Quantitative evaluation of positive ϕ angle propensity in flexible regions of proteins from three-bond J couplings†
Abstract
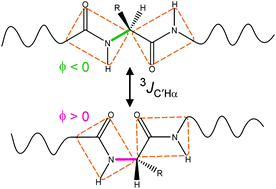
3 J HNHα and 3JC′C′ couplings can be readily measured in isotopically enriched proteins and were shown to contain precise information on the backbone torsion angles, ϕ, sampled in disordered regions of proteins. However, quantitative interpretation of these couplings required the population of conformers with positive ϕ angles to be very small. Here, we demonstrate that this restriction can be removed by measurement of 3JC′Hα values. Even though the functional forms of the 3JC′Hα and 3JHNHα Karplus equations are the same, large differences in their coefficients enable accurate determination of the fraction of time that positive ϕ angles are sampled. A four-dimensional triple resonance HACANH[C′] E.COSY experiment is introduced to simultaneously measure 3JC′Hα and 3JHNC′ in the typically very congested spectra of disordered proteins. High resolution in these spectra is obtained by non-uniform sampling (in the 0.1–0.5% range). Application to the intrinsically disordered protein α-synuclein shows that while most residues have close-to-zero positive ϕ angle populations, up to 16% positive ϕ population is observed for Asn residues. Positive ϕ angle populations determined with the new approach agree closely with consensus values from protein coil libraries and prior analysis of a large set of other NMR parameters. The combination of 3JHNC′ and 3JC′C′ provides information about the amplitude of ϕ angle dynamics.
- This article is part of the themed collection: Exploring the conformational heterogeneity of biomolecules: theory and experiments

 Please wait while we load your content...
Please wait while we load your content...