Highlights from Faraday discussion 170: Challenges and opportunities of modern mechanochemistry, Montreal, Canada, 2014
Tomislav
Friščić
*a,
Stuart L.
James
b,
Elena V.
Boldyreva
cd,
Carsten
Bolm
e,
William
Jones
f,
James
Mack
g,
Jonathan W.
Steed
h and
Kenneth S.
Suslick
i
aDepartment of Chemistry and the Centre for Green Chemistry and Catalysis, McGill University, 801 Sherbrooke St. W., H3A 0B8 Montreal, Canada. E-mail: tomislav.friscic@mcgill.ca
bSchool of Chemistry and Chemical Engineering, Queen's University, Belfast, UK
cNovosibirsk State University, ul. Pirogova, 2, Novosibirsk, Russia
dInstitute of Solid State Chemistry and Mechanochemistry SB RAS, ul. Kutateladze, 18, Novosibirsk 630128, Russia
eInstitut für Organische Chemie der RWTH Aachen University, Landoltweg 1, 52056 Aachen, Germany
fDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK
gDepartment of Chemistry, University of Cincinnati, 301 Clifton Court, Cincinnati, OH 45221-0172, USA
hDepartment of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK
iSchool of Chemical Sciences, University of Illinois at Urbana-Champaign, 600 S. Mathews Av., Urbana, IL 61801, USA
First published on 18th March 2015
Abstract
The Faraday Discussion Mechanochemistry: From Functional Solids to Single Molecules which took place 21–23 May 2014 in Montreal, Canada, brought together a diversity of academic and industrial researchers, experimentalists and theoreticians, students, as well as experienced researchers, to discuss the changing face of mechanochemistry, an area with a long history and deep connections to manufacturing, that is currently undergoing vigorous renaissance and rapid expansion in a number of areas, including supramolecular chemistry, smart polymers, metal–organic frameworks, pharmaceutical materials, catalytic organic synthesis, as well as mineral and biomass processing and nanoparticle synthesis.
In May 2014, the New Residence Hall of McGill University provided a home to a unique gathering of approximately 100 experts, novices and aficionados of mechanochemistry (Fig. 1).1 Entitled Mechanochemistry: From Functional Solids to Single Molecules, the 256th Faraday Discussion was inspired by the rising importance of mechanochemistry in making, understanding and manipulating molecules, and was envisioned as a first step towards bridging the perceived gap between researchers in different sub-areas of mechanochemistry.
 | ||
| Fig. 1 Macdonald Physics Building, a hallmark of McGill University, and the site of Ernest Rutherford's pioneering studies on α-, β- and γ-radiation. | ||
Grinding and milling of bulk materials have traditionally been associated with metals,2 inorganic materials,3 specifically minerals,4 and polymers,5 but has now also become the focus of chemists wishing to develop solvent-free and energy-efficient approaches to molecules and molecular materials, or who are simply interested in exploring the properties and advantages of a non-conventional reaction medium. Mechanochemistry has found followers in broad areas of chemistry and industrial development, ranging from inorganic chemistry6 to organic7 and supramolecular chemistry,8 with the most recent additions including the synthesis of coordination polymers (including metal–organic frameworks, MOFs)9 and pharmaceutical solids (e.g. making and screening polymorphs, cocrystals, amorphous phases).10 In contrast, ultrasonic radiation provides another way to expose molecules and particles to high shear forces suitable for materials processing and fundamental studies of chemical bonding.11 The most clear-cut approach to mechanochemistry is manipulation of individual molecules and atoms by tools of high precision, such as the tip of the atomic force microscope (AFM), allowing the determination of fundamental properties of individual molecules (e.g. flexibility of protein structures), as well as precise surface patterning and modification.12
Another, very different aspect of mechanochemistry is understanding the processes which occur in different types of mechanical devices, e.g. by in situ monitoring of reaction progress,13 or theoretical modelling of distribution of stress and temperature throughout the sample being milled, with the ultimate purpose to scale-up and optimise the type of mechanical action for a specific chemical transformation.
Therefore, it was clear from the outset of this meeting that mechanochemistry is of exceptional, as well as growing interest for chemists, physicists, theoreticians and industrial researchers. This broadness was reflected in the composition of participants who spent three days discussing fundamentals, applications and problems that distinguish or, perhaps, connect different aspects of mechanochemistry. The participants included researchers from across North & South America (USA, Canada, Mexico, Colombia, Brazil), Europe (United Kingdom, France, Germany, Poland, Slovakia, Croatia, Switzerland) and Asia (India, China, Russia), representatives of chemical, mining, pharmaceutical and food industries, as well as representatives of manufacturers of milling equipment (e.g. Retsch US, Spex).
Opening lecture
The conference was opened by William Jones (University of Cambridge), who provided a short but elaborate overview of the history of mechanochemistry (noting that some of the seminal experiments in mechanochemistry were reported by Michael Faraday) and its future opportunities (DOI: 10.1039/C4FD00162A). The opening lecture highlighted the importance of mechanical processing in the context of industrial and materials manufacture, and especially in pharmaceuticals materials science. Prof. Jones described how sophisticated mechanochemical techniques, such as liquid-assisted grinding, bring about improvements in generating and controlling new solid forms of pharmaceuticals, such as pharmaceutical cocrystals,12 while at the same time the behaviour required of materials and processing parameters during large-scale mechanical processing remain an open problem. This inspiring and exciting opening lecture was followed by the first of the four topical sessions, chaired by Stuart L. James (Queen's University Belfast) and Tomislav Friščić (McGill University).Session 1: mechanochemistry of organic molecules, soft materials and pharmaceuticals
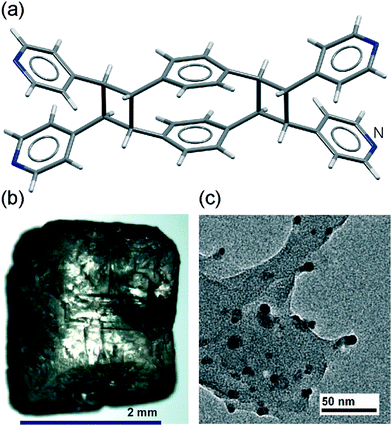
The session was off to a dynamic start with the presentation by Leonard R. MacGillivray (University of Iowa) who described a novel and experimentally simple strategy for coupling photochemistry with mechanochemical milling (DOI: 10.1039/C4FD00006D). This vortex grinding strategy14 allows mechanochemical grinding of photoactive solids under continuous exposure to ultraviolet light, leading to completely solvent-free and quantitative synthesis of a para-cyclophane (Fig. 2a). The discussion that ensued, and that was facilitated by the student hosts Patrick A. Julien and Igor Huskić (McGill University), established that this technique resolves a long-standing problem of light penetration in solid-state photochemical synthesis.† The second presentation, by Graeme M. Day (University of Southampton), described efforts in computationally addressing reversible covalent reactions by mechanical milling (DOI: 10.1039/C4FD00162H), specifically the base-catalyzed equilibration of aromatic disulfides.15 Outcomes of these computational studies show that lattice energy calculations, combining anisotropic atom–atom potentials with molecular density functional theory (DFT), open a way to rationalise or even predict the course of reversible mechanochemical transformations. However, successful modelling and prediction of mechanochemical reactivity under thermodynamic equilibrium are still at a very early stage, with the principle hurdle being the conformational flexibility of explored molecules. The discussion† that followed was highly positive about this major computational advance in modelling mechanochemical reactions, and highlighted the modelling of reaction kinetics as the next big challenge. In the final presentation of this session Tamara D. Hamilton described the application of mechanochemistry to one of the persistently highly challenging problems of synthetic organic chemistry: the synthesis of porphyrins (DOI: 10.1039/C4FD00140G). Prof. Hamilton presented a two-step methodology to access a variety of meso-substituted porphyrins by mechanochemical reaction of suitable aldehydes and pyrroles, followed by a mechanochemical oxidation step. This protocol gave porphyrin targets with significant benefits compared to solution-based protocols: reactions are much faster (less than an hour compared to several hours) and the use of high temperatures and corrosive reagents is avoided (reflux in propionic acid is the conventional synthetic alternative). A mechanochemical first step followed by oxidation in chloroform gives yields comparable to those obtained from an entirely solution-based process, while eliminating a large amount of solvent and reducing reaction time. The subsequent discussion† revealed that the ‘green’ nature of this methodology can be further improved by the using solvent-free entrainment sublimation for product purification. The benefits and simplicity of this mechanochemical approach are reflected in all metrics relevant to sustainability and environmentally-friendly nature of chemical reactions.The effect of different types of mechanical action on chemical reactivity was demonstrated in the contribution of Adam Michalchuk and co-workers (University of Edinburgh and Novosibirsk State University) (DOI: 10.1039/C4FD00150D). Mixtures of α-glycine and β-malonic acid were shown to produce two unique reaction products when submitted to isolated impact and shear treatments. This leads to the formation of zones with distinct chemical reactivity within a single milling vessel,16 an observation of particular importance when considering the purity and control of industrialized mechanochemical processes. Thermal investigation suggested that reactivity under shear treatment may be induced due to heating, while a tablet-induced pressure model was suggested as a potential mechanism for reactions under impact treatment. The first session was followed by a Lightning Session of 14 rapid flash presentations selected by the organizers to highlight the most outstanding poster contributions.
Flash presentations
The flash presentations were given by Katharina Holz (University of Kiel) on “A 1,2,3-triazole for mechanically induced 1,3-dipolar cycloreversion”, who described mechanical reversal of the popular ‘click reaction’ of an alkyne and an azide by pulling on a triazole fragment using the AFM tip. Next, Robert Schmidt (University of Jena) highlighted his work on assessing energetics of the milling aldol reaction between barbituric acid and vanillin. Filip Topić (University of Jyväskylä) described a new strategy for the preparation of ternary (three-component) cocrystals held by a combination of hydrogen and halogen bonds, enabled by mechanochemical cocrystal screening. The next presenter was Manuel Jörres (RWTH Aachen University) who presented the use of ball milling for solvent-free organocatalytic Michael additions. Qiwen Su (Barry University) gave a highlight of a novel mechanochemical technique for the preparation of porphyrins, while Daniel Prochowicz outlined an unprecedented technique for the synthesis of isoreticular MOFs (IRMOFs), including the archetypal material MOF-5, that have so far resisted synthesis by mechanochemical means.17 The next presentation was from Canada, given by George Margoutidis (Memorial University, Newfoundland), who described ball milling for depolymerisation of chitin into small amino-substituted molecules. Gurpaul Kochhar (Queen’s University, Kingston) gave a brief overview of his work on theoretical modelling of mechanically-induced distortions in molecular structure and ring opening of cyclobutene mechanophore. Frédéric Lamaty (Institut des Biomolécules Max Mousseron, Montpellier) gave a summary of his poster “Mechanochemistry in organic synthesis”, describing stoichiometric and catalytic mechanochemistry in the synthesis of amides, amino acids and (oligo)peptides. Next, Vladimir Stilinović (University of Zagreb) gave details of his systematic investigation of the role of acetone vapor on forming new crystalline phases in thin layers. Aileen Nielsen (Columbia University) highlighted her poster “High resolution single-molecule force spectroscopy probes inter-molecular interactions”, on using AFM to evaluate the use of intermolecular recognition and non-covalent bonding in making two-molecule junctions. Cristina Mottillo (McGill University) presented a novel approach for the synthesis of solid-state fluorescent materials, by entrapment of BODIPY dyes into MOFs using either mechanochemistry or the recently developed accelerated aging technique. Next, Matej Baláž described the synthesis of L-cysteine-capped core–shell luminescent quantum dot nanoparticles CdS@ZnS by a rapid three-step milling procedure, delineated in his poster “Characterization of mechanochemically synthesized CdS@ZnS quantum dots: focus on surface properties and potential interaction with capping agent”. The final presentation was given by Louis Cuccia (Concordia University), whose poster “Enantiomer-specific oriented attachment in conglomerate crystals of guanidine carbonate” demonstrated interparticle collisions as a mechanism of crystal growth and spontaneous deracemization in Viedma ripening (Fig. 2b). The rapidly concluded Lightning Session was followed by a rich and social poster session, accompanied by an apparently almost inexhaustible wine reception and a free evening that saw a large number of meeting participants spontaneously gathering at the McGill University Graduate Student Club, the (in)famous Thomson House pub.Session 2: mechanochemistry of inorganic compounds and coordination-based materials
The excitements of the previous night did not hinder the timely beginning of the second session on Thursday morning. The session, chaired by Leonard MacGillivray and Laszlo Takacs, was opened by David W. Peters (ATMI, USA), who described the first large-scale synthesis of an important organometallic compound bis(n-propyltetramethylcyclopentadienyl)strontium (SrCp′2), by mechanical agitation of SrI2 and KCp′ in diethyl ether (EtOEt) (DOI: 10.1039/C4FD00157A). Development of this first large-scale organometallic mechanosynthetic procedure was based on the unexpected discovery that small scale reactions, stirred by a magnetic stirrer, reproducibly gave the product (SrCp′2·EtOEt), whereas large batch reactions stirred by an overhead stirrer did not result in any product. This revealed that the reaction takes place by mechanical crushing of the reagent solids in the presence of ether. Realisation of the mechanochemical nature of this process enabled scale-up to at least 0.5 kg amounts using a specially designed large-volume glass reactor equipped with steel milling balls. The discussion focused on the importance of ether which, although a poor solvent for the reactants, performs several roles in this process: as a ‘lubricant’ for kinetic acceleration of the reaction, as a heat sink for this exothermic reaction, as a dispersant for product removal from the reaction sites, and as a suspending agent for the reactants.† Next, Lucia Maini (University of Bologna) highlighted mechanochemical synthesis and discovery of new luminescent copper(I) iodide (CuI) materials based on the ligand tris(2-pyridyl)phosphine (PN) (DOI: 10.1039/C4FD00164D). Ball milling was demonstrated as a simple means, complementary to solution synthesis, to screen for supramolecular architectures with attractive properties, such as phosphorescence.18 While conventional solution chemistry yields three different CuI architectures, application of ball milling readily expanded this library of luminescent CuI motifs. Solution-achievable phases are based either on a monomeric CuI motif in CuI(PN)3, a dimer in Cu2I2(PN)3 or a tetramer in Cu4I4(PN)2. In addition to these, ball milling also made accessible new materials CuI(PN)2, of still unknown structure, and CuIPN0.5 with a polymeric CuI∞ structure. Her presentation also tackled the problem of structurally characterising mechanochemical products, either by crystal growth in solvothermal synthesis or by structure solution from X-ray powder diffraction data using direct space methods. Lisa Tröbs (BAM Federal Institute for Materials Research and Testing in Berlin) outlined mechanistic studies of mechanochemical synthesis of bismuth(III) coordination frameworks, conducted by rapid stepwise analysis using Raman spectroscopy and X-ray powder diffraction (DOI: 10.1039/C4FD00163F). The results demonstrated time resolution in seconds, so far unprecedented in stepwise studies of mechanochemical reactions. In a twist particularly relevant for supramolecular solid-state chemistry and pharmaceutical materials, the presentation also highlighted the use of rapid stepwise analysis for monitoring the cocrystallisation of two pharmaceutical ingredients, carbamazepine and indomethacin.Next, the discussions switched to inorganic materials, such as magnetic oxides and hydrides for hydrogen storage.† The first presentation was given by Vladimir Šepelák (Karlsruhe Institute for Technology), whose group19 has pioneered multi-faceted investigations of mechanochemically prepared materials, combining Mössbauer and solid-state NMR (SSNMR) spectroscopy with X-ray diffraction and high-resolution electron microscopy. The presentation gave a detailed structural explanation for the modification of magnetism in barium hexaferrite by milling (DOI: 10.1039/C4FD00137G). Milling was found to induce amorphization and distortion of the crystal structure of the material down to ca. 2 nm below the milled particle surface. The ability to observe surface deformations in milled particles with nanometer resolution, and provide a detailed description of such deformations by combining spectroscopy, diffraction and microscopy gave an excellent illustration of the power of modern analytical techniques and an inspiration for further investigations in inorganic, metal–organic and organic systems. Further in the spirit of mechanochemistry in the service of inorganic chemistry, Shalabh Gupta and Vitalij Pecharsky (Iowa State University) presented a new, mechanochemical methodology for the direct generation of non-solvated alane, AlH3, a highly unstable compound of considerable appeal for its high gravimetric hydrogen content (DOI: 10.1039/C4FD00161J). Although mechanochemical synthesis of alane has been achieved previously by milling LiAlH4 with AlCl3, such procedures were vexed by in situ degradation of reactive intermediates into metallic aluminium. The Ames group circumvented this obstacle by performing AlCl3 addition in several small portions, in that way ensuring a continuous excess of hydride reagent. At the same time, the procedure was ingeniously simplified by replacing the highly reactive and difficult to handle LiAlH4 with LiH resulting in the first room-temperature, solvent-free approach to generate alane from stoichiometric reagents and without the need for stabilizing organic solvents.
The next two presentations addressed the hot and rapidly developing area of nanoparticle20 mechanosynthesis. Audrey Moores (McGill University) unveiled a methodology for mechanochemical production of nanoparticles of copper, gold, palladium, ruthenium or rhenium embedded within a reductive organic matrix (DOI: 10.1039/C4FD00053F). The mechanochemical synthesis of these nanoparticles is exceedingly simple, achieved without extensive dilution normally required by conventional procedures. Milling with Kraft lignin led to in situ reduction of metal precursors to elementary metal nanoparticles with sizes from 2.8 nm (from palladium(II) acetate) to 14.8 nm (from HAuCl4), that remain immobilised in the organic lignin matrix. Besides providing a rapid, simple and inexpensive approach to metal nanoparticles, this work also revealed the ability to direct the organisation of nanoparticles either within or onto the organic matrix by judicious choice of precursor: inorganic precursors led largely to nanoparticles associated with lignin surface, while more hydrophobic precursors preferred intimate nanoparticle–lignin composites (Fig. 2c). Development of a mechanochemical bottom-up procedure21 for solvent-free assembly of nanoparticles of binary compounds was presented by Peter Baláž (Institute of Geotechnics, Slovak Academy of Sciences) who outlined the synthesis of monodisperse PbS nanoparticles (32–34 nm diameter) by milling lead(II) acetate with L-cystine (disulfide dimer of the aminoacid L-cysteine). High-resolution transmission electron microscopy revealed that nanoparticles are highly crystalline, with clearly observable lattice fringes, and faceted, adopting the morphology of truncated octahedra. Excess cystine was found attached to particle surface, highlighting a simple methodology to obtain group II–VI nanoparticles with biocompatible surfaces.
All four presentations in the inorganic materials session inspired active discussions that addressed different aspects of particle structure, formation and advanced techniques for product characterisation in mechanochemical reactions. The presentations formed a neat, high-impact overview of technological and methodological advances in making and understanding mechanochemically synthesised nanoparticles.22 The effectiveness and simplicity of mechanochemistry in generating monodisperse nanoparticles are particularly impressive when considered from the perspective of extensive research invested in developing conventional approaches to such systems in solution, which demand careful control of reagent choice and addition, and depend on extensive use of solvent and additional energy input (e.g. rapid shaking or sonication).
Session 3: mechanistic understanding, catalysis and scaling up
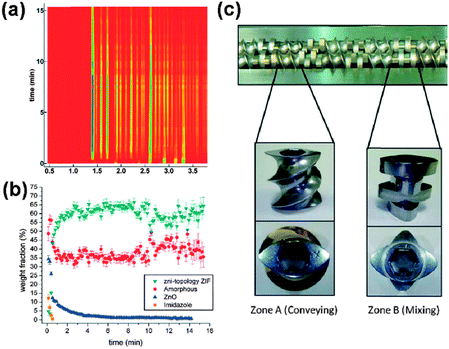
After the lunch break, which gave extensive opportunity for further interaction between presenters and exhibitors, the afternoon session, chaired by Carsten Bolm and Peter Baláž, began by addressing the latest development of a technique for quantitative real-time monitoring of mechanochemical reactions. Ivan Halasz (Institute Ruđer Bošković) described the use of high energy synchrotron X-ray powder diffraction for quantitative in situ and real-time monitoring of mechanochemical reaction pathways (DOI: 10.1039/C4FD00013G). The presented studies, which focused on mechanosynthesis of zeolitic imidazolate frameworks (ZIFs), demonstrated that kinetic behaviour of mechanochemical reactions can strongly resemble 1st or 2nd order reaction kinetics of solution reactions (Fig. 3a and b).This confirmed the notion that rapidly milled reaction mixtures can behave as ‘pseudo-fluid’ reaction environments.23 In addition, the ability to conduct a quantitative Rietveld analysis of in situ mechanochemical reaction data provided an unprecedented opportunity to monitor the participation of amorphous phases in the reactions. Next, Richard Blair (University of Central Florida) addressed scaling-up of mechanochemical reactions, with particular focus on differences between different types of milling equipment, such as shaker, planetary and attrition mills, and how differences in design of milling equipment affect energy and materials transfer in mechanochemical processes (DOI: 10.1039/C4FD00007B).† The contribution dealt with two industrially relevant processes: mechanochemical degradation of cellulose and mechanochemical edge oxidation and delamination of graphene. A particularly eye-catching aspect of the presentation was the simulation of the motion of milling media and of the distribution of high-energy impacts, obtained by discrete element modelling. Such modelling, a novelty to a large number of participants, offers a means not only to understand the motion of milling media in different experiments, but also to rationalise and even predict thermal effects in mechanochemical reactions.
Reaction scale-up was also the topic of the presentation by Karthik Nagapudi (Amgen Inc.), who outlined details of large scale mechanochemical process for the synthesis of pharmaceutical cocrystals (DOI: 10.1039/C4FD00153A). This team previously described the first application of extrusion24 for scaling up of mechanochemical cocrystal formation and the current presentation was the first to highlight the details of the design, focusing on the synthesis of cocrystals of AMG-517, a potent and selective TRPV1 antagonist, with sorbic acid (Fig. 3c). Twin screw extrusion provides an efficient, scalable, and environmentally-friendly process for the continuous production of cocrystals. The presentation, however, also initiated a lively discussion on the importance of amorphous phases or eutectics as either intermediates25 or necessary activated phases in mechanochemical reactions.†
Understanding and modelling of thermal effects and energy transfer are among the central challenges of mechanochemistry. This topic was addressed by Laszlo Takacs (University of Maryland, Baltimore County) who presented studies of mechanochemically-induced self-propagating reactions (MSRs) (DOI: 10.1039/C4FD00133D). The MSRs are a unique subset of mechanochemical reactions in which the ratio of reaction enthalpy and heat capacity (ΔH/C) is sufficiently large to allow the reaction to become a self-sustained thermal process. Such behavior is found in milling thermite mixtures, as well as binary mixtures leading to the formation of borides, carbides, or chalcogenides.26 One aspect of MSRs that particularly captured the attention of the audience was the induction period, whereupon milling leads to little initial chemical change but is followed by a rapid onset of the reaction. It was noted during the vigorous discussion† that this delay, although still poorly understood, corresponds to a mechanochemical activation of starting materials, which can take place by different mechanisms simultaneously, including particle comminution, mixing, surface amorphisation, structure distortion and creation of defects. Such a view of mechanical activation is supported by detailed investigations of milled particles, presented by the Šepelák group. Consequently, MSRs provide clear examples of mechanochemical transformations in which mechanical agitation is primarily a means to activate, rather than sustain, a chemical reaction.
In the final talk of the session Achim Stolle (Friedrich-Schiller University Jena) described a systematic investigation of the experimental parameters (conveniently divided into chemical, technological and process parameters) important for reaction optimisation, scaling-up and process intensification in planetary milling.27 This study provided a unique overview of the richness of experimental parameters that can be varied for fine-tuning a mechanochemical organic reaction, such as the diameter of milling balls, rotation frequency, as well as the filling degree of the milling vessel (with respect to packing of milling media and/or the amount of substrate). This work, which is of particular relevance to industrial researchers, is very likely to become a central reference point in the design of mechanochemical processes in planetary mills.
The evening after the afternoon session saw all participants gather once again, this time for the Conference Dinner held in the classical environment of the McGill Faculty Club ballroom. An excellent and high-spirited mood extended throughout the evening, enhanced by the traditional Loving Cup ceremony of the Faraday Society (Fig. 4). The dinner was also an excellent environment for the acting president of the Faraday Society, Prof. Peter Skabara (University of Strathclyde) to announce the winner of the Best Poster Award, graduate student Gurpaul S. Kochhar, for his work Modelling mechanical processes under constant force and constant extension conditions, performed under the supervision of Prof. Nicholas J. Mosey at Queen's University, Kingston. Following an outstanding four-course meal, the scientific discussions (Fig. 4) continued over music and pool at the Bar de Pins, a popular venue of McGill graduate students. conveniently located across the road from the conference site.
Session 4: sonication and macromolecular mechanochemistry
The final session of the meeting took place in the morning of the third day of the conference and was dedicated to approaches to mechanochemistry other than milling: molecular cleavage under ultrasonic irradiation, and manipulation of individual chemical bonds using a tip of the atomic force microscope.The duty of chairing this morning session belonged to James Mack (University of Cincinnati) and Richard Blair, and the opening presentation was given by Stephen L. Craig (Duke University), who described a highly efficient design (DOI: 10.1039/C4FD00001C) for incorporating mechanophores, i.e. functional groups sensitive to mechanical tug,28 into polymers, leading to materials responsive to mechanical deformation. In particular, it was discovered that the incorporation of polybutadiene containing mechanophoric gem-dibromocyclopropane groups as a central component in a triblock ABA copolymer with polystyrene leads to at least a 7-fold increase in mechanochemical response compared to mechanophore-rich poly(butadiene) only. Next, Martin Beyer (Leopold-Franzens-Universität Innsbruck) presented the use of atomic force microscope spectroscopy (AFMS) for evaluating energetics and mechanisms of covalent bond scission. The presentation compared dynamic (or force-ramp) AFMS (DOI: 10.1039/C4FD00119A), in which bond cleavage is recorded while ramping the force on a molecule stretched between a surface and the AFM tip, to force-clamp AFMS, which monitors bond cleavage events with the molecule under constant stress.29 While both methodologies gave similar bond dissociation parameters for acid hydrolysis of amylose esters, which confirmed the previously proposed degradation mechanism, the force-clamp technique gave a greater ability to identify fine differences in the structure of the reactive ester site. The discussions then switched to bulk behaviour of mechanically treated polymers, with the talk of Fernando Galembeck (National Center for Energy and Materials Research, Campinas, Brazil) on the triboelectric effect, i.e. the appearance and distribution of electrical charge on rubbed polymer surfaces (DOI: 10.1039/C4FD00118K) (Fig. 5a).
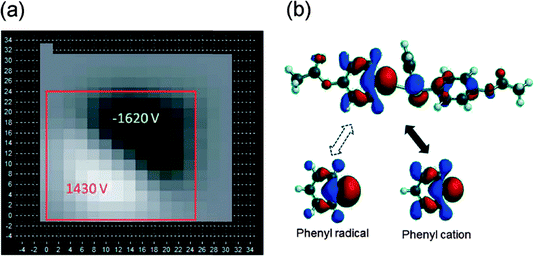 | ||
| Fig. 5 (a) Electrostatic potential maps of PTFE film (left) sheared with low-density polyethylene foam slabs, presented by F. Galembeck (DOI: 10.1039/C4FD00118K) and (b) LUMO of the triphenylsulfinium mechanophore compared to the LUMO of the phenyl radical and cation, calculated in Spartan'10 at the B3LYP/6-31G* level of theory, presented by C. E. Diesendruck (DOI: 10.1039/C4FD00027G). | ||
Prof. Galembeck provided a detailed analysis of chemical composition and charge on surfaces of rubbed polymers, pointing to the formation of highly mobile macromolecular fragments that readily diffuse across the surface or can even be transferred in the form of nanoparticles. Whereas this study provided an explanation why it has so far not been possible to establish a triboelectrical series of polymers, a particularly excited discussion developed around the demonstrated presence of nitrogen on the surface of initially completely nitrogen-free polymers, such as poly(tetrafluoroethylene) (PTFE) or polyethylene.† Prof. Galembeck explained that the extensive coverage of polymer surface by nitrogen species is most likely the result of chemisorption from air, sparking speculations on possible development of processes for binding atmospheric nitrogen. The final talk of the session was delivered by Charles E. Diesendruck from the Moore group (University of Illinois at Urbana – Champaign), on the development of a new triphenylsulfinium-based mechanophore (DOI: 10.1039/C4FD00027G). Theoretical investigation of mechanochemical scission of this mechanophore indicated that heterolytic bond breaking, generating phenyl cations, would be slightly preferred to the homolytic cleavage, which would give rise to phenyl cations (Fig. 5b). The predictions were confirmed by sonochemically-induced cleavage of the mechanophore placed between poly(methylacrylate) chains, with the formation of phenyl cations detected by 1H NMR and UV/Vis spectroscopy in trapping experiments. The work clearly illustrated the ability to use theoretical modelling for developing new mechanophores for applications in self-healing materials. Anatoly Politov and Olga Golyazimova (Novosibirsk Institute of Solid State Chemistry and Mechanochemistry, Russian Academy of Sciences) addressed the efficiency of converting mechanical energy into useful chemical energy. Focusing on the application of ultrasound to lignocellulosic substrates, with the intention to facilitate subsequent fermentative hydrolysis, it was shown that judicious selection of mechanical treatment allows ten-fold increases in reaction yield, even at small inputs of energy. Development of such a targeted mechanochemical approach depends on detailed studies of how mechanical action can be optimised to generate defects and radicals central for a particular transformation.
Closing lecture
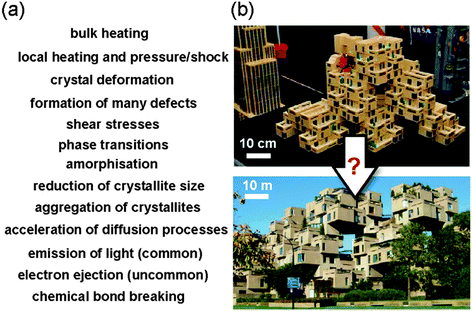
The Closing Lecture (DOI: 10.1039/C4FD00148F) was delivered by Kenneth Suslick (University of Illinois at Urbana-Champaign), a renowned expert and a pioneer in the development of sonochemistry of molecules and materials.30 The closing lecture provided an excellent and light-spirited overview of the development and recent advances in sonochemistry, and at the same time highlighted connections to grinding or milling. Prof. Suslick strongly stressed the analogies and connections between different areas of mechanochemistry, such as ball milling and sonic irradiation. Although these areas may appear unconnected on superficial inspection, a careful examination of physical and chemical consequences of sonochemical and grinding processing on inorganic materials reveals exactly the same list (Fig. 6a).Prof. Suslick also noted that modern mechanochemistry, despite a tremendous growth of interest, remains a highly underdeveloped area, poorly understood by experimentalists and theoreticians alike. This, to an extent, can be attributed to the complex nature of mechanochemical processes. While these can be divided into surface modification, crack propagation, surface electrification, plasma-like transients, dislocations and amorphisation, appearance of metastable polymorphs and localised heating, it is important to remember that no single mechanism can explain all of mechanochemistry. Indeed, a more realistic picture is obtained by understanding that all these mechanisms operate all at once and at very different timescales, from nanoseconds to days or weeks. Different mechanisms will be more dominant in some reactions and for some configurations than others. The closing lecture also noted the importance of developing the models and understanding of mechanochemistry that will enable rational, rapid and efficient scaling up (Fig. 6b).
Conclusions
The very end of conference was dedicated to a discussion of the current state and future developments of mechanochemistry. Several developments in mechanochemical research were identified as particularly promising for its development:
(1) exploration of the materials at very small length scales, such as in microscopy and nanoindentation studies;
(2) the increased use of concepts of supramolecular chemistry and intermolecular interactions in understanding mechanochemical activation and defects;
(3) the development of new methodologies for observing mechanochemical reactions as they take place, at different length scales, from bulk to molecular level.
(4) the development of theoretical models and simulations addressing mass and energy transfer in mechanochemical reactions at different scales.
(5) the development of smart mechanochemistry, i.e. a highly selective approach to optimise reactivity by judicious application of one or more types of mechanical treatment, based on systematic investigation of how different types of mechanical action affect chemical reactivity.
Conference attendees also attempted to outline several questions and potential directions for the future development of mechanochemistry. The first one addressed the definition of mechanochemistry. While IUPAC briefly defines a mechano-chemical reaction as a chemical reaction that is induced by the direct absorption of mechanical energy,31 contributions at the 256th Faraday Discussion illustrated that mechanochemistry is a much broader area which includes, besides conventional modes of organic and inorganic reactivity, processes of molecular organisation and self-assembly in two or three dimensions. It may, therefore, be beneficial to include in the definition of mechanochemistry aspects of supramolecular chemistry and self-assembly that underlie processes of mechanochemical activation (e.g. amorphisation) or certain types of reactions (e.g. cocrystallisation or polymorphic transformations).8,10,32 Such an attempt to improve the definition of mechanochemistry should describe mechanical force (including agitation) as a means to enable, either directly or through an intermediate activation process, the assembly or dissociation of objects ranging in dimensions from nanometers (molecules, molecular complexes) to millimeters (bulk materials).
Next, it was obvious that understanding of the mechanisms remains a central problem of modern mechanochemistry and it may be that future studies should take into account several interacting pathways (for example, vapour phase diffusion simultaneous to amorphisation) rather than interpret mechanochemical behaviour according to one particular mechanism. The complexity of such future studies will require the researchers of different backgrounds to communicate and increasingly work together.21 In that context, the conference had brilliantly served its purpose, to bring together researchers with different interests and background, and encourage their understanding, exchange of ideas, collaboration and communication. In that spirit, Prof. Lamaty has kindly invited the attendees to a future mechanochemistry meeting, planned in Montpellier, France, in the summer of 2015.
Acknowledgements
We acknowledge Prof. L. R. MacGillivray, University of Iowa (USA), Prof. G. M. Day, Southampton University (UK), Prof. T. D. Hamilton, Barry University (USA), Dr D. W. Peters (ATMI), Dr L. Maini (University of Bologna), Prof. A. H. Moores (McGill University), Dr L. Batzdorf (née Tröbs), Prof. R. G. Blair (University of Central Florida); Prof. V. Šepelák (Karlsruhe Institute for Technology), Dr S. Gupta (Iowa State University), Prof. V. Pecharsky (Iowa State University), A. Michalchuk (University of Edinburgh and Novosibirsk State University), Prof. A. Politov (Novosibirsk Institute of Solid State Chemistry and Mechanochemistry, Russian Academy of Sciences) for help in composing this report. Ms Sandra Aerssen, McGill University is acknowledged for professional assistance in meeting organisation and Prof. D. N. Harpp in help in organisation of the event at the McGill University Faculty Club. Warm thanks go to the McGill University Faculty Club staff Pierre Marjois, Luigi Foschini, Nicolas Zrihen and Adrian Chu.Notes and references
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- (a) C. Suryanarayana, Prog. Mater. Sci., 2001, 46, 1–184 CrossRef CAS; (b) C. Suryanarayana, E. Ivanov and V. V. Boldyrev, Mater. Sci. Eng., A, 2001, 304–306, 151–158 CrossRef.
- E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719–7738 RSC.
- (a) P. Baláž, Int. J. Miner. Process., 2003, 72, 341–354 CrossRef; (b) P. Baláž, A. Aláčová, M. Achimovičová, J. Ficeriová and E. Godočiková, Hydrometallurgy, 2005, 77, 9–17 CrossRef PubMed.
- (a) M. M. Caruso, D. A. Davis, Q. Shen, S. A. Odom, N. R. Sottos, S. R. White and J. S. Moore, Chem. Rev., 2009, 109, 5755–5798 CrossRef CAS PubMed; (b) A. L. Black, J. M. Lenhardt and S. L. Craig, J. Mater. Chem., 2011, 21, 1655–1663 RSC.
- P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- (a) A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC; (b) L. Chen, B. E. Lemma, J. S. Rich and J. Mack, Green Chem., 2014, 16, 1101–1103 RSC; (c) D. C. Waddell, T. D. Clark and J. Mack, Tetrahedron Lett., 2012, 53, 4510–4513 CrossRef CAS PubMed.
- (a) T. Friščić, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC; (b) A. N. Sokolov, D.-K. Bučar, J. Baltrusaitis, S. X. Gu and L. R. MacGillivray, Angew. Chem., Int. Ed., 2010, 49, 4273–4277 CrossRef CAS PubMed.
- (a) A. Lazuen-Garay, A. Pichon and S. L. James, Chem. Soc. Rev., 2007, 36, 846–855 RSC; (b) D. Braga, S. L. Giaffreda, F. Grepioni, A. Pettersen, L. Maini, M. Curzi and M. Polito, Dalton Trans., 2006, 1249–1263 RSC.
- (a) D. Braga, L. Maini and F. Grepioni, Chem. Soc. Rev., 2013, 42, 7638–7648 RSC; (b) A. Delori, T. Friščić and W. Jones, CrystEngComm, 2012, 14, 2350–2362 RSC.
- C. R. Hickenboth, J. S. Moore, S. R. White, N. R. Sottos, J. Baudry and S. R. Wilson, Nature, 2007, 446, 423–427 CrossRef CAS PubMed.
- M. K. Beyer and H. Clausen-Schaumann, Chem. Rev., 2005, 105, 2921–2948 CrossRef CAS PubMed.
- (a) T. Friščić, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. J. Kimber, V. Honkimäki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 CrossRef PubMed; (b) I. Halasz, A. Puškarić, S. A. J. Kimber, P. J. Beldon, A. M. Belenguer, F. Adams, V. Honkimäki, R. E. Dinnebier, B. Patel, W. Jones, V. Štrukil and T. Friščić, Angew. Chem., Int. Ed., 2013, 52, 11538–11541 CrossRef CAS PubMed.
- J. Stojaković, B. S. Farris and L. R. MacGillivray, Chem. Commun., 2012, 48, 7958–7960 RSC.
- A. M. Belenguer, T. Friščić, G. M. Day and J. K. M. Sanders, Chem. Sci., 2011, 2, 696–700 RSC.
- A. A. L. Michalchuk, I. A. Tumanov and E. V. Boldyreva, CrystEngComm, 2013, 15, 6403–6412 RSC.
- D. Prochowicz, K. Sokołowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friščić and J. Lewinski, Chem. Commun., 2015, 51, 4032–4035 RSC.
- L. Maini, D. Braga, P. P. Mazzeo and B. Ventura, Dalton Trans., 2012, 41, 531–539 RSC.
- V. Šepelák, S. Bégin-Colin and G. Le Caër, Dalton Trans., 2012, 41, 11927–11948 RSC.
- M. J. Rak, N. K. Saade, T. Friščić and A. Moores, Green Chem., 2014, 16, 86–89 RSC.
- L. Takacs, Chem. Soc. Rev., 2013, 42, 7649–7659 RSC.
- (a) M. Ojeda, A. Pineda, A. A. Romero, V. Barrón and R. Luque, ChemSusChem, 2014, 7, 1876–1880 CrossRef CAS PubMed; (b) M. Baláž, P. Baláž, G. Tjuliev, A. Zubrík, M. J. Sayagués, A. Zorkovská and N. Kostova, J. Mater. Sci., 2013, 48, 2424–2432 CrossRef.
- X. Ma, W. Yuan, S. E. J. Bell and S. L. James, Chem. Commun., 2014, 50, 1585–1587 RSC.
- C. Medina, D. Daurio, K. Nagapudi and F. Alvarez-Nunez, J. Pharm. Sci., 2010, 99, 1693–1696 CAS.
- T. Friščić and W. Jones, Cryst. Growth Des., 2009, 9, 1621–1637 Search PubMed.
- L. Takacs, Prog. Mater. Sci., 2002, 47, 355–414 CrossRef CAS.
- A. Stolle, R. Schmidt and K. Jacob, Chem. Soc. Rev., 2014, 170, 267–286 CAS.
- (a) P. A. May and J. S. Moore, Chem. Soc. Rev., 2013, 42, 7497–7506 RSC; (b) J. M. Lenhardt, A. L. Black and S. L. Craig, J. Am. Chem. Soc., 2009, 131, 10818–10819 CrossRef CAS PubMed.
- S. W. Schmidt, P. Filippov, A. Kersch, M. K. Beyer and H. Clausen-Schaumann, ACS Nano, 2012, 6, 1314–1321 CrossRef CAS PubMed.
- H. Xu, B. W. Zeiger and K. S. Suslick, Chem. Soc. Rev., 2013, 42, 2555–2567 RSC.
- IUPAC Compendium of Chemical Terminology, 2nd edn (the “Gold Book”), compiled by A. D. McNaught and A. Wilkinson, Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins, ISBN 0-9678550-9-8, DOI: 10.1351/goldbook, http://goldbook.iupac.org/MT07141.html.
- (a) T. N. Drebuschak, A. A. Ogienko and E. V. Boldyreva, CrystEngComm, 2011, 13, 4405–4410 RSC; (b) D. Braga, F. Grepioni and G. I. Lampronti, CrystEngComm, 2011, 13, 3122–3124 RSC; (c) M. C. Etter, S. M. Reutzel and C. G. Choo, J. Am. Chem. Soc., 1993, 115, 4411–4412 CrossRef CAS.
Footnote |
| † Transcripts of all discussions are also published in the Faraday Discussions volume 170. |
| This journal is © The Royal Society of Chemistry 2015 |