Structure and dynamics of the fibronectin-III domains of Aplysia californica cell adhesion molecules†
Abstract
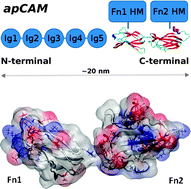
Due to their homophilic and heterophilic binding properties, cell adhesion molecules (CAMs) such as integrin, cadherin and the immunoglobulin superfamily CAMs are of primary importance in cell–cell and cell–substrate interactions, signalling pathways and other crucial biological processes. We study the molecular structures and conformational dynamics of the two fibronectin type III (Fn-III) extracellular domains of the Aplysia californica CAM (apCAM) protein, by constructing and probing an atomically-detailed structural model based on apCAM's homology with other CAMs. The stability and dynamic properties of the Fn-III domains, individually and in tandem, are probed and analysed using all-atom explicit-solvent molecular dynamics (MD) simulations and normal mode analysis of their corresponding elastic network models. The refined structural model of the Fn-III tandem of apCAM reveals a specific pattern of amino acid interactions that controls the stability of the β-sheet rich structure and could affect apCAM's response to physical or chemical changes of its environment. It also exposes the important role of several specific charged residues in modulating the structural properties of the linker segment connecting the two Fn-III domains, as well as of the inter-domain interface.


 Please wait while we load your content...
Please wait while we load your content...