Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green synthesis of zirconium-MOFs†
Helge
Reinsch
a,
Bart
Bueken
b,
Frederik
Vermoortele
b,
Ivo
Stassen
b,
Alexandra
Lieb
c,
Karl-Petter
Lillerud
a and
Dirk
De Vos
*b
aDepartment of Chemistry, University of Oslo, P.O. Box 1033 Blindern, 0315 Oslo, Norway
bCentre for Surface Chemistry and Catalysis, University of Leuven, Kasteelkpark Arenberg 23, 3001 Heverlee, Belgium. E-mail: dirk.devos@biw.kuleuven.be; Fax: +32 16 3 21998; Tel: +32 16 32 16 39
cInstitut für Chemie, Universitätsplatz 2, 39106 Magdeburg, Germany
First published on 30th April 2015
Abstract
The synthesis of Zr-MOFs under green, industrially feasible conditions was investigated. Two new compounds with bcu-topology and the fluorinated analogue of UiO-66 exhibiting fcu-topology were obtained and characterised. All products exhibit permanent porosity. In the bcu-frameworks the interaction with sulfate anions apparently induces an unusual eightfold connectivity of the Zr cluster.
Metal–organic frameworks (MOFs) are one of the most intensely investigated classes of materials in fields like catalysis,1 separation2 or luminescence.3 Among the thousands of different compounds, Zr4+-based MOFs clearly occupy an outstanding position. These MOFs often exhibit an extremely high thermal and chemical stability4 while having large specific surface areas and being excellent catalysts in various reactions.5 Except the MIL-140 series of compounds,6 most carboxylate-based Zr-MOFs are based on a hexanuclear Zr-oxo-cluster which coordinates up to twelve carboxylate moieties. This inorganic node is for example incorporated in the versatile series of compounds denoted as UiO-66-X (where UiO stands for University of Oslo and the X represents respective functional groups).7 A great leap forward for the synthesis of Zr-MOFs was the utilization of modulating reagents during the synthesis.8 This gave control over the crystal size9 but resulted as well in a number of new compounds based on different linker molecules, which can be only obtained by adding such “modulators” like formic, acetic or benzoic acid.10 These organic additives are not necessarily incorporated in the framework but can strongly affect the connectivity of the inorganic node.11
The synthesis of Zr-MOFs is commonly carried out in N,N-dimethylformamide or similar organic solvents. This represents a major drawback for the transfer of MOFs into applications since the employed amides are flammable, toxic and teratogenic. Recently, the synthesis of a functionalised Zr-MOF in water was reported.12 This inspired us to further investigate the aqueous chemistry of Zr4+-carboxylates. For two reasons we focussed on the use of zirconium sulphate. First of all, it is the only sufficiently water soluble Zr4+-source that could be employed under large-scale industrial conditions.13 In addition, it was already shown that SO42−-anions can be utilised to guide the connectivity of MOFs14 and therefore can be considered as potential inorganic modulators. This is supported by the structural diversity of sulphate based Zr-oxo-species.15 Furthermore we limited the synthesis conditions to parameters which would be suitable for a straightforward transfer to industrial scale, as described recently by BASF (Fig. 1).13 These parameters comprise the synthesis below the boiling point of the solvent or under reflux in an open system, in order to avoid autogenous pressure in the reactor. This enables the transfer to larger facilities, for example pilot plant stations, which are usually not compatible for high pressure syntheses. Moreover, the utilisation of nitrates or perchlorates is excluded due to their oxidising potential which impedes their use at larger scale. Chlorides should not be used either since such compounds can cause corrosion. In addition, water should be used as solvent since organic solvents are more expensive and in some cases very difficult to recycle.
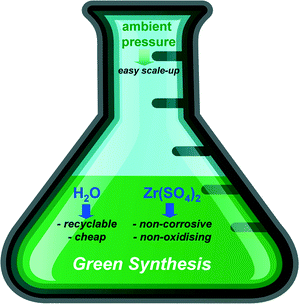 | ||
| Fig. 1 Illustration for the green synthesis of zirconium MOFs based on the cornerstones defined by BASF.13 | ||
Reacting 2-aminoterephthalic acid (H2BDC-NH2) and Zr(SO4)2·4H2O in water at 98 °C, a highly crystalline compound could be obtained under mild synthesis conditions. The PXRD data showed strong similarities to the pattern of UiO-66 but indexing indicated a lower symmetry with different unit cell parameters (extinction conditions corresponding to I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) , a = b = 14.2302(25) Å, c = 21.4142(22) Å; for UiO-66: space group Fm
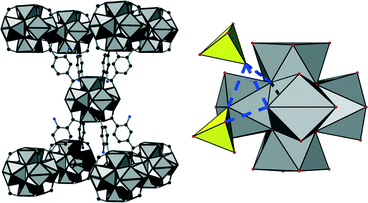
, a = b = 14.2302(25) Å, c = 21.4142(22) Å; for UiO-66: space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = b = c = 20.7004(2) Å). The observed space group is related to the one of UiO-66 by a supergroup-subgroup relationship. Utilising this relationship, a structural model and a chemical formula in agreement with further analytical results (EDX and IR spectroscopy, thermogravimetry) could be established; it was confirmed by Rietveld refinement (for details about deduction of the formula see ESI†). The crystal structure of [Zr6(OH)10.8(SO4)3.6(BDC-NH2)3(H2O)7.4]·nH2O (1) exhibits the same spatial arrangement of inorganic building units as UiO-66. However, due to the reduced connectivity (idealised eightfold instead of idealised twelvefold like in UiO-66) a bcu-topology is observed (Fig. 2 left). The inorganic nodes are interconnected via linker molecules only along the crystallographic c-axis while they are not interconnected but decorated with sulphate anions around the equatorial plane of the Zr6-cluster (Fig. 3). A fraction of the anions is directly coordinated to the inorganic node in a monodentate η1 fashion while another fraction is interacting via hydrogen bonds with μ3-OH-groups of the inorganic building unit (Fig. 2 right).
m, a = b = c = 20.7004(2) Å). The observed space group is related to the one of UiO-66 by a supergroup-subgroup relationship. Utilising this relationship, a structural model and a chemical formula in agreement with further analytical results (EDX and IR spectroscopy, thermogravimetry) could be established; it was confirmed by Rietveld refinement (for details about deduction of the formula see ESI†). The crystal structure of [Zr6(OH)10.8(SO4)3.6(BDC-NH2)3(H2O)7.4]·nH2O (1) exhibits the same spatial arrangement of inorganic building units as UiO-66. However, due to the reduced connectivity (idealised eightfold instead of idealised twelvefold like in UiO-66) a bcu-topology is observed (Fig. 2 left). The inorganic nodes are interconnected via linker molecules only along the crystallographic c-axis while they are not interconnected but decorated with sulphate anions around the equatorial plane of the Zr6-cluster (Fig. 3). A fraction of the anions is directly coordinated to the inorganic node in a monodentate η1 fashion while another fraction is interacting via hydrogen bonds with μ3-OH-groups of the inorganic building unit (Fig. 2 right).
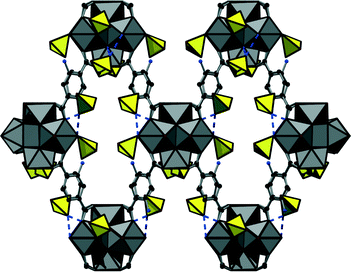
The positions of the sulphate anions as well as those of the linker molecules are only partially occupied which is a common phenomenon in Zr-MOFs. Thus a sulphate-decorated open framework with bcu-topology is obtained (Fig. 3) in which intersecting rhombic channels can be observed.
Employing pyromellitic acid (1,2,4,5-benzenetetracarboxylic acid or H4BTEC) under similar reaction conditions, a new compound with the approximate composition [Zr6(OH)14(BDC(CO2H)2)4(H2O)2(SO4)]·nH2O (2) could be obtained (for details see ESI†). The framework of 2 represents an isoreticular analogue of 1 bearing functional CO2H-groups instead of NH2-groups. Thus the use of sulfate in the synthesis induces again the formation of an eightfold connected bcu-framework. A similar structure directing effect was already observed for the synthesis of zirconium adipates.16 However, the amount of inorganic anions in 2 is much lower than in 1 (about one SO42− per inorganic node) and therefore the anions could not be located inside the framework by XRD methods. In this context it should be also mentioned that we assume that the intracluster oxygen atoms are solely part of OH− ions, which was previously reported for a molecular compound exhibiting similar hexanuclear cluster geometry obtained under similar conditions.17 Nevertheless, these intracluster atoms can be also part of O2− ions and thus the number of water molecules bound to the Zr4+ ions might be higher.
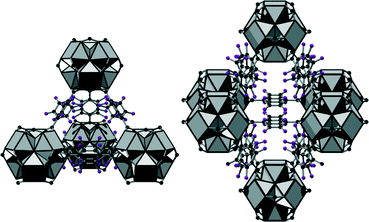
Employing tetrafluoroterephthalic acid (H2BDC-F4), we obtained UiO-66-F4 (3) with the approximate formula [Zr6O2(OH)6(BDC-F4)6(SO4)]·nH2O·1.2H2BDC-F4, the perfluorinated analogue of UiO-66. The framework of this compound exhibits the fcu-topology. Due to the F-functionalization, the aromatic rings are not arranged in plane with the carboxylate groups but disordered over two possible orientations (Fig. 4). The framework contains cavities of tetrahedral and octahedral shape with reduced size compared to UiO-66 (diameters of ≈3.6 and 6 Å in 3, respectively) which are interconnected by trigonal windows (diameter between 2.4 and 4.6 Å). Thus the employed green synthesis conditions do not only lead to the formation of compounds with bcu-topology but allow as well the formation of fcu-frameworks with UiO-66-structure.
EDX spectroscopy indicates the presence of sulphate in all three compounds. The sulphate ions in 1 could be localised by crystallographic methods and can only partially be removed by treatment in water at elevated temperature (≈3 SO42−-ions per Zr6-cluster after washing instead of 3.6 in the as-synthesized form), indicating a strong interaction between anions and framework. In the isoreticular framework 2, the sulphate ions are only present in very small amount and could therefore not be localised crystallographically. Moreover the sulphate ions can be completely removed by aqueous treatment, indicating only weak interaction with the framework. Thus the functional groups of the linker molecules clearly affect the binding strength of the anions to the bcu-framework, possibly due to the larger amount of missing linker molecules in 1 compared to 2. Another reason could be the fact that SO42− ions can only leave as uncharged moieties, i.e. H2SO4 molecules, which might be deprotonated by the weakly basic NH2-groups and therefore retained in the framework. In 3, sulphate ions are found as well. It can be conjectured that these ions could occupy positions resulting from the existence of missing-linker defects. This is supported by a recent report on the coordination of sulphate ions to the inorganic unit in a zirconium MOF with sixfold connecting inorganic building units.18 As in 2, the anions could not be localised due to the lower sulphate content (≈1 SO42−-ion per Zr6-cluster).
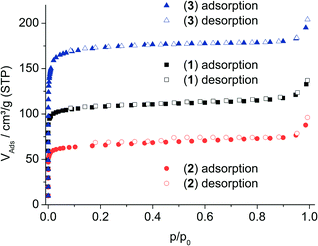
Upon thermal activation (150 °C under vacuum), all three compounds exhibit permanent porosity towards N2 at 77 K (Fig. 5) with isotherms characteristic for microporous materials. The apparent specific surface areas are SBET ≈ 420 m2 g−1 for 1, 250 m2 g−1 for 2 and 640 m2 g−1 for 3. The corresponding micropore volumes are VMIC = 0.15 cm3 g−1 for 1, 0.08 cm3 g−1 for 2 and 0.24 cm3 g−1 for 3, respectively, as calculated from the amount adsorbed at p/p0 = 0.5.
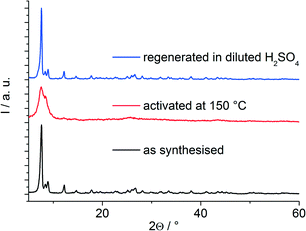
While compound 3 exhibits a rigid framework upon thermal activation and does not show any structural changes according to PXRD data, the frameworks of 1 and 2 exhibit a strong structural change upon removal of the occluded solvent molecules. The PXRD data (Fig. 6) after activation indicate a drastic decrease in long-range order. This amorphisation is fully reversible and a treatment in diluted sulphuric acid leads to a complete recovery of the compound's crystallinity already at a comparably low temperature (60 °C). Instead of sulphuric acid, diluted formic acid can be utilized as well. Thus the regeneration of the framework is in general induced by acids. We assume that this remarkable behaviour originates mostly from the partial occupancy of the positions of the organic building units and the presence of sulphate anions. A perfect bcu-framework could show structural changes as well, but in this case transitions between different crystalline framework conformations would be expected. The relatively high degree of structural imperfection, especially in the framework of 1, implies that the coordination environments of different inorganic nodes are rather heterogeneous and therefore the structural changes due to dehydration should be diverse across the framework structure. In addition to this effect, we also observed the formation of anhydrides in the structure of 2 which form upon condensation of two functional carboxylic acid groups (see ESI†). This was reported previously for other MOFs with free CO2H-groups,19 and might in this case also affect the crystallinity of the compound.
Since MOFs of the UiO-66-series are versatile Lewis-acidic catalysts,5 we examined also the presence of acid sites by IR-spectroscopy via adsorption of deuterated acetonitrile for 3. MOFs with UiO-66-structure are well known to possess active Lewis acid sites upon dehydration of the inorganic node at temperatures above 200 °C.20 In the new framework of UiO-66-F4 we observed Brønsted- as well as Lewis-acid sites after evacuation at 220 °C (ESI,† Fig. S7). We attribute the Brønsted sites to the inclusion of unreacted linker molecules in the pores of the framework. The inorganic cluster itself is dehydrated at high temperatures, resulting in sevenfold coordinated Zr-ions with Lewis acid sites. However, these sites could exist as well due to the presence of defects in the framework structure.21 The position of the signal for the CN-vibration at 2305 cm−1 indicated a much stronger Lewis acidity compared to the unfunctionalised parent framework UiO-66 as it was reported in ref. 5, further confirming the inductive effect of functional groups on the Lewis acidity of the Zr-cluster.5
Summarising our findings, three different new Zr-MOFs could be synthesised under green, industrially feasible synthesis conditions. The bcu-framework compounds show a reversible structural disarrangement which can be attributed to the high density of defects in the structures. Such findings could not only enable the synthesis of Zr-MOFs at large scale but moreover might also result in an extension of modulator-based MOF chemistry to inorganic modulating anions.
Acknowledgements
DDV thanks IWT (Vlaanderen) for support in the SBO project MOFShape, KU Leuven for CASAS Methusalem funding, FWO for research projects G.0486.12 and G.0256.14 and for fellowships to IS and BB and Belspo for funding in IAP 7/05.Notes and references
- A. Corma, H. García, F. X. Llabrés and I. Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS PubMed; P. Valvekens, F. Vermoortele and D. De Vos, Catal. Sci. Technol., 2013, 3, 1435 Search PubMed.
- B. Van de Voorde, M. Boulhout, F. Vermoortele, P. Horcajada, D. Cunha, J. S. Lee, J.-S. Chang, E. Gibson, M. Daturi, J.-C. Lavalley, A. Vimont, I. Beurroies and D. De Vos, J. Am. Chem. Soc., 2013, 135, 9849 CrossRef CAS PubMed; Q. Yang, A. D. Wiersum, H. Jobic, V. Guillerm, C. Serre, P. L. Llewellyn and G. Maurin, J. Phys. Chem. C, 2011, 115, 13768 Search PubMed.
- J. Heine and K. Müller-Buschbaum, Chem. Soc. Rev., 2013, 42, 9232 RSC.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632 CrossRef CAS.
- F. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. De Vos, J. Am. Chem. Soc., 2013, 135, 11465 CrossRef CAS PubMed; C. Gomes Silva, I. Luz, F. X. Llabres i Xamena, A. Corma and H. Garcia, Chem. – Eur. J., 2010, 17, 11133 CrossRef PubMed.
- V. Guillerm, F. Ragon, M. Dan-Hardi, T. Devic, M. Vishnuvarthan, B. Campo, A. Vimont, G. Clet, Q. Yang, G. Maurin, G. Ferey, A. Vittadini, S. Gross and C. Serre, Angew. Chem., Int. Ed., 2012, 51, 9267 CrossRef CAS PubMed; W. Liang, R. Babarao and D. M. D'Alessandro, Inorg. Chem., 2013, 52, 12878 CrossRef PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem. – Eur. J., 2011, 17, 6643 CrossRef CAS PubMed.
- G. Wißmann, A. Schaate, S. Lilienthal, I. Bremer, A. M. Schneider and P. Behrens, Microporous Mesoporous Mater., 2012, 152, 64 CrossRef PubMed.
- W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443 CrossRef CAS PubMed; D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10453 CrossRef PubMed; H.-L. Jiang, D. Feng, K. Wang, Z.-Y. Gu, Z. Wei, Y.-P. Chen and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 13934 CrossRef PubMed; B. Bueken, H. Reinsch, N. Reimer, I. Stassen, F. Vermoortele, R. Ameloot, N. Stock, C. E. A. Kirschhock and D. De Vos, Chem. Commun., 2014, 50, 10055 RSC.
- V. Bon, I. Senkovska, I. A. Baburin and S. Kaskel, Cryst. Growth Des., 2013, 13, 1231 Search PubMed; V. Bon, V. Senkovskyy, I. Senkovska and S. Kaskel, Chem. Commun., 2012, 84, 8407 RSC.
- Q. Yang, S. Vaesen, F. Ragon, A. D. Wiersum, D. Wu, A. Lago, T. Devic, C. Martineau, F. Taulelle, P. L. Llewellyn, H. Jobic, C. Zhong, C. Serre, G. De Weireld and G. Maurin, Angew. Chem., Int. Ed., 2013, 52, 10316 CrossRef CAS PubMed.
- M. Gaab, N. Trukhan, S. Maurer, R. Gummaraju and U. Müller, Microporous Mesoporous Mater., 2012, 157, 131 CrossRef CAS PubMed.
- A. C. Sudik, A. P. Cote, A. G. Wong-Foy, M. O'Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2013, 45, 2528 CrossRef PubMed.
- P. J. Squattrito, P. R. Rudolf and A. Clearfield, Inorg. Chem., 1987, 26, 4240 CrossRef CAS; M. Hansson, Acta Chem. Scand., 1973, 27, 2614 CrossRef PubMed; D. B. McWhan and G. Lundgren, Inorg. Chem., 1966, 5, 284 CrossRef; Y.-J. Hu, K. E. Knope, S. Skanthakumar, M. G. Kanatzidis and J. F. Mitchell, J. Am. Chem. Soc., 2013, 135, 14240 CrossRef PubMed.
- H. Reinsch, I. Stassen, B. Bueken, A. Lieb, R. Ameloot and D. De Vos, CrystEngComm, 2015, 17, 331 RSC.
- L. Pan, R. Heddy, J. Li, C. Zheng, X.-Y. Huang, X. Tang and L. Kilpatrick, Inorg. Chem., 2008, 47, 5537 CrossRef CAS PubMed.
- J. Jiang, F. Gándara, Y.-B. Zhang, K. Na, O. M. Yaghi and W. G. Klemperer, J. Am. Chem. Soc., 2014, 136, 12844 CrossRef CAS PubMed.
- N. Reimer, B. Gil, B. Marszalek and N. Stock, CrystEngComm, 2012, 14, 4119 RSC; F. Ragon, B. Campo, Q. Yang, C. Martineau, A. D. Wiersum, A. Lago, V. Guillerm, C. Hemsley, J. F. Eubank, M. Vishnuvarthan, F. Taulelle, P. Horcajada, A. Vimont, P. L. Llewellyn, M. Daturi, S. Devautour-Vinot, G. Maurin, C. Serre, T. Devic and G. Clet, J. Mater. Chem. A, 2015, 3, 3294 Search PubMed.
- F. Vermoortele, M. Vandichel, B. Van de Voorde, R. Ameloot, M. Waroquier, V. Van Speybroeck and D. De Vos, Angew. Chem., Int. Ed., 2012, 51, 4887 CrossRef CAS PubMed.
- G. C. Shearer, S. Chavan, J. Ethiraj, J. G. Vitillo, S. Svelle, U. Olsbye, C. Lamberti, S. Bordiga and K. P. Lillerud, Chem. Mater., 2014, 26, 4068 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthesis and structure determination, TG data and IR spectra and structural information files. See DOI: 10.1039/c5ce00618j |
| This journal is © The Royal Society of Chemistry 2015 |