Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dinuclear planar chiral ferrocenyl gold(I) & gold(II) complexes†
Marta
Ayerbe Garcia
a,
Wolfgang
Frey
a,
Mark R.
Ringenberg
b,
Max
Schwilk
c and
René
Peters
*a
aUniversität Stuttgart, Institut für Organische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany. E-mail: rene.peters@oc.uni-stuttgart.de
bUniversität Stuttgart, Institut für Anorganische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany
cUniversität Stuttgart, Institut für Theoretische Chemie, Pfaffenwaldring 55, 70569 Stuttgart, Germany
First published on 5th October 2015
Abstract
Oxidation of Au(I) in the presence of Fe(II) allowed for the synthesis of unique dinuclear ferrocenyl Au(II) complexes via the first reported enantiopure planar chiral ferrocenyl Au(I) complex. (Spectro)electrochemical studies show that oxidation at Fe(II) is favoured, but DFT studies suggest that the energy differences for oxidation of one or the other metal should be quite small.
Metallocene based palladacycles are established as catalysts for asymmetric synthesis1 enabling a number of catalytic asymmetric applications,2,3 for which no other catalyst has surpassed. Related metallacycles, like the corresponding platinacycles, have been less studied, but recently they also demonstrated high potential as carbophilic catalysts.4 In contrast, planar chiral enantiopure ferrocenyl gold complexes are not known so far and only a few achiral or racemic complexes have been reported.5 In addition, one planar chiral ruthenocenyl Au(I)PPh3 complex has been reported, but isolation of pure material was not achieved.6
Due to the lack of metallocenyl gold complexes, little is known about their reactivity. Based on the many applications of Au(I) catalysts7 and the catalytic efficiency of metallocene based metallacycles,1–4 the study of metallocenyl gold complexes has attracted our interest. This report puts forward the first case for the asymmetric synthesis and structural elucidation of planar chiral ferrocenyl Au complexes including auracycles featuring Au(II) centers involved in short Au–Au bonds.
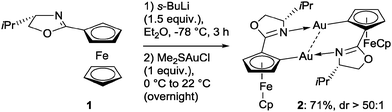
Our targets were ferrocenyl Au complexes in which phosphines as additional ligands would be avoided as they might impede future catalytic applications by their high affinity to Au. ortho-Lithiation of 18 and subsequent trapping with (Me2S)AuCl proceeded smoothly and gave the dinuclear Au(I) complex 2 with high diastereoselectivity in good yield (Scheme 1).
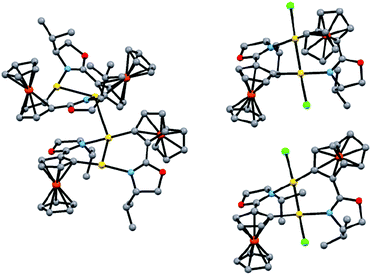
X-ray analysis of 2 shows a tetranuclear solid state structure (Fig. 1, left), in which two dinuclear complexes containing a bridging pair of anionic C,N-ligands are connected via an intermolecular Au(I)⋯Au(I) interaction (separation: 3.0651(3) Å).9 The Me2S-ligands have been replaced by oxazoline N-donors resulting in a head-to-tail ligand arrangement to form helically chiral 10-membered rings featuring intramolecular Au(I)⋯Au(I) interactions (distances: 2.8449(3) & 2.8452(3) Å). The 1H-/13C-NMR spectra of 2 show single sets of signals pointing to dinuclear species in solution, which is supported by ESI-MS studies.
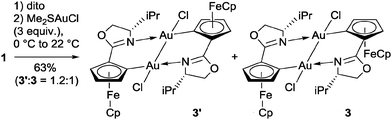
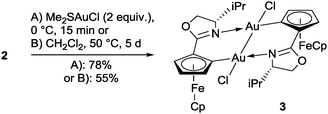
With an excess of (Me2S)AuCl a mixture of diastereomeric Au(II)-dimers 3 and 3′ was produced (Scheme 2), while a Au mirror was formed in the reaction vessel. Disproportionation of Au(I) is otherwise rather uncommon.10–12
X-ray crystal structure analysis of the C1 symmetric dimer 3′ has confirmed that both gold centers have been oxidised to Au(II)Cl with a square planar coordination geometry featuring a Au(II)–Au(II) bond, which results in a notable decrease of the intermetallic distance to 2.5333(5) Å (Fig. 1, top right).9 This short separation is in good agreement with the small number of bimetallic Au(II)-complexes carrying linker ligands.13 Usually, the latter are 3 atom-linkers, while 3/3′ are to our knowledge the first bimetallic Au(II)-complexes possessing two 4-atom C,X-linkers.14 Oxidation of the intermediately formed 2 results in the formation of two 6-membered metallacycles which are bridged by the Au(II)–Au(II) bond. Surprisingly, in 3′ one FeCp fragment has changed the face of the disubstituted Cp-ligand to which it is coordinated, while the C,N-ligands retain their head-to-tail arrangement already present in 2. The solid state structure of the C2 symmetric 3 (Fig. 1, bottom right) closely resembles that of its epimers 3′ with a slightly longer Au(II)–Au(II) separation of 2.5654(3) Å. Few examples are known for other N ligands in Au(II) complexes,13d while arylcyclometallated Au(II)-complexes with bridging anionic C,N-ligands have to our knowledge been unknown so far.15
It might be surprising that a relatively inert metal atom such as Au(I) is oxidised to the less common Au(II)13 oxidation state in the presence of the redox-active ferrocene core. On the other hand we have recently found that treatment of pentaphenylferrocenyl palladacycles with Ag(I) salts resulted in the oxidation of Pd(II) to give Pd(III) centers, while the ferrocene core remained unchanged.16 Oxidation of 2 to 3/3′ might be facilitated by electronic cooperation between both gold centers in the bimetallic entities, finally resulting in an intermetallic bond offering a lower barrier oxidation pathway than for monometallic complexes.13
Oxidation of isolated 2 by (Me2S)AuCl proceeded smoothly (eqn (1)). After 15 min at 0 °C, 3 is formed in diastereomerically pure form, whereas longer reaction times in the presence of (Me2S)AuCl resulted in ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of 3 and 3′.
1 mixtures of 3 and 3′.
 | (1) |
Epimerisation thus appears to be promoted by the Au source, but is also observed in CDCl3 solutions containing traces of DCl.
In addition, isomerically pure 3 can also be formed simply by heating a solution of 2 in CH2Cl2 under N2 for several days in a closed vial. Dichloromethane thus also serves as the oxidising agent (eqn (1)).17,18 In contrast, trials to oxidise 2 by different silver salts just resulted in decomposition.
Cyclic voltammetry (CV) and spectroelectrochemical (SEC)19 properties for 1, 2 and 3 were studied to get more insight into their redox behaviour. The ligand (1) redox is highly reversible and consistent with a ferrocene/ferrocenium (Fc0/+) couple (ESI†). The CVs for 2 and 3 both contain two 1e− reversible oxidations separated by 137 mV and 160 mV, respectively. The processes for 2 are anodically shifted by an average of 135 mV compared to 3, which is consistent with the overall higher oxidation state of the Au(II) species.
The voltammogram of 2 also contains an irreversible oxidation at 0.9 V vs. Fc0/+. While 3 does not have this irreversible oxidation, the CV does have an irreversible reduction wave at − 1.2 V vs. Fc0/+ (ESI†). Due to the relatively high potentials and irreversible nature of these processes they are associated with the direct oxidation and reduction of the Au centers, respectively.
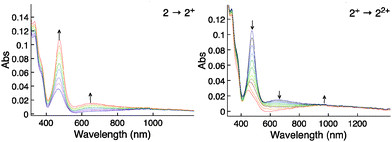
The two reversible processes in the CV for 2 and 3 could be differentiated in the UV-Vis-NIR SEC (Fig. 2). The in situ oxidation of 2 to 2+ resulted in the growth of an absorption band at 475 nm, and a lower energy band at 660 nm. The second oxidation, 2+ to 22+, results in the quenching of the band at 475 to a band at 446 nm. The two reversible processes in the CV are therefore the oxidation of the iron in the ferrocene moiety. The strength of the band in the UV-Vis-NIR and the separation of the oxidation events in the CV indicate weak communication between the ferrocene moieties.20
The UV-Vis-NIR SEC for the two oxidations of 3 contains only the shift of the band at 544 nm to a lower energy band at 586 nm, the second oxidation also shifts the band to a lower energy at 615 nm. The spectra are consistent with ferrocenium.
As shown above, chemical formation of the Au(II) species is selectively possible without affecting the ferrocene core. Both solid 3 and 3′ proved to be quite stable towards air and moisture, as no decomposition was observed in air after several weeks at 22 °C. The deep purple colour of 3/3′ in solution is quite uncommon for ferrocene derivatives.21 Known dinuclear aryl Au(II) complexes display bands in the range of 356–416 nm, which have been interpreted to originate from the Au(II)–Au(II) bonds.15 The intense purple colour of 3/3′ in solution probably results from d–d iron transitions and displays the electron-withdrawing character of the Au(II) centers. The latter might also facilitate the observed partial epimerisation of the planar chiral ferrocene entities in the presence of an excess of carbophilic Au(I) or DCl.22 We assume that the Au source and the Brønsted acid interact with the CpFe fragments (like in π-complexes) and thus promote the reversible CpFe decomplexation from the disubstituted Cp ligands.
For a computational analysis, the crystal structures of 2 and 3 as well as of (Me2S)AuCl have been optimised using the Kohn–Sham density functional theory (DFT) at the TPPS23 level of theory with Grimme's D3 dispersion correction24 using the def2-TZVP25 basis set in the TURBOMOLE program package.26 Solvation in diethylether was modelled using the COSMO continuum solvation model.27
For a better understanding of the reactivity of 2, the Kohn–Sham orbitals of the structure were analysed. The theoretical justification of the interpretation of eigenvalues in terms of approximate orbital energies has very recently been worked out by Chan et al.28 The HOMO down to the HOMO−6 of 2 lie quite tightly together between −4.12 eV and −4.81 eV (ESI†) and are located either on both gold and iron atoms or on the iron atoms, showing that gold and iron centers are in principle both prone to oxidation.
Intrinsic Bond Orbitals (IBOs)29 were calculated using the same parameters as for the calculation of the Kohn–Sham orbitals using the Molpro program package.30 IBOs have the advantage of reliably yielding an orbital picture in terms of Lewis structures, best suited for chemical interpretation.29,31 IBO analysis of the C–Au bond in 2 and 3 exhibits a distinct polarisation towards the C atom. Only 0.584 (i.e. 27%) and 0.743 (37%), respectively, of the bond charge is located on the gold atom. Furthermore, the difference of these charges is an indicator for the electron withdrawing character of the Au centers after oxidation to Au(II) in 3. Whereas in 2 no covalent character in the Au⋯Au interaction can be observed through the IBO analysis (the interaction evidently is of pure aurophilic32 character), the Au–Au bond in 3 has a clear σ character (Fig. 3). Interestingly, the Au–Cl bond in 3 is partly located on the remote gold atom (respective location of the bond on the centers of the Cl–Au–Au axis: 83%, 8%, and 7%), which can be interpreted as a strong contribution of a +M effect from the Cl atom towards the Au–Au moiety (see Fig. 3).
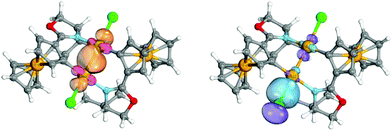 | ||
| Fig. 3 Intrinsic bond orbitals for the Au–Au and Cl–Au bonds in 3 (the iso-surface encloses 85% of the electron density, visualisation realised using IboView33). | ||
This clearly indicates a strong electronic cooperation of the two gold centers. Hashmi et al.34 very recently showed that IBO locations on atomic centers correlate very well with electrophilic substituent constants (Hammett parameters),35 which encouraged us to deduce bond stabilities from IBO partial charges. The Au–N bond has a clear dative character in both 2 and 3, since it is chiefly located on the ligand (87% and 86% of the bond respectively). For comparison, in the dative S–Au bond of (Me2S)AuCl 79% of the bond is located on the ligand. Kohn–Sham orbitals and IBOs of the optimised geometries have also been calculated with three other DFT functionals (TPSSH, PBE36 and PBE0), without deviating from the conclusions that have been drawn from the results using the TPSS functional.‡
We have reported the first asymmetric syntheses of ferrocenyl gold complexes. A dinuclear Au(I) complex featuring two bridging monoanionic C,N-ligands was generated by diastereoselective ortho-lithiation and trapping with (Me2S)AuCl. A ten-membered helically chiral (N–C–C–C–Au)2 ring was formed, which contains aurophilic Au(I)⋯Au(I) interactions. The latter might facilitate the smooth oxidation to the corresponding dinuclear auracycles featuring a short Au(II)–Au(II) bond. While the helical chirality and the head-to-tail ligand arrangement are conserved in the Au(II) species, we noticed a partial epimerisation of one ferrocene fragment, which seems to be caused by excess of (Me2S)AuCl. The latter also acts as the oxidising agent, but even CH2Cl2 can be applied demonstrating the ease of oxidative additions to this kind of Au(I) species. This might create possibilities for Au(I/II) catalysis, which is currently investigated in our laboratory. While (spectro)electrochemical studies show that there is a preference for oxidation at Fe, the theoretical studies suggest that the energy gaps between the highest molecular orbitals are quite small and that the latter are located either mainly on Fe, or on both Fe and Au.
This work was financially supported by the Deutsche Forschungsgemeinschaft (DFG, PE 818/6-1) and F. Hoffmann-La Roche. M.S. would like to thank Dr Enrique Abad González for useful discussions.
Notes and references
- (a) C. J. Richards, in Chiral Ferrocenes in Asymmetric Catalysis, ed. L.-X. Dai and X.-L. Hou, Wiley-VCH, Weinheim, 2010, pp. 337–368 Search PubMed; (b) R. Peters, D. F. Fischer and S. Jautze, Top. Organomet. Chem., 2011, 33, 139 CrossRef CAS.
- For monopalladacycles, see e.g.: (a) Y. Donde and L. E. Overman, J. Am. Chem. Soc., 1999, 121, 2933 CrossRef CAS; (b) R. Peters, Z.-q. Xin and F. Maier, Chem. – Asian J., 2010, 5, 1770 CrossRef CAS PubMed; (c) M. Weber and R. Peters, J. Org. Chem., 2012, 77, 10846 CrossRef CAS PubMed; (d) S. H. Eitel, S. Jautze, W. Frey and R. Peters, Chem. Sci., 2013, 4, 2218 RSC; (e) J. M. Bauer, W. Frey and R. Peters, Angew. Chem., Int. Ed., 2014, 53, 7634 CrossRef CAS PubMed; (f) T. Hellmuth, W. Frey and R. Peters, Angew. Chem., Int. Ed., 2015, 54, 2788 CrossRef CAS PubMed; (g) C. Schrapel and R. Peters, Angew. Chem., Int. Ed., 2015, 54, 10289 CrossRef CAS PubMed ; for related Co-sandwich complexes, see e.g.: ; (h) L. E. Overman, C. E. Owen, M. M. Pavan and C. J. Richards, Org. Lett., 2003, 5, 1809 CrossRef CAS PubMed; (i) H. Nomura and C. J. Richards, Chem. – Eur. J., 2007, 13, 10216 CrossRef CAS PubMed.
- For bispalladacycles, see e.g.: (a) S. Jautze and R. Peters, Angew. Chem., Int. Ed., 2008, 47, 9284 CrossRef CAS PubMed; (b) M. Weber, S. Jautze, W. Frey and R. Peters, Chem. – Eur. J., 2012, 18, 14792 CrossRef CAS PubMed; (c) M. Weber, W. Frey and R. Peters, Chem. – Eur. J., 2013, 19, 8342 CrossRef CAS PubMed; (d) M. Weber, W. Frey and R. Peters, Angew. Chem., Int. Ed., 2013, 52, 13223 CrossRef CAS PubMed; (e) T. Hellmuth, S. Rieckhoff, M. Weiss, K. Dorst, W. Frey and R. Peters, ACS Catal., 2014, 4, 1850 CrossRef CAS; (f) M. Weiss and R. Peters, ACS Catal., 2015, 5, 310 CrossRef CAS.
- (a) H. Huang and R. Peters, Angew. Chem., Int. Ed., 2009, 48, 604 CrossRef CAS PubMed; (b) M. Weiss, W. Frey and R. Peters, Organometallics, 2012, 31, 6365 CrossRef CAS.
- (a) A. N. Nesmeyanov, E. G. Perevalova, D. A. Lemenovskii, A. N. Kosina and K. I. Grandberg, Izv. Akad. Nauk SSSR, Ser. Khim., 1969, 2030 CAS; (b) A. N. Nesmeyanov, E. G. Perevalova, K. I. Grandberg, D. A. Lemenovskii, T. V. Baukova and O. B. Afanassova, J. Organomet. Chem., 1974, 65, 131 CrossRef CAS; (c) K. I. Grandberg and V. P. Dyadchenko, J. Organomet. Chem., 1994, 474, 1 CrossRef CAS; (d) K. Jacob, F. Voigt, K. Merzweiler and C. Pietzsch, J. Organomet. Chem., 1997, 545–546, 421 CrossRef CAS; (e) K. Jacob, F. Voigt, K. Merzweiler, C. Wagner, P. Zanello, M. Fontani and C. Pietzsch, J. Organomet. Chem., 1998, 552, 265 CrossRef CAS; (f) F. Voigt, A. Fischer, C. Pietzsch and K. Jacob, Z. Anorg. Allg. Chem., 2001, 627, 2337 CrossRef CAS.
- G. E. Herberich, U. Englert and T. Wirth, Eur. J. Inorg. Chem., 2005, 4924 CrossRef CAS PubMed.
- Selected recent reviews: (a) Modern Gold Catalyzed Synthesis, ed. A. S. K. Hashmi and F. D. Toste, Wiley-VCH, Weinheim, 2012 Search PubMed; (b) A. S. K. Hashmi, Acc. Chem. Res., 2014, 47, 864 CrossRef CAS PubMed; (c) Y.-M. Wang, A. D. Lackner and F. D. Toste, Acc. Chem. Res., 2014, 47, 889 CrossRef CAS PubMed; (d) C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902 CrossRef CAS PubMed; (e) I. Braun, A. M. Asiri and A. S. K. Hashmi, ACS Catal., 2013, 3, 1902 CrossRef CAS; (f) N. Krause, in Organometallics in Synthesis: Fourth Manual, ed. B. H. Lipshutz, 2013, pp. 429–540 Search PubMed; (g) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef PubMed; (h) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed.
- (a) T. Sammakia, H. A. Latham and D. R. Schaad, J. Org. Chem., 1995, 60, 10 CrossRef CAS; (b) C. J. Richards, T. Damalidis, D. E. Hibbs and M. B. Hursthouse, Synlett, 1995, 74 CrossRef CAS; (c) Y. Nishibayashi and S. Uemura, Synlett, 1995, 79 CrossRef CAS.
- Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre for compounds 2, 3 and 3′ as depositions 1056862, 1056863 and 1056865.
- F. A. Cotton, Basic Inorganic Chemistry, Wiley, New York, vol. 3, 1995 Search PubMed.
- Formation of AuII compounds by comproportionation: J. Coetzee, W. F. Gabrielli, K. Coetzee, O. Schuster, S. D. Nogai, S. Cronje and H. G. Raubenheimer, Angew. Chem., Int. Ed., 2007, 46, 2497 CrossRef CAS PubMed.
- (a) A. A. Mohamed, H. E. Abdou and J. P. Fackler Jr., Coord. Chem. Rev., 2010, 354, 1253 CrossRef PubMed . For Au(II) compounds formed by other metal oxidants, see e.g.: ; (b) H. H. Murray, A. M. Mazany and J. P. Fackler, Jr., Organometallics, 1985, 4, 154 CrossRef CAS; (c) V. W.-W. Yam, S. W.-K. Choi and K.-K. Cheung, J. Chem. Soc., Chem. Commun., 1996, 1173 RSC; (d) V. W.-W. Yam, C.-K. Li, C.-L. Chan and K.-K. Cheung, Inorg. Chem., 2001, 40, 7054 CrossRef CAS PubMed; (e) M. D. Irwin, H. E. Abdou, A. A. Mohamed and J. P. Fackler, Jr., Chem. Commun., 2003, 2882 RSC; (f) D. Y. Melgarejo, G. M. Chiarella, J. P. Fackler, Jr., L. M. Perez, A. Rodrigue-Witchel and C. Reber, Inorg. Chem., 2011, 50, 4238 CrossRef CAS PubMed.
- (a) D.-A. Rosca, D. A. Smith, D. L. Hughes and M. Bochmann, Angew. Chem., Int. Ed., 2012, 51, 10643 CrossRef CAS PubMed; (b) D. Zopes, C. Hegemann, W. Tyrra and S. Mathur, Chem. Commun., 2012, 48, 8805 RSC; (c) D. Y. Melgarejo, G. M. Chiarella, J. P. Fackler, Jr., L. M. Perez, A. Rodrigue-Witchel and C. Reber, Inorg. Chem., 2011, 50, 4238 CrossRef CAS PubMed; (d) see ref. 12a.
- M. A. Bennett, S. K. Bhargava, D. C. R. Hockless, F. Mohr, K. Watts, L. L. Welling and A. C. Willis, Z. Naturforsch., B: J. Chem. Sci., 2004, 59, 1563 CAS.
- N. Mirzadeh, M. A. Bennett and S. K. Bhargava, Coord. Chem. Rev., 2013, 257, 2250 CrossRef CAS PubMed.
- S. H. Eitel, M. Bauer, D. Schweinfurth, N. Deibel, B. Sarkar, H. Kelm, H.-J. Krüger, W. Frey and R. Peters, J. Am. Chem. Soc., 2012, 134, 4683 CrossRef CAS PubMed.
- It has been reported that bis-Au(II) complexes can be formed from bis-Au(I) precursors by oxidation with chlorinated solvents like 1,2-dichloroethane, see ref. 13d.
- Oxidative additions of Au(I) giving Au(III): (a) J. H. Teles, Angew. Chem., Int. Ed., 2015, 54, 5556 CrossRef CAS PubMed; (b) C.-Y. Wu, T. Horibe, C. B. Jacobsen and F. D. Toste, Nature, 2015, 517, 449 CrossRef CAS PubMed; (c) M. Joost, L. Estévez, K. Miqueu, A. Amgoune and D. Bourissou, Angew. Chem., Int. Ed., 2015, 54, 5236 CrossRef CAS PubMed.
- W. Kaim and J. Fiedler, Chem. Soc. Rev., 2009, 38, 3373 RSC.
- (a) C. J. Ziegler, K. Chanawanno, A. Hasheminsasab, Y. V. Zatsikha, E. Maligaspe and V. N. Nemykin, Inorg. Chem., 2014, 53, 4751 CrossRef CAS PubMed; (b) U. Pfaff, A. Hildebrandt, M. Korb and H. Lang, Polyhedron, 2015, 86, 2 CrossRef CAS PubMed; (c) H. H. Shah, R. A. Al-Balushi, M. K. Al-Suti, M. S. Khan, F. Marken, A. L. Sudlow, G. Kociok-Köhn, C. H. Woodall, P. R. Raithby and K. C. Molloy, Dalton Trans., 2014, 43, 9497 RSC.
- See, e.g.: B. N. Ahamed, M. Arunachalam and P. Ghosh, Inorg. Chem., 2010, 49, 4447 CrossRef CAS PubMed.
- (a) D. W. Slocum, S. P. Tucker and T. R. Engelmann, Tetrahedron Lett., 1979, 621 Search PubMed; (b) H. Falk, H. Lehner, J. Paul and U. Wagner, J. Organomet. Chem., 1971, 28, 115 CrossRef CAS.
- J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 CrossRef.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- (a) F. Weigend, M. Häser, H. Patzelt and R. Ahlrichs, Chem. Phys. Lett., 1998, 294, 143 CrossRef CAS; (b) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- (a) R. Ahlrichs, M. Bär, M. Häser, H. Horn and C. Kölmel, Chem. Phys. Lett., 1989, 162, 165 CrossRef CAS; (b) TURBOMOLE V6.4, TURBOMOLE GmbH, 2012 Search PubMed.
- A. Klamt and G. Schürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799 RSC.
- H. v. Aggelen and G. K.-L. Chan, Mol. Phys., 2015, 113, 2018 CrossRef PubMed.
- G. Knizia, J. Chem. Theory Comput., 2013, 9, 4834 CrossRef CAS.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 242 CrossRef CAS PubMed.
- G. Knizia and J. E. M. N. Klein, Angew. Chem., Int. Ed., 2015, 54, 5518 CrossRef CAS PubMed.
- (a) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370 RSC; (b) P. Pyykkö, Chem. Rev., 1997, 97, 597 CrossRef PubMed; (c) P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412 CrossRef PubMed; (d) F. Mendizabal, S. Miranda-Rojas and L. Barrientos-Poblete, Comput. Theor. Chem., 2015, 1057, 74 CrossRef CAS PubMed.
- G. Knizia, IboView; see http://www.iboview.org.
- L. Nunes dos Santos Comprido, J. E. M. N. Klein, G. Knizia, J. Kästner and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2015, 54, 10336 CrossRef CAS PubMed.
- H. C. Brown and Y. Okamoto, J. Am. Chem. Soc., 1958, 80, 4979 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data for new products, and details about the electrochemical and computational investigations. CCDC 1056862, 1056863 and 1056865. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc07018j |
| ‡ For the complete data set of computational results and for a more detailed discussion of the parameters used and tested in the calculations we refer to the ESI.† |
| This journal is © The Royal Society of Chemistry 2015 |