Abstract
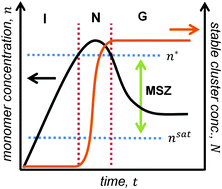
We introduce a comprehensive quantitative treatment for burst nucleation (BN)—a kinetic pathway toward self-assembly or crystallization defined by an extended post-supersaturation induction period, followed by a burst of nucleation, and finally the growth of existing stable assemblages absent the formation of new ones—based on a hybrid mean field rate equation model incorporating thermodynamic treatment of the saturated solvent from classical nucleation theory. A key element is the inclusion of a concentration-dependent critical nucleus size, determined self-consistently along with the subcritical cluster population density. The model is applied to an example experimental study of crystallization in tetracene films prepared by organic vapor–liquid–solid deposition, where good agreement is observed with several aspects of the experiment using a single, physically well-defined adjustable parameter. The model predicts many important features of the experiment, and can be generalized to describe other self-organizing systems exhibiting BN kinetics.

 Please wait while we load your content...
Please wait while we load your content...