Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stability and its manifestation in the chemical and biological worlds
Robert
Pascal
 a and
Addy
Pross
*bc
a and
Addy
Pross
*bc
aInstitut des Biomolécules Max Mousseron, UMR5247 CNRS-University of Montpellier-ENSCM, CC17006, Place E. Bataillon, Montpellier F-34095, France
bDepartment of Chemistry, Ben Gurion University of the Negev, Be'er Sheva 84105, Israel. E-mail: pross@bgu.ac.il
cNYU Shanghai, 1555 Century Avenue, Pudong New Area, Shanghai, China 200122
First published on 7th October 2015
Abstract
Bridging between the phenomenologically distinct biological and physical worlds has been a major scientific challenge since Boltzmann's probabilistic formulation of the second law of thermodynamics. In this review we summarize our recent theoretical attempts to bridge that divide through analysis of the thermodynamic-kinetic interplay in chemical processes and the manner in which that interplay impacts on material stability. Key findings are that the term ‘stability’ manifests two facets – time and energy – and that stability's time facet, expressed as persistence, is more general than its energy facet. That idea, together with the proposed existence of a logical law of nature, the persistence principle, leads to the mathematically-based insight that stability can come about through either Boltzmann's probabilistic considerations or Malthusian kinetics. Two mathematically-based forms of material persistence then lead directly to the physical likelihood of two material forms, animate and inanimate. Significantly, the incorporation of kinetic considerations into the stability concept appears to bring us closer to enabling two of the central theories in science – the second law of thermodynamics and Darwin's theory of evolution – to be reconciled within a single conceptual framework.
Introduction
The second law of thermodynamics, dealing as it does with the nature and direction of changes in the universe, constitutes one of the central laws in science.1,2 Being probabilistic in its essence, the second law governs all material systems, living and non-living, so that changes in the biological world, though strikingly different to those in the physical/chemical world, also necessarily conform to the universal thermodynamic directive. Yet, somehow the behavior of biological systems seems to be strangely incompatible with the second law leading several of the great physicists of the 20th century to speculate on the possible existence of physical laws yet to be discovered.3 Darwinian theory, the theory that revolutionized our understanding of the biological world and the basis for change in that world, seems oddly detached from thermodynamic considerations, a disconnect that epitomizes the glaring conceptual gap that continues to divide the animate from the inanimate. In addressing the paradox inherent in the existence of living systems, Lotka pointed out almost a century ago that thermodynamics only tells us what cannot happen, not what does happen.4 The ‘what is life’ question remains stubbornly resistant to a generalized thermodynamic approach and recent developments in non-equilibrium thermodynamics,5 though insightful in themselves, have not added materially to a resolution of the animate–inanimate dichotomy.In recent years the authors, together and individually, have addressed the issue through the formulation of an alternative stability kind, dynamic kinetic stability (DKS).6 A central conclusion from those studies is that the concept of stability, normally understood in thermodynamic terms, can be extended in scope and relevance through the incorporation of kinetic considerations. Of course the role of kinetics in governing reaction processes has long been recognized. However it is specifically within the world of replicative processes that the relative importance of kinetic and thermodynamic factors can change dramatically, leading to the strikingly different material behavior observed for living systems.7 Through a kinetic perspective it can be demonstrated that the process of life's emergence, as well as its subsequent evolution, seemingly inconsistent, if not at odds with thermodynamic logic, actually have a mathematical/logical basis. And, significantly, as will be discussed, once kinetic considerations are taken into account, the explanatory power of the stability concept as it applies to replicative systems, may offer insights beyond those traditionally provided by the prime thermodynamic principle, the second law of thermodynamics.
Discussion
The concept of stability is a central one in science but the term is utilized in two quite different ways. Following the establishment of thermodynamics as a quantitative discipline, the term can be used in an energy/entropy sense. Thus a system of lower enthalpy and/or higher entropy is considered more stable. However, the term can also be used in a time sense, in the sense of persistence. A system that is persistent, unchanging over time, is also considered stable, though it is not necessarily stable in an energy sense. Indeed Lotka, almost a century ago, used the term ‘stable’ in that time/persistence sense when he proposed that Darwin's ‘survival of the fittest’ could be expressed as the principle of “persistence of stable forms”.4 Dawkins also alluded to the importance of the time/persistence definition of stability in evolution and reworded the Lotka principle as “survival of the most stable”,8 while Grand has further built on the ‘stability as persistence’ idea with a general principle of existence expressed as: “Things that persist, persist; things that don't, don't”.9But the fact that the stability concept has two quite distinct facets – energy and time – has chemical consequences, in particular, when the two facets operate in a contradictory fashion, and such a situation is common. For example, a mixture of H2 and O2 is unstable in a Gibbs (free) energy sense, as it can readily react to give the thermodynamically stable product, water, but it can be highly stable in a time/persistent sense (kinetically stable) if the H2 + O2 mixture is kept under appropriate conditions (low temperature and the absence of a catalyst).
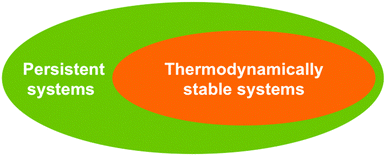
So how then do these two distinct facets of stability – time and energy – relate to one another? The relationship is illustrated in the Venn diagram of Fig. 1. From the diagram it can be seen that the energy facet of stability, expressed as thermodynamic stability, is actually a sub-group of the more general set of persistent systems. This is because thermodynamically stable systems are necessarily persistent. Having reached the lowest Gibbs energy equilibrium state (corresponding to maximal entropy for isolated systems), the system remains unchanged over time. In other words once a system has attained thermodynamic stability, it becomes persistent; systems that are stable in a Gibbs energy sense also manifest stability in a time sense.
 | ||
| Fig. 1 Diagram illustrating the set of thermodynamically stable systems as a sub-set of the more general set of persistent systems. | ||
Notice, however, that the Venn diagram, as formulated, indicates that there is a category of systems that are persistent without being thermodynamically stable, and the H2 + O2 mixture mentioned previously exemplifies such a system. Through the existence of a kinetic barrier, the H2 + O2 mixture may be unable to overcome that barrier to form the more thermodynamically stable H2O product. The concept of kinetic stability is well understood in chemistry and any system that finds itself in a so-called kinetic trap expresses stability of this kind. But before proceeding to characterize yet another stability kind that fits within the general persistence category, we can now make a significant observation – stability in the sense of persistence is more general than stability in an energetic sense (thermodynamic stability). Stability in its most fundamental sense is about time rather than about energy; persistent systems may or may not be thermodynamically stable, however thermodynamically stable systems will necessarily be persistent.
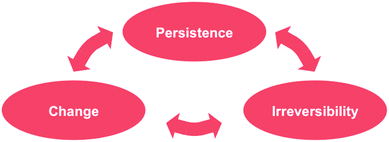
Once it is appreciated that the more general facet of stability is its time facet, the one that extends beyond the more limited thermodynamic description, it leads directly to a logical law of nature, which we term the persistence principle: systems will tend from less stable (persistent) to more stable (persistent) forms,10 or, alternatively, and more concisely: nature seeks persistent forms. Note that the principle is logically true, in effect axiomatic, regardless of the reasons for the system's persistence, thermodynamic or otherwise. The principle derives directly from its time formulation; systems will tend over time to a cascade towards ones of greater persistence. This point might be clarified by the following comment which merely paraphrases the principle, though in a way that emphasizes its axiomatic/logical character: changing things change, until they change into things that don't. Thus there exists a logical relationship, illustrated schematically in Fig. 2, between persistence, change and irreversibility. Stability/persistence in material systems is induced through the process of change, change that (as will be discussed below) is necessarily irreversible.
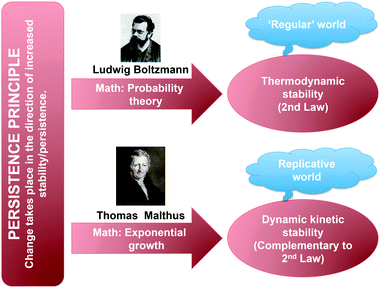
The above qualitative rule, while logically compelling, offers little operational insight as to how change in real systems actually takes place. However Ludwig Boltzmann's landmark contribution to statistical mechanics a little over a century ago is able to quantify that rule thereby formally confirming the principle's validity. Boltzmann's enormous insight was to recognize that energetic stability has a logical/mathematical basis and is a direct outcome of probability theory; systems that can be described by a greater number of microstates are preferred probabilistically to those described by a smaller number of microstates. Thus ‘less stable to more stable’ is just ‘less probable to more probable’. But in the context of our discussion, those two statements lead directly to the time variant manifestation: ‘less persistent to more persistent’. Systems at equilibrium, in their most probable state, are necessarily persistent. In other words, Boltzmann's probabilistic analysis leads not just to an understanding of stability in terms of energy, but also in terms of time/persistence. Furthermore, the (effective) irreversibility associated with all changes also derives directly from the Boltzmann probabilistic analysis; for macroscopic systems, involving an inordinately large number of particles, the probability of the reversible change becomes effectively zero.†
The Boltzmann mathematical description of stability, described above, applies specifically to thermodynamic stability, a limited sub-set of the set of persistent systems illustrated in Fig. 1. But is there a mathematical basis for stability in the time/persistence sense beyond the one offered by the Boltzmann formulation? The answer is yes. For a particular group of systems lying outside of the thermodynamically stable sub-group of Fig. 1, there also exists a mathematical basis for stability/persistence. However, it is not probabilistic, but rather Malthusian,11 as it rests on the kinetic power of exponential growth. Let us summarize how this comes about.
An alternative form of stability in the persistence sense, one applicable solely to certain replicating systems, is the aforementioned dynamic kinetic stability, DKS.6 In simplest terms that stability kind is the stability associated with entities able to make copies of themselves at a rate that results in a non-equilibrium steady-state population of replicating entities being maintained over time – persistence through self-replication. Living systems, of course, exemplify this kind of stability in that populations of living things, whether humans, camels, mosquitoes, or bacteria, are maintained over time, due to the continual production of new entities (though in limited quantities due to resource limitations), which replace existing ones. Individual entities are continually being degraded by various processes, termed death (for biological systems), or degradation (for chemical systems). So it is the population of replicators that is stable/persistent, rather than the individual replicators that make up the population at any given moment. Thus DKS, being a kinetic form of stability, expresses a form of persistence that also lies outside the thermodynamic stability region of Fig. 1. DK stable systems are inherently thermodynamically unstable and therefore necessarily depend on a continual input of energy essential for maintaining that steady-state population. They therefore correspond to the definition of dissipative structures emerging under far from equilibrium conditions.5
Crucially, however, the qualitative ‘less persistent to more persistent’ rule operates in the replicative world as well. As pointed out by Eigen and Schuster,12 and Lifson,13 the mathematical logic associated with exponentially driven replicating systems results in the same qualitative (and irreversible) direction of the change – from less stable/persistent to more stable/persistent. If a particular replicating system replicates with some variation leading to competing replicating entities, then the competitive kinetics is explicit – competing exponential replicators cannot coexist. The more stable one in a DKS sense drives the less stable one into extinction. Thus there is a natural evolutionary process from DK less stable/persistent to DK more stable/persistent, the one governed by the math of exponential growth.
We discover therefore that irreversible change in the material world, as expressed through the persistence principle, can manifest itself in two discrete ways based on two mathematically governed processes (illustrated in Fig. 3). In the so-called ‘regular’ (non-replicative) chemical world, it manifests itself through the second law, i.e., systems are driven toward more probable states, while in the replicative world it manifests itself through the drive toward more successful (DK stable) replicating systems, though, of course, that evolutionary process must also be consistent with the strict requirements of the second law. And now the reason for two distinct material forms all around us becomes clear. Simply, there are currently just two known mathematical formulations of persistence – Boltzmann's probabilistic way and Malthus's exponential growth way. Two persistence kinds mean two distinct chemistries resulting in the two material forms that surround us.
Note that Darwin's theory of evolution can now be seen as the more limited biological expression of the persistence principle, reaffirming Lotka's early attempt to place Darwinian evolution within a broader physical framework. ‘Survival of the fittest’, biological in its expression, is contained within the more general ‘nature seeks persistent forms’.‡ Furthermore, the principle offers insight into the chemical process of life's emergence, one of science's great mysteries. Through the principle, abiogenesis and Darwinian biological evolution can be understood as one continuous process.6 The entire evolutionary process of the replicative change, beginning with some prebiotic persistent chemical replicating system and proceeding through to complex life, manifests the logical and irreversible drive toward greater persistence (stability) within the replicative world. Through the math of Malthusian kinetics we identify life as just the more evolved replicative expression of the persistence principle.
The unorthodox conclusion which follows: that the living state, far from being a mysterious and seemingly inexplicable material state of matter, may be understood as a logically predictable state, no more phenomenological puzzling than the established and ubiquitous physical states of matter – solid, liquid and gas. The puzzle that is life lies not at the biological level, or at the conceptual physical level, but rather at the intermediate chemical level. It lies in the types and nature of simple chemical replicative systems, and the chemical means by which such systems, still poorly understood, might naturally emerge. We discuss this point subsequently. (For reviews of simple replicative chemical systems, see ref. 14.)
Role of complexification in the replicative world
While we have made clear that within both ‘regular’ and replicative worlds all systems tend toward greater stability/persistence, the organizational means by which that greater persistence is achieved are very different. In the ‘regular’ world the probabilistic drive toward greater persistence manifests through increasing entropy. Entropy is the state function by which probabilistic progress is measured. In the replicative world, however, where the mathematical underpinning is based on the kinetic power of exponential growth, the measure of progress is less obvious. In contrast to the ‘regular’ chemical world, there is no state function which characterizes the DKS state. DKS systems are dynamic steady-state systems, necessarily open to energy and resource input6 so that the stability of such systems depends not just on the system itself but on factors outside the system. Yet, despite that fundamental distinction, there does appear to be a parameter that is able, at least qualitatively, to reflect ‘progress toward greater replicative stability’, and that parameter is complexity. Let us now discuss this issue in more detail.The subject of complexity, to put it somewhat awkwardly, and circularly, is complex.15–17 The concept is notoriously difficult to define and no less difficult to measure. For any real entity complexity measures can be context-dependent, dependent on the required level of detail, and even the language employed.16 And then there are different kinds of complexity – structural,18 informational,19 ecological,20 and functional,21,22 all adding to the uncertainty, not to mention confusion, which envelops the topic. Importantly though, in the biological context there is a broad agreement that complexity increased over evolutionary time, whatever complexity kind is considered.19,23,24 Initially, some primordial system, being chemical, would have been relatively simple. However the evolutionary process then led to the emergence of prokaryotic life, already inordinately complex, then to more complex eukaryotic life, and subsequently, to multi-cellular life. Despite a popular view that complexity per se could be inherently undesirable, there is little doubt that it can be highly favorable when it leads to an increase in what we might term replicative efficiency.
A counter-argument that is commonly raised, which questions the evolutionary drive toward greater complexity, is that the organizationally more complex eukaryotic life, whether single cell or multi-cell did not eliminate prokaryotic life, thereby questioning the thesis that complexity continually increased during evolution. However it is important to recognize that complexity is not just measured by the complexity of those individual replicating entities, prokaryotes or eukaryotes, but also by the complexity of the networks that these entities generate. All living things live in communities and the network of individuals that make up that community also contributes to the system's complexity. And, of course, most living organisms serve as resources for other living forms so that an integrated network that incorporates interspecies dependence and multiple levels of integration is constituted. Indeed there is little doubt that ecological complexity continually increases with time, and should be viewed as part of the general process of evolutionary complexification. But, as we will now discuss, the kinetic stability of the system as a whole depends crucially on that network aspect and helps explain the basis for the existence of a general stability–complexity relationship.
The underlying reason for the evolutionary process of complexification derives from the existence of a complexity-function relationship; an increase in efficiency of the replicative function almost invariably requires an increase in complexity.21 That relationship extends beyond biology and constitutes a fundamental basis for technological advance. The Wright Brothers airplane of 1903 was simpler and less functional than a Boeing 747. The function of flight is enhanced by increasing the complexity and the evolutionary process of flight capability therefore largely involved a continuing process of complexification. Broadly speaking, that general relationship underpins most technological advance and complexification tends to continue until some optimum level of complexity is reached with respect to available material resources.
But just as any engineer understands and exploits that complexity-function relationship, the evolutionary process, from some unknown relatively simple replicating system to simple life and then complex life, also exploits that same relationship. In other words the physical manifestation of greater persistence in the replicative world over evolutionary time is primarily brought about through increasing complexity, functional complexity. In fact in a limited way complexity in the replicative world may be thought of as a rough analog of entropy in the ‘regular’ world though there are key differences. As noted earlier, complexity is difficult to quantify, in contrast to entropy. Moreover, though in the ‘regular’ chemical world entropy can reach a maximum value, beyond which a further increase is not possible, in the replicative world the degree of complexity has no formal upper limit – replicative function can always, at least potentially, be increased. It is no surprise therefore that life processes express a seemingly insatiable appetite for expansion and conquest. The complexification process, continually tested and examined for increased dynamic kinetic stability, and driven by the kinetic power of exponential growth, continues unabated.
Contingency in the emergence of life
The preceding discussion might lead one to the conclusion that the emergence of life from inanimate matter is effectively deterministic, that the persistence principle points to the emergence of persistent complex replicating entities as a logical and necessary chemical outcome. However that is not the case as the earliest steps along that evolutionary path toward increasingly DKS stable entities would also depend on contingent chemical factors, and these remain poorly understood. The emergence of life from non-living matter would in the first instance depend on the natural emergence of some relatively simple, but minimally persistent, evolvable replicating system, but the likelihood of such an event is unknown at the present time. Simply, our chemical knowledge of such systems remains rudimentary. How common is the kind of replicative chemistry that would support the existence of such systems? Are chemical systems other than the nucleic acid–protein duality feasible? And even if the chemical possibilities are many, how likely are such systems to emerge spontaneously? What chemical environment would be necessary? We do not know. Till now chemists have been unable to generate persistent chemical replicating systems of any kind, though van Esch et al.25 have been able to generate experimental dynamic non-equilibrium systems, though not replicative in nature. In that respect systems chemistry, the relatively new area of chemical research that deals with, inter alia, replicating chemical systems and the networks they establish, is still a virgin area of study.26 Until a substantial body of experimental data on dynamic replicative non-equilibrium systems and the networks they establish is available, we will remain in the dark. Paradoxically, however, given the innate difficulties in our ability to identify extra-terrestrial life processes should they exist, it may well be through experiments conducted on the earth that the likely answer to the perennial ‘are we alone?’ question may be obtained. Until we better understand the detailed chemical character of replicative chemistry – how readily replicative systems are able to emerge from diverse chemical environments, and then how readily these systems can evolve – the answer to that tantalizing ‘are we alone’ question will likely remain out of reach.Conclusions
By extending the stability concept to include kinetic, and not just thermodynamic, considerations, the nature of the change in the material world, embracing both animate and inanimate systems, can be accommodated within a single unified framework. First and foremost, that framework rests on a logical law of nature that we have termed the persistence principle, one which can express itself in two mathematically distinct ways (probability theory and the kinetic power of replication). The principle, expressed as ‘nature seeks persistent forms’, is based on the recent revelation10 that the most general expression of stability in the material world is through its time aspect – persistence – not its energy/entropy aspect. Accordingly, the persistence principle provides a means of describing stability in nature beyond that offered by the second law and Boltzmann's probabilistic thinking.10 Once it is recognized that stability/persistence can emerge from Malthusian kinetics and that a distinct and alternative kind of stability/persistence is physically possible, then it follows that irreversible change in the universe can come about in two fundamentally distinct ways – toward most probable states, toward heat death, or, alternatively (while an energy source is available), and in a local context, toward ever more effective (persistent) replicating systems, toward what we term life. The seeming paradox of two starkly distinct worlds, so puzzling from a purely thermodynamic perspective, appears to have been resolved. One might even venture to add that through the logic of the persistence principle, two foundational scientific theories – the second law of thermodynamics and Darwin's theory of evolution – appear reconcilable within a simple rational framework. Through the perspective offered by that principle, the process of evolution is just the expression of the principle's operation within the replicative world, and life itself, as the anticipated consequence of the principle in action.Note also that the principle offers insight into the perennial origin of life problem. By governing the nature of material change in the replicative world, whether chemical or biological, the principle reaffirms that the emergence of life and its subsequent evolution constitute one single continuous physicochemical process.6 Thus biology's central concept, natural selection, can be reduced to chemistry (as kinetic selection), then to physics (as dynamic kinetic stability), and finally to logic (drive toward persistent forms). Remarkably, given the reality of continuing change in the material world, life is actually in some sense a logically expected phenomenon. Furthermore, given the ongoing debate between reductionist and holistic approaches to the problem of biocomplexity,27 it is noteworthy that our analysis indicates that biological complexity – both with regard to its emergence and its evolution – may, after all, be amenable to classic reductionist thinking. Thus, though life is undoubtedly complex, the underlying reasons for its complexity appear to be reassuringly simple.
Nevertheless, before closing, some qualification is necessary. While the logic inherent within the persistence principle may indicate the physics of life to be quite unremarkable, even logically dictated, the actual chemistry that led to life's emergence continues to remain uncertain, effectively terra incognita. The current challenge is to extend our knowledge of replicative chemistry, the virgin area of systems chemistry dealing with replicating chemical systems and the networks they establish.26 How general is the chemistry underpinning the formation of simple, but persistent, replicative chemical systems? How contingent would the early evolutionary processes acting on simple replicative systems have been? Despite the logical insight the persistence principle may offer, until more definitive answers to these chemical questions become available, the generality of the life phenomenon within a cosmic context will continue to remain uncertain.
Acknowledgements
We thank Prof. Peter Strazewski for helpful discussions and comments on an earlier draft of the paper. This work is the result of exchanges facilitated through the COST Actions CM1304 Emergence and Evolution of Complex Chemical Systems and TD1308 Origins and Evolution of Life on Earth and in the Universe (ORIGINS). RP acknowledges support from the Agence Nationale de la Recherche for the PeptiSystems project (grant ANR-14-CE33-0020).Notes and references
- C. H. Lineweaver and C. A. Egan, Phys. Life Rev., 2008, 5, 225 CrossRef PubMed.
- D. Kondepudi and I. Prigogine, Modern thermodynamics: from heat engines to dissipative structures, Wiley, Chichester, UK, 1998 Search PubMed.
- (a) E. Schrödinger, What is life?, Cambridge University Press, Cambridge, UK, 1944 Search PubMed; (b) E. Wigner, Found. Phys., 1970, 1, 35 CrossRef; (c) N. Bohr, Nature, 1933, 131, 421 CrossRef PubMedN. Bohr, Nature, 1933, 133, 457 CrossRef PubMed.
- A. Lotka, Proc. Natl. Acad. Sci. U. S. A., 1922, 8, 151 CrossRef CAS.
- G. Nicolis and I. Prigogine, Self-organization in nonequilibrium systems, Wiley, New York, 1977 Search PubMed.
- (a) A. Pross and V. Khodorkovsky, J. Phys. Org. Chem., 2004, 17, 312 CrossRef CAS PubMed; (b) R. Pascal, J. Syst. Chem., 2012, 3, 3, DOI:10.1186/1759-2208-3-3; (c) R. Pascal, A. Pross and J. D. Sutherland, Open Biol., 2013, 3, 130156, DOI:10.1098/rsob.130156; (d) R. Pascal, Isr. J. Chem., 2015, 55, 865–874 CrossRef CAS PubMed; (e) A. Pross, J. Syst. Chem., 2011, 2, 1 CrossRef CAS; (f) A. Pross, Chem. – Eur. J., 2009, 15, 8374 CrossRef CAS PubMed; (g) A. Pross, Pure Appl. Chem., 2005, 77, 905 CrossRef; (h) A. Pross and R. Pascal, Open Biol., 2013, 3, 120190, DOI:10.1098/ rsob.120190; (i) A. Pross, What is life? How chemistry becomes biology, Oxford University Press, Oxford, 2012 Search PubMed.
- A. Pross, J. Theor. Biol., 2003, 220, 393 CrossRef.
- R. Dawkins, The selfish gene, Oxford University Press, Oxford, 1989, p. 12 Search PubMed.
- S. Grand, Creation: Life and how to make it, Harvard University Press, Cambridge, USA, 2003 Search PubMed.
- A. Pross and R. Pascal, J. Syst. Chem., 2014, 5, 3 CrossRef.
- T. Malthus, An essay on the principle of population; Printed for J. Johnson, in St. Paul's Church-Yard, London, 1798, http://www.esp.org/books/malthus/population/malthus.pdf.
- M. Eigen and P. Schuster, The hypercycle. A principle of natural self-organization, Springer, Berlin, 1979 Search PubMed.
- S. Lifson, J. Mol. Evol., 1997, 44, 1 CrossRef CAS.
- (a) R. Plasson, A. Brandenburg, L. Jullien and H. Bersini, J. Phys. Chem. A, 2011, 115, 8073 CrossRef CAS PubMed; (b) A. J. Bissette and S. P. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800 CrossRef CAS PubMed; (c) A. J. Meyer, J. W. Ellefson and A. D. Ellington, Acc. Chem. Res., 2012, 45, 2097 CrossRef CAS PubMed; (d) V. Vasas, C. Fernando, M. Santos, S. Kauffman and E. Szathmáry, Biol. Direct, 2012, 7, 1, DOI:10.1186/1745-6150-7-1.
- P. A. Corning and E. Szathmáry, J. Theor. Biol., 2015, 371, 45 CrossRef PubMed.
- M. Gell-Mann, Complexity, 2015, 1, 16 CrossRef PubMed.
- C. Adami, BioEssays, 2002, 24, 1085 CrossRef PubMed.
- D. W. McShea and R. N. Brandon, Biology's first law: the tendency for diversity and complexity to increase in evolutionary systems, University of Chicago Press, Chicago, 2010 Search PubMed.
- (a) M. Gell-Mann and S. Lloyd, Complexity, 1996, 2, 44 CrossRef; (b) C. Adami and N. J. Cerf, Phys. D, 2000, 137, 62 CrossRef.
- W. B. Arthur, in Complexity: metaphors, models, and reality, ed. G. Cowan, et al., Addison Wesley, Redwood City, 1994, vol. XIX of Santa Fe institute studies in the science of complexity, pp. 65–78 Search PubMed.
- A. Pross, J. Mol. Evol., 2013, 76, 185 CrossRef CAS PubMed.
- D. W. McShea, Biol. Philos., 2000, 15, 641 CrossRef.
- P. Schuster, Complexity, 1996, 2, 22 CrossRef.
- J. Maynard Smith and E. Szathmáry, The major transitions in evolution, Oxford University Press, Oxford, 1995 Search PubMed.
- (a) J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2010, 49, 4825 CrossRef CAS PubMed; (b) J. Boekhoven, W. E. Hendriksen, G. J. M. Koper, R. Eelkema and J. H. van Esch, Science, 2015, 349, 1075 CrossRef CAS PubMed.
- (a) G. von Kiedrowski, S. Otto and P. Herdewijn, J. Syst. Chem., 2010, 1, 1, DOI:10.1186/1759-2208-1-1; (b) R. Ludlow and S. Otto, Chem. Soc. Rev., 2008, 37, 101–108 RSC; (c) J. J. P. Peyralans and S. Otto, Curr. Opin. Chem. Biol., 2009, 13, 705–713 CrossRef CAS PubMed; (d) E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119, DOI:10.1038/nnano.2014.337.
- (a) M. H. V. Van Regenmortel, EMBO Rep., 2004, 5, 1016, DOI:10.1038/sj.embor.7400284; (b) F. Mazzocchi, Wiley Interdiscip. Rev.: Syst. Biol. Med., 2012, 4, 413, DOI:10.1002/wsbm.1181.
- G. M. Wang, E. M. Sevick, E. Mittag, D. J. Searles and D. J. Evans, Phys. Rev. Lett., 2002, 89, 050601 CrossRef CAS.
Footnotes |
| † For sufficiently small systems over short time scales, measurable violations of the second law have been observed. However, these theoretically predictable violations should be viewed as exceptions that prove the rule.28 |
| ‡ Noteworthily, the subtitle of Darwin's “The Origin of Species” was “The Preservation of Favoured Races in the Struggle for Life”. The term ‘preservation’ is similar to ‘persistence’ suggesting that Darwin himself was thinking about evolution in broader non-biological terms. This observation was brought to our attention by Guillaume Lecointre. |
| This journal is © The Royal Society of Chemistry 2015 |