Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoswitching in nanoporous, crystalline solids: an experimental and theoretical study for azobenzene linkers incorporated in MOFs†
Zhengbang
Wang
a,
Lars
Heinke
*a,
Jelena
Jelic
b,
Murat
Cakici
ce,
Marcel
Dommaschk
d,
Reinhard J.
Maurer
 b,
Harald
Oberhofer
b,
Sylvain
Grosjean
cg,
Rainer
Herges
d,
Stefan
Bräse
cfg,
Karsten
Reuter
b and
Christof
Wöll
a
b,
Harald
Oberhofer
b,
Sylvain
Grosjean
cg,
Rainer
Herges
d,
Stefan
Bräse
cfg,
Karsten
Reuter
b and
Christof
Wöll
a
aKarlsruher Institut für Technologie (KIT), Institut für Funktionelle Grenzflächen (IFG), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. E-mail: Lars.Heinke@KIT.edu
bLehrstuhl für Theoretische Chemie, Technische Universität München, Lichtenbergstr. 4, 85747 Garching, Germany
cInstitute of Organic Chemistry (IOC), Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany
dInstitute of Organic Chemistry, University Kiel, Otto-Hahn-Platz 4, 24118 Kiel, Germany
eFaculty of Science, Department of Chemistry, Ataturk University, 25240 Erzurum, Turkey
fInstitute of Toxicology and Genetics, Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany
gInstitute of Biological Interfaces (IBG), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 13th May 2015
Abstract
In this article, we use the popular photoswitchable molecule, azobenzene, to demonstrate that the embedding in a nanoporous, crystalline solid enables a precise understanding of light-induced, reversible molecular motion. We investigate two similar azobenzene-containing, pillared-layer metal–organic frameworks (MOFs): Cu2(AzoBPDC)2(BiPy) and Cu2(NDC)2(AzoBiPy). Experimental results from UV-vis spectroscopy and molecular uptake experiments as well as theoretical results based on density-functional theory (DFT) show that in the Cu2(AzoBPDC)2(BiPy) MOF structure, the azobenzene side groups undergo photoisomerization when irradiated with UV or visible light. In a very similar MOF structure, Cu2(NDC)2(AzoBiPy), the experimental studies show an unexpected absence of photoisomerization. The DFT calculations reveal that in both MOFs the initial and final states of the photoswitching process (the trans and the cis conformation) have similar energies, which strongly suggests that the reason for the effective blocking of photoswitching in the AzoBiPy-based MOFs must be related to the switching process itself. More detailed calculations show that in Cu2(NDC)2(AzoBiPy) a naphthalene linker from the molecular framework blocks the photoisomerization trajectory which leads from the trans to the cis conformation. For Cu2(AzoBPDC)2(BiPy), as a result of the different geometry, such a steric hindrance is absent.
Metal–organic frameworks (MOFs) are crystalline, porous solids, self-assembled from metal knots and organic linker molecules.1 Due to their large structural variety, MOFs enable many different applications, ranging from gas storage and separation2 over catalysis3 to potential applications, such as in biomedicine4 and sensor techniques.5 One particularly interesting property of MOFs is the availability of free space, which allows the incorporation of photoswitchable molecules. This may enable the switching of physical or chemical properties in a fully remote-controlled way, i.e. without any (e.g. electrical) contacts. So far, only a few MOFs, which contains linker molecules with photoswitchable azobenzene side groups have been synthesized.6–12 Some proof-of-principle studies show how the adsorption capacity of gas molecules, like methane and carbon dioxide, can be varied.7,10 Enabled by the photoswitching of the azobenzene in the MOF structure, other pioneering experiments demonstrated the remote-controlled release of guest molecules.11,12
In order to have a successful incorporation of photoswitchable molecules into solids, a detailed analysis of the dynamics of the photoswitching process itself is necessary. In this context it should be noted that pure azobenzene (C12H10N2) shows no switching behavior in the condensed, crystalline form.13 However, for azobenzene derivatives, like azobenzene functionalized with various halogens,14 photoswitching can be observed also in the condensed, crystalline phase. When pure azobenzene is in solution, switching can typically be observed with precise dynamics (switching speed, relaxation times, etc.) depending on the properties (viscosity, polarizability, etc.) of the liquid. In the gas phase, switching can also be observed directly.15 When azobenzene units are embedded into polymers16 or bound to other materials like graphene,17 photoswitching is often enabled, but the properties are affected by the environment.16,18,19 Switching is only possible if sufficient space is available within the polymer. A number of studies showed that the switching, i.e. the achieved cis percentage, can vary between more than 80% in rather porous polymers20,21 and virtually zero in densely packed polymers,22,23 where the photoswitching is sterically hindered.
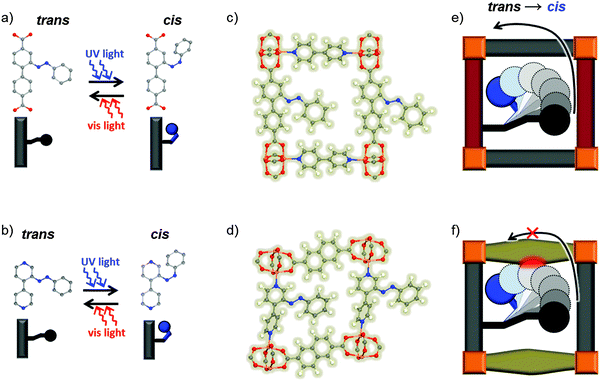
In this work, we study azobenzene units that have been incorporated into crystalline, nanoporous solids. The use of metal–organic frameworks (MOFs) allows us to perform detailed experimental and theoretical studies of the switching process since the structure of these compounds is well-defined. We used thin, epitaxially grown films of two different azobenzene-containing pillared-layer MOF structures which were already published: Cu2(NDC)2(AzoBiPy)9 and Cu2(AzoBPDC)2(BiPy),11 see Fig. 1 and Fig. S1 (ESI†) (NDC: 2,6-naphthalenedicarboxylic acid; BiPy: 4,4′-bipyridine; AzoBiPy: 3-azobenzene-4,4′-bipyridine; AzoBPDC: 3-azobenzene 4,4′-biphenyldicarboxylic acid. Cu2(NDC)2(AzoBiPy) is also known as Cu-CAU-5.6,9). For these MOF-types, we were able to fabricate well-ordered SURMOFs, which is not always the case.24 These thin surface-mounted MOF films (SURMOFs) were grown on a functionalized solid surface in a layer-by-layer fashion using liquid-phase epitaxy.25 The out-of-plane and in-plane X-ray diffraction (XRD) patterns (Fig. S2, ESI†) verify the highly crystalline and oriented SURMOF growths (Fig. S2, ESI†). By exploiting UV-vis spectroscopy, uptake experiments using a quartz crystal microbalance (QCM) and density-functional theory (DFT) calculations, we are able to show that the azobenzene units within the Cu2(AzoBPDC)2(BiPy) MOF undergo photoisomerization when irradiated with UV light, whereas the other MOF structure cannot be photoswitched due to steric hindrance.
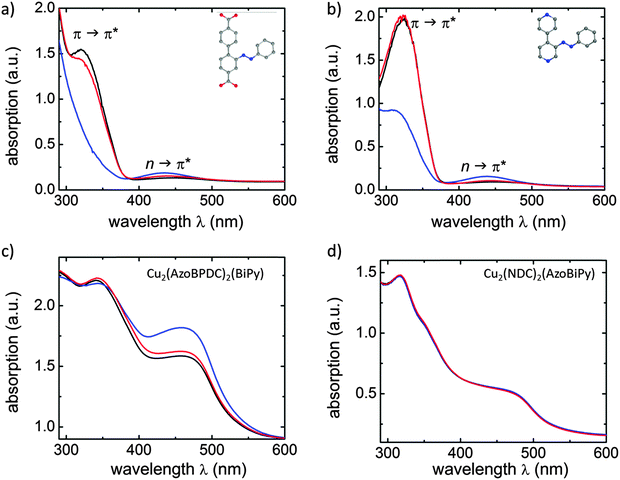
The UV-vis spectra of both linkers recorded in solution (ethanol) and after incorporation into the corresponding MOF films are shown in Fig. 2. When irradiated with UV light, the photoswitching of the linker molecules in solution, i.e. the light-induced isomerization of the azobenzene side groups from the planar trans to the bent cis state, is clearly visible as a decrease of the π → π* band (at about 320 nm) and an increase of the n → π* band (at about 440 nm; Fig. 2(a) and (b)). The intensity shifts of both bands are generally contributed to the photoswitching process.26,27 By irradiating these solutions with visible light (in this case a 560 nm LED, which usually results in an efficient cis-to-trans photoisomerization27), the n → π* band decreases and the π → π* band increases again, indicating that the molecules are switched back to the trans state. Thus, in ethanolic solution both linker molecules, AzoBiPy and AzoBPDC, are clearly photoswitchable.
With azobenzene-linkers incorporated in thin MOF films, the situation is quite different. In this case, only the MOF of type Cu2(AzoBPDC)2(BiPy) shows comparable shifts of the π → π* and n → π* band intensities when irradiated with UV light (see Fig. 2(c)). Similar to the linker molecules in solution, irradiation of Cu2(AzoBPDC)2(BiPy) with visible light results in an n → π* band decrease and the π → π* band increases again. This indicates that the azobenzene moieties in Cu2(AzoBPDC)2(BiPy) can be switched by UV light from the trans to the cis state and back again by visible light. However, when thin films of type Cu2(NDC)2(AzoBiPy) are irradiated with UV or visible light, no changes in the UV-vis spectra could be observed (see Fig. 2(d)). Even after irradiating the sample with UV or visible light for long periods of time of up to 12 h, the UV-vis spectrum did not significantly change. This is a clear indication that no photoswitching occurs in Cu2(NDC)2(AzoBiPy).
By means of X-ray diffraction (Fig. S3, ESI†), it was shown that the crystallinities of the SURMOF samples are not affected by the UV irradiation.
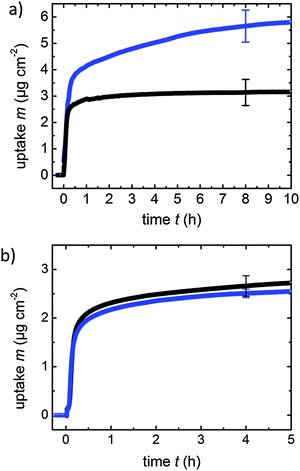
An independent way to investigate the switching of the azo-based linkers used here is to monitor the effect of the transport of guest molecules inside the MOF. Due to the different conformation in the trans and the cis state, the corresponding diffusion coefficients and adsorption capacities are expected to be different. A convenient way to study these effects in thin MOF films is to monitor the molecular uptake of small guest molecules using an appropriate sensor. We used 1,4-butanediol as a probe molecule and studied the loading by the corresponding SURMOFs by means of a quartz crystal microbalance (QCM).11 The corresponding results for the uptake by the thin MOF film in the pristine state (trans) and by the thin MOF film after UV irradiation are shown in Fig. 3. While the UV irradiation resulted in a significant increase of the uptake amount and a decrease of the uptake rate for Cu2(AzoBPDC)2(BiPy), no significant change of the uptake was observed for Cu2(NDC)2(AzoBiPy) after irradiation with UV light. Thus, the uptake data verify the findings from UV-vis spectroscopy that Cu2(AzoBPDC)2(BiPy) is photoswitchable and can perform isomerization when irradiated with UV light, while Cu2(NDC)2(AzoBiPy) cannot.
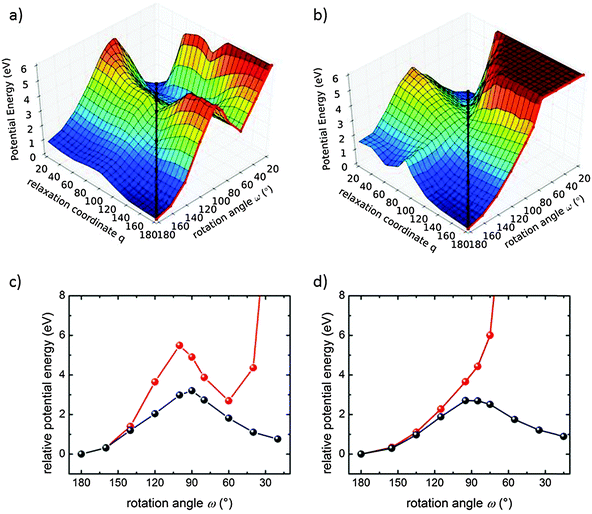
For a better understanding of why in one MOF structure the photoinduced isomerization is enabled and in the other it is not, we performed DFT calculations for the optimized trans and cis states in each compound. The geometries of these two states are characterized by values of the dihedral reaction coordinate of ω = 180° (trans) and ω = ∼20° (cis), respectively. Like in plain azobenzene, both states are (meta-)stable for the two MOF structures, with trans being the ground state at a lower potential energy than the cis state (ΔECu2(AzoBPDC)2(BiPy) = 0.76 eV and ΔECu2(NDC)2(AzoBiPy) = 0.89 eV). A similarly minute influence of the MOF structures is seen in the vertical excitation energies at the two metastable states as calculated with time-dependent DFT. There is no significant difference when the azobenzene in the two MOF structures are compared to each other or when they are compared to plain azobenzene. With respect to the ground-state energetics, this similarity also extends beyond these two states. As shown in Fig. 4, both MOFs exhibit similar minimum energy pathways along the reaction coordinate ω, i.e. when all other degrees of freedom are filly optimized for a given value of ω (black lines in Fig. 4(c) and (d) for Cu2(AzoBPDC)2(BiPy) and Cu2(NDC)2(AzoBiPy), respectively).
Since the initial and the final states as well as the minimum energy pathways show no indication of the absence of the photoswitching in Cu2(NDC)2(AzoBiPy), we focus on the dynamics of the process itself. This pertains specifically to the trajectories of the molecular fragments during the course of the photoswitching process. Such investigations would be hardly meaningful in the liquid or in amorphous solids. In contrast, these trajectories are rather well defined in MOFs due to their crystalline nature. Here, we focused on the rotation mechanism, which seems to be the prevailing isomerization mechanism.28,29 In Fig. 4(a) and (b), 2D projections of the corresponding high-dimensional ground-state potential energy hypersurface (PES) are plotted for the two MOFs. Next to the azobenzene-rotation angle ω as reaction coordinate, these projections also consider a second generalized coordinate q, which describes the relaxation of all other coordinates in the MOFs due to changes in ω. Thereby, coordinates ω = q correspond to the respective trans ↔ cis minimum energy pathways, which are discussed above, i.e. all other coordinates can optimize perfectly for each value of ω. Coordinates q = 180° (20°) indicate that all other coordinates remain as they are in the trans (cis) state. As shown in Fig. 4(c), the azobenzene group in Cu2(AzoBPDC)2(BiPy) can be brought from the trans to the cis energy minimum by a simple rotation along ω with little influence from other degrees of freedom (red line). This means, even if all other coordinates were frozen at the trans configuration, a cis-like local minimum along ω exists. This minimum can be reached through the photoswitching, after which further relaxation of q directly leads to the actual cis state. On the other hand, in Cu2(NDC)2(AzoBiPy), the situation is quite different. Here, only a concerted motion of ω and q could lead to a stable cis state. Starting from a structure with q frozen at the trans state, a simple rotation along ω alone does not lead to the cis state's energetic minimum (red line in Fig. 4(d)). This qualitative difference in the ground-state energetics is a result of the steric hindrance for the rotation of the azo bond. Whereas in Cu2(AzoBPDC)2(BiPy) this rotation can proceed relatively unhindered, the PES of Cu2(NDC)2(AzoBiPy) requires a substantial reorganization of other (MOF-) degrees of freedom in order to avoid collision with neighboring NDC linkers of the framework. For a successful switching, energy would need to be transferred within the excitation lifetime (typically in the order of pico-seconds26) from the photo-excited ω mode to other modes, including modes of the MOF itself. The experimentally observed absence of switching capability in the Cu2(NDC)2(AzoBiPy) framework suggests that the photo-excitation and relaxation in the azobenzene is too fast for this rearrangement to happen. Therefore, it is the Cu2(NDC)2(AzoBiPy) framework itself that blocks the photoisomerization of the azobenzene side group.
It can be inferred that photoswitching in Cu2(NDC)2(AzoBiPy) should be enabled by modifying the linker in such a way that there is more free space around the azobenzene moiety. This can be achieved, for instance, by extending the linear backbone of the AzoBiPy linker by one phenyl ring resulting in AzoBiPyB linker (see ESI 2,†). It was shown by UV-vis spectroscopy and QCM uptake experiments that pillared-layer MOFs containing AzoBiPyB (this means MOFs of type Cu2(BDC)2(AzoBiPyB)) enables photoswitching (see S4 and S5, ESI†).
In conclusion, the incorporation of photoactive compounds into MOFs enables the fabrication of functional, smart materials, where properties such as the uptake of guest molecules can be remote-controlled. However, for the design of such materials, it is not sufficient to study whether the corresponding cis and trans states known from gas-phase or solution are compatible with the MOF-structure. Instead, the entire trajectories of the switching unit have to be considered. In this paper, we exemplified this for the photoswitching in two similar, azobenzene-containing pillared-layer MOFs. By means of UV-vis spectroscopy and QCM uptake experiments, we showed that the photoswitching is enabled in one MOF structure, while it is not possible in the other. DFT calculations indicate that steric hindrance along the switching trajectory oppresses the photoisomerization process in the non-switching MOF. The results obtained here demonstrate that the highly crystalline structure of MOFs allows for straightforward experimental and theoretical investigations of photoswitching. In the future, we plan to perform such studies in a time-resolved fashion to gain deeper insights into the photoswitching process.
Methods
Sample syntheses
The investigated MOF films were prepared by liquid-phase epitaxy (LPE) on a functionalized solid substrate in a well-defined layer-by-layer fashion.25 These thin MOF films, called surface-mounted MOFs (SURMOFs), are oriented and highly-crystalline. SURMOFs are prepared by the sequential immersion of the substrate in each of the two reactant solutions, i.e. the solutions of the metal complexes and of the organic linker molecules in ethanol. For Cu2(AzoBPDC)2(BiPy), the solutions were 0.5 mM copper acetate as well as 0.1 mM BiPy and AzoBPDC. For Cu2(NDC)2(AzoBiPy), the solutions were 0.5 mM copper acetate as well as 0.1 mM NDC and AzoBiPy in ethanol. After each immersion step, the substrate was rinsed with ethanol to remove any unreacted, weakly adsorbed reactants. To nucleate the growth on the substrate surface (here: 150 nm gold on a silicon wafer) and to control the SURMOF's crystal orientation, the substrate surface was functionalized with an 11-mercapto-1-undecanol self-assembled monolayer (MUD SAM). Here, all samples were synthesized in 80 cycles.UV-vis spectroscopy
The UV-vis spectra were recorded by means of a Cary5000 spectrometer with a UMA unit from Agilent. The linker molecules were studied in ethanolic solution in transmission mode. The UV-vis spectra of the SURMOFs on the gold substrate were measured in reflection with an angle of 20° to the normal.UV irradiation was performed with a 365 nm LED with a power of 150 mW. The visible light irradiation was performed with a 560 nm LED with 100 mW. The distance between the sample and the LED was about 10 cm.
Uptake experiments using a quartz crystal microbalance
The uptake of the guest molecules by the thin MOF films was studied by using a quartz crystal microbalance (QCM).11,30 The QCM cell is connected to a gas flow system with Argon as carrier gas. With this gas flow system, it is possible to switch instantly between a pure argon flow and a argon flow enriched with the guest molecule, here 1,4-butanediol, at room temperature (25 °C). This results in a butanediol partial pressure of roughly 1 Pa. All QCM experiments were performed at a constant temperature of 30 °C, which is precisely controlled by the Q-Sense setup. Before each experiment, the samples were activated in a pure argon flow at 65 °C overnight.Computational details
All DFT calculations were performed with the all-electron code FHI-aims.31,32 The MOF structures were fully relaxed (residual forces below 10−4 eV Å−1) in periodic boundary conditions and Γ-point sampling of the Brillouin zone. All relaxations and single point energy calculations were conducted non spin-polarized with the PBE generalized gradient exchange–correlation (XC) functional.33 Electron orbitals and density were expanded in a numeric atomic orbital basis at the “tier1” level using “light” integration grids. Convergence checks with respect to basis set and integration grid size, spin-polarization and XC-functional (against hybrid functional calculations) showed no appreciable influence of these factors on the relative ground-state energetics used to identify the steric hindrance. Dispersion interactions were accounted for using the correction scheme of Tkatchenko and Scheffler.34 The time-dependent DFT calculations were performed with TURBOMOLE35,36 based on the B3LYP hybrid functional with an optimized triple-zeta basis (def-TZVPP).Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Acknowledgements
Z.W. is grateful for a PhD fellowship donated by the China Scholarship Council (CSC). L.H. is indebted to the Baden-Württemberg Stiftung for the financial support of this research project by the Eliteprogramm for Postdocs. J.J., R.J.M., H.O. and K.R. gratefully acknowledge support through the Solar Technologies Go Hybrid initiative of the State of Bavaria.References
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Ferey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2011, 112, 1105–1125 CrossRef PubMed.
- A. Modrow, D. Zargarani, R. Herges and N. Stock, Dalton Trans., 2011, 40, 4217–4222 RSC.
- A. Modrow, D. Zargarani, R. Herges and N. Stock, Dalton Trans., 2012, 41, 8690–8696 RSC.
- S. Bernt, M. Feyand, A. Modrow, J. Wack, J. Senker and N. Stock, Eur. J. Inorg. Chem., 2011, 5378–5383 CrossRef CAS PubMed.
- A. Modrow, M. Feyand, D. Zargarani, R. Herges and N. Stock, Z. Anorg. Allg. Chem., 2012, 638, 2138–2143 CrossRef CAS PubMed.
- J. Park, D. Q. Yuan, K. T. Pham, J. R. Li, A. Yakovenko and H. C. Zhou, J. Am. Chem. Soc., 2012, 134, 99–102 CrossRef CAS PubMed.
- L. Heinke, M. Cakici, M. Dommaschk, S. Grosjean, R. Herges, S. Bräse and C. Wöll, ACS Nano, 2014, 8, 1463–1467 CrossRef CAS PubMed.
- J. Brown, B. L. Henderson, M. D. Kiesz, A. C. Whalley, W. Morris, S. Grunder, H. Deng, H. Furukawa, J. I. Zink, J. F. Stoddart and O. M. Yaghi, Chem. Sci., 2013, 4, 2858 RSC.
- M. Tsuda and K. Kuratani, Bull. Chem. Soc. Jpn., 1964, 37, 1284–1288 CrossRef CAS.
- O. S. Bushuyev, A. Tomberg, T. Friščić and C. J. Barrett, J. Am. Chem. Soc., 2013, 135, 12556–12559 CrossRef CAS PubMed.
- L. Duarte, R. Fausto and I. Reva, Phys. Chem. Chem. Phys., 2014, 16, 16919–16930 RSC.
- A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139–4175 CrossRef CAS PubMed.
- Y. Feng, H. Liu, W. Luo, E. Liu, N. Zhao, K. Yoshino and W. Feng, Sci. Rep., 2013, 3, 3260 Search PubMed.
- Y. Imai, K. Naka and Y. Chujo, Macromolecules, 1999, 32, 1013–1017 CrossRef CAS.
- S. Deshmukh, L. Bromberg, K. A. Smith and T. A. Hatton, Langmuir, 2009, 25, 3459–3466 CrossRef CAS PubMed.
- T. Kondo, K. Yoshii, K. Horie and M. Itoh, Macromolecules, 2000, 33, 3650–3658 CrossRef CAS.
- M. Ueda, H. B. Kim, T. Ikeda and K. Ichimura, Chem. Mater., 1992, 4, 1229–1233 CrossRef CAS.
- P.-c. Che, Y.-n. He and X.-g. Wang, Chin. J. Polym. Sci., 2012, 30, 478–486 CrossRef CAS PubMed.
- K. Kinashi and Y. Ueda, Mol. Cryst. Liq. Cryst., 2006, 445, 223–230 CAS.
- O. Shekhah, H. Wang, T. Strunskus, P. Cyganik, D. Zacher, R. Fischer and C. Wöll, Langmuir, 2007, 23, 7440–7442 CrossRef CAS PubMed.
- O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Wöll, J. Am. Chem. Soc., 2007, 129, 15118–15119 CrossRef CAS PubMed.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- E. Uchida and N. Kawatsuki, Polym. J., 2006, 38, 724–731 CrossRef CAS.
- A.-H. Gao, B. Li, P.-Y. Zhang and J. Liu, Comput. Theor. Chem., 2014, 1031, 13–21 CrossRef CAS PubMed.
- J. C. Corchado, M. Luz Sanchez, I. F. Galvan, M. Elena Martin, A. Munoz-Losa, R. Barata-Morgado and M. A. Aguilar, J. Phys. Chem. B, 2014, 118, 12518–12530 CrossRef CAS PubMed.
- L. Heinke and C. Wöll, Phys. Chem. Chem. Phys., 2013, 15, 9295–9299 RSC.
- X. Ren, P. Rinke, V. Blum, J. Wieferink, A. Tkatchenko, A. Sanfilippo, K. Reuter and M. Scheffler, New J. Phys., 2012, 14, 053020 CrossRef.
- V. Blum, R. Gehrke, F. Hanke, P. Havu, V. Havu, X. Ren, K. Reuter and M. Scheffler, Comput. Phys. Commun., 2009, 180, 2175–2196 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- A. Tkatchenko and M. Scheffler, Phys. Rev. Lett., 2009, 102, 073005 CrossRef.
- M. V. Arnim and R. Ahlrichs, J. Comput. Chem., 1998, 19, 1746–1757 CrossRef.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: MOF structures, X-ray diffractograms, the synthesis description of AzoBiPyB as well as UV-vis and QCM data of the photoswitching in Cu2(BDC)2(AzoBiPyB). See DOI: 10.1039/c5cp01372k |
| This journal is © the Owner Societies 2015 |