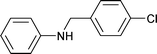
| 13 |
|
|
|
1.5 | 90 | 99 | 26.5 | 0.1 | 73.4 |
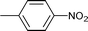
| 14 |
|
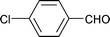
|
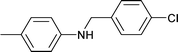
|
1.5 | 80 | 96 | 17.2 | 0.8 | 82 |
| 15 | 1.5 | 90 | 98 | 16.0 | 0.1 | 83.9 | |||
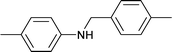
| 16 |
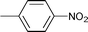
|
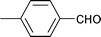
|
|
1.5 | 80 | 99 | 22.5 | 0.5 | 77.0 |
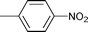
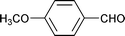
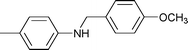
| 17 |
|
|
|
1.5 | 70 | 90 | 23 | n.d. | 77 |
| 18 | 1.5 | 80 | 98 | 23 | n.d. | 77 | |||
Comparison of the reactions of nitrobenzene and 4-nitrotoluene with benzaldehyde (Table 1, entries 4 and 6) allowed one to see that the yield of secondary aromatic amine was higher when 4-nitrotoluene was used as the starting reagent. The same feature was observed in the reaction of nitrobenzene and 4-nitrotoluene with n-heptaldehyde (Table 1, entries 8 and 11) and 4-chlorobenzaldehyde (Table 1, entries 13 and 15), pointing out the probable beneficial effect of the methyl group on the reactivity of nitroarene. The yields of the target amines decreased when substituted benzaldehydes were used in the reaction with 4-nitrotoluene (Table 1, entries 6, 14, 16 and 18), probably due to the higher rate of their hydrogenation to alcohols.
Aliphatic n-heptaldehyde gave higher yields of secondary amine in reductive amination with nitrobenzene (Table 1, entries 7–9) or 4-nitrotoluene (Table 1, entries 10–12) in comparison with the examples where benzaldehyde and its derivatives were used (Table 1, entries 4, 6 and 13–18). So, the reaction of n-heptaldehyde with nitrobenzene and 4-nitrotoluene occurred with 94% and more than 99% yields of the corresponding secondary amines (Table 1, entries 8 and 11). Excellent yields of N-heptylanilines can be explained by the fast reaction of n-heptaldehyde with anilines 3.4d In these cases, almost all aniline molecules 3 are converted to imines 4 which are further reduced to target secondary amines 5. It was found that variation of the reaction temperature in the range of 70–90 °C had little effect on the yield of secondary amines (Table 1).
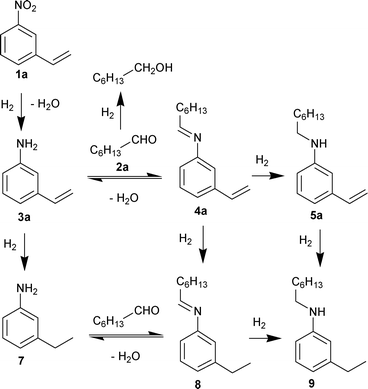
Due to the high chemoselectivity of the Au/Al2O3 catalyst in the hydrogenation of nitro aromatic compounds,8b we managed to realize the synthesis of secondary aromatic amine containing a C–C double bond by one-pot one-step reductive amination of aldehyde with nitroarene. N-Heptyl-3-vinylaniline 5a was synthesized in 74% yield by reductive amination of n-heptaldehyde with 3-nitrostyrene under hydrogenation conditions over the Au/Al2O3 catalyst at 60 °C (Table 2). In addition to 5a, 3-vinylaniline 3a, 3-ethylaniline 7, imines 4a, 8 and N-heptyl-3-ethylaniline 9 were found in the reaction mixtures at the reactor outlet. Decreasing the reaction temperature led to a decrease in the formation of the C–C double bond reduction products, but at the same time, significant amounts of 3-vinylaniline 3a and imine 4a were formed at lower temperatures. The temperature increase promoted the reduction of the double bond and decreased the yield of the targeted product. Thus, the Au/Al2O3 catalyst in our conditions gave a maximal yield of the targeted product, N-heptyl-3-vinylaniline, at 60 °C. A similar reaction between nitrobenzene and 3-vinylbenzaldehyde was realized previously in one vessel but using two stage procedures: the hydrogenation of the nitroaromatic compound was performed at 120 °C under H2 pressure until 95–98% conversion level, and then the reactor was depressurized and left at the same temperature until completion of the cascade process.6b
| Entry | T, °C | Conversion of 2a, % | Yield, % | |||||
|---|---|---|---|---|---|---|---|---|
| 3a | 4a | 5a | 7 | 8 | 9 | |||
| a Concentrations of 1a and 2a: 0.025 mol L−1 and 0.0375 mol L−1, respectively, pressure = 50 bar. | ||||||||
| 1 | 50 | 64 | 23.5 | 7.5 | 65 | 0.9 | 0.3 | 3 |
| 2 | 60 | 71 | 13 | 3 | 74 | 1.5 | 0.3 | 8.5 |
| 3 | 70 | 81 | 6.5 | 1.5 | 68 | 2 | 0.4 | 22 |
| 4 | 80 | 88 | 5 | 0.5 | 51.5 | 3.5 | 0.4 | 39 |
The time-dependent investigation of the Au/Al2O3 catalyst in one-pot reductive amination of aldehydes with nitroarenes showed slight decrease in secondary amine yields with time-on-stream (Fig. 1 and ESI†). A reduction in the yields of N-heptylaniline and N-benzylaniline at 80 °C from 95% to 90% and from 84% to 82% occurred after 135 minutes on-stream in the reaction of nitrobenzene with n-heptaldehyde and benzaldehyde, respectively (reaction conditions: 0.025 mol L−1 nitrobenzene, 0.0375 mol L−1 aldehyde, 50 bar H2). It was shown that the activity of the spent Au/Al2O3 catalyst can be recovered after the treatment in methanol flow without significant loss of secondary amine yields. After the reaction of nitrobenzene and n-heptaldehyde at 135 min, the cartridge CatCart®30 was washed with methanol flow (1 mL min−1) for 30 min and introduced in the next reaction cycle.
After the first and second runs, a slight decay in the yield of N-heptylaniline was observed (Fig. 1). Importantly, the leaching of Au from the Au/Al2O3 catalyst and the increase in gold particle size were not detected after three cycles of the reaction and regeneration, while the formation of some amounts of carbon deposition was observed by differential thermal analysis (ESI†). Thus, the decrease in secondary amine yields with time-on-stream can be explained by the formation and accumulation of carbon deposition on the surface of the Au/Al2O3 catalyst. The development of more efficient regeneration procedures will be one of the aims of further investigation.
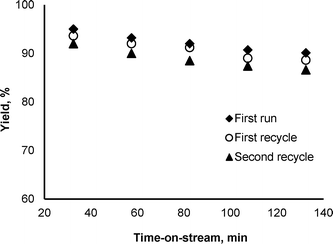 | ||
| Fig. 1 Recycling experiments of the reductive amination of n-heptaldehyde with nitrobenzene over the Au/Al2O3 catalyst at 80 °C. | ||
Conclusions
In conclusion, various substituted secondary aromatic amines were synthesized by Au/Al2O3-catalyzed one-pot reductive amination of aromatic and aliphatic aldehydes with nitroarenes in a continuous flow reactor using molecular hydrogen as a reducing agent in good to excellent yields.Acknowledgements
The authors thank M. V. Shashkov, A. V. Ishchenko and G. S. Litvak for their help in carrying out this work. This work was supported in part by the Skolkovo Foundation (Grant Agreement No. 3 of 25.12.2014) and the grant from the RF President for support to Leading Scientific Schools (grant SS-5340.2014.3).Notes and references
- (a) A. Seayad, M. Ahmed, H. Klein, R. Jackstell, T. Gross and M. Beller, Science, 2002, 297, 1676 CrossRef CAS PubMed; (b) R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785 CrossRef CAS.
- (a) D. M. Roundhill, Chem. Rev., 1992, 92, 1 CrossRef CAS; (b) A. Corma, T. Ródenas and M. J. Sabater, Chem. – Eur. J., 2010, 16, 254 CrossRef CAS PubMed; (c) K. Shimizu, N. Imaiida, K. Kon, S. M. A. Hakim Siddiki and A. Satsuma, ACS Catal., 2013, 3, 998 CrossRef CAS.
- (a) A. F. Abdel-Magid and S. Mehrman, J. Mol. Pharm. Org. Process Res., 2006, 10, 971 CrossRef CAS; (b) M. E. Domine, M. C. Hernandez-Soto and Y. Perez, Catal. Today, 2011, 159, 2 CrossRef CAS PubMed; (c) K. N. Gusak, Zh. V. Ignatovich and E. V. Koroleva, Russ. Chem. Rev., 2015, 84, 288 CrossRef CAS PubMed.
- (a) L. Li, Z. Niu, S. Cai, Y. Zhi, H. Li, H. Rong, L. Liu, L. Liu, W. He and Y. Li, Chem. Commun., 2013, 49, 6843 RSC; (b) L. Hu, X. Cao, D. Ge, H. Hong, Z. Guo, L. Chen, X. Sun, J. Tang, J. Zheng, J. Lu and H. Gu, Chem. – Eur. J., 2011, 17, 14283 CrossRef CAS PubMed; (c) M. O. Sydnes and M. Isobe, Tetrahedron Lett., 2008, 49, 1199 CrossRef CAS PubMed; (d) M. O. Sydnes, M. Kuse and M. Isobe, Tetrahedron, 2008, 64, 6406 CrossRef CAS PubMed; (e) M. M. Dell'Anna, P. Mastrorilli, A. Rizzuti and C. Leonelli, Appl. Catal., A, 2011, 401, 134 CrossRef PubMed; (f) M. Nasrollahzadeh, New J. Chem., 2014, 38, 5544 RSC; (g) B. Sreedhar, P. S. Reddy and D. K. Devi, J. Org. Chem., 2009, 74, 8806 CrossRef CAS PubMed; (h) H. Li, Z. P. Dong, P. Wang, F. Zhang and J. Ma, React. Kinet., Mech. Catal., 2013, 108, 107 CrossRef CAS; (i) S. Wei, Z. Dong, Z. Ma, J. Sun and J. Ma, Catal. Commun., 2013, 30, 40 CrossRef CAS PubMed; (j) J. Zhou, Z. Dong, P. Wang, Z. Shi, X. Zhou and R. Li, J. Mol. Catal. A: Chem., 2014, 382, 15 CrossRef CAS PubMed.
- (a) F. G. Cirujano, A. Leyva-Perez, A. Corma and F. X. Llabres i Xamena, ChemCatChem, 2013, 5, 538 CrossRef CAS PubMed; (b) M. Pintado-Sierra, A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sanchez, J. Catal., 2013, 299, 137 CrossRef CAS PubMed.
- (a) Y. Yamane, X. Liu, A. Hamasaki, T. Ishida, M. Haruta, T. Yokoyama and M. Tokunaga, Org. Lett., 2009, 11, 5162 CrossRef CAS PubMed; (b) L. L. Santos, P. Serna and A. Corma, Chem. – Eur. J., 2009, 15, 8196 CrossRef CAS PubMed.
- (a) T. Stemmler, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, ChemSusChem, 2014, 7, 3012 CrossRef CAS PubMed; (b) T. Stemmler, F. A. Westerhaus, A.-E. Surkus, M.-M. Pohl, K. Junge and M. Beller, Green Chem., 2014, 16, 4535 RSC.
- (a) A. Corma and P. Serna, Science, 2006, 313, 332 CrossRef CAS PubMed; (b) K. Shimizu, Y. Miyamoto, T. Kawasaki, T. Tanji, Y. Tai and A. Satsuma, J. Phys. Chem. C, 2009, 113, 17803 CrossRef CAS.
- (a) M. Irfan, T. N. Glasnov and C. O. Kappe, ChemSusChem, 2011, 4, 300 CrossRef CAS PubMed; (b) V. Hessel, Chem. Eng. Technol., 2009, 32, 1655 CrossRef CAS PubMed; (c) I. R. Baxendale, L. Brocken and C. J. Mallia, Green Process. Synth., 2013, 2, 211 CAS; (d) C. Wiles and P. Watts, Green Chem., 2014, 16, 55 RSC.
- (a) J. Liu, A. E. Fitzgerald and N. S. Mani, Synthesis, 2012, 44, 2469 CrossRef CAS; (b) S. K. Sharma, J. Lynch, A. M. Sobolewska, P. Plucinski, R. J. Watson and J. M. J. Williams, Catal. Sci. Technol., 2013, 3, 85 RSC; (c) N. Zotova, F. J. Roberts, G. H. Kelsall, A. S. Jessiman, K. Hellgardt and K. K. Hii, Green Chem., 2012, 14, 226 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental rig and procedure, preparation and characterization of the catalysts, GC chromatograms, NMR data. See DOI: 10.1039/c5cy00964b |
| ‡ The investigation of the catalytic properties of the Au/Al2O3 catalyst in the one-pot reductive amination of aldehydes with nitroarenes was performed using the commercially available continuous flow device H-Cube Pro™ (Thalesnano, Hungary). The liquid feed was introduced into the reactor by an HPLC pump in up-flow mode after mixing with the hydrogen flow, generated by a built-in electrolytic cell. The packed bed cartridge-like reactors CatCart®30 with an inner diameter of 4.0 mm and length of 30 mm were used in the experiments. Before the experiment, the cartridge containing the catalyst (0.200 g of particles with a size of 250–500 μm) was installed in the heated block and pure solvent was fed to the reactor until the required reaction parameters (temperature, pressure, hydrogen and reactant flow) were reached. Afterwards, the inlet was switched to the flask with the substrate's solution and the reaction was started. The solution of nitroarene (0.025 M) and aldehyde (0.025 M or 0.0375 M) in toluene was used in the experiments with n-decane as the internal standard. In the standard experiment, the reaction was carried out at 50 bar of hydrogen pressure, 0.5 mL min−1 liquid feed rate, 60 mL min−1 hydrogen feed rate, and the temperature was changed to 50–90 °C interval. The performance of the catalysts was evaluated by averaging the 2 samples taken at 30–35 min and 55–60 min after the start of the experiment. The reaction products were analyzed by gas chromatography (Agilent 6890 N instrument with a 19091S-416 HP 5-MS capillary column 60.0 m × 320 μm × 0.25 μm) and GC-MS. The conversion of aldehydes was calculated using n-decane as the internal standard. Yields of N-containing products were calculated by GC as the ratio of the product concentration to the sum of the concentrations of all products. |
| This journal is © The Royal Society of Chemistry 2015 |