Open Access Article
Open Access ArticleAccess to pure and highly volatile hydrochalcogenide ionic liquids†‡
L. H.
Finger
 ,
F.
Wohde
,
E. I.
Grigoryev
,
A.-K.
Hansmann
,
R.
Berger
,
B.
Roling
and
J.
Sundermeyer
*
,
F.
Wohde
,
E. I.
Grigoryev
,
A.-K.
Hansmann
,
R.
Berger
,
B.
Roling
and
J.
Sundermeyer
*
Fachbereich Chemie and Materials Science Center, Philipps-Universität, Hans-Meerwein-Str. 4, 35043 Marburg, Germany. E-mail: JSU@staff.uni-marburg.de
First published on 3rd September 2015
Abstract
The reaction of methylcarbonate ionic liquids with H2S or H2Se offers a highly selective synthesis of analytically pure, well-defined and soluble hydrosulphide and hydroselenide organic salts of general interest. Among them, imidazolium hydrochalcogenides show an astonishingly high volatility for cation-aprotic ILs, which allows their quantitative sublimation below 100 °C/10−2 mbar and actually results in ionic single crystal growth from the gas phase. Vaporisation and decomposition characteristics were investigated by isothermal TGA measurements and DFT calculations.
Tetraalkyl ammonium hydrosulphides, despite being known since the 1960s, were never characterised in detail.1 They are important building blocks in the synthesis of chalcogenide metal complexes2 and clusters.3 More recently, the synthesis of imidazolium and pyrrolidinium hydrosulphides was published.4 The assumed ionic liquids (ILs) were used as starting materials for organic polysulphide salts, which were investigated as redox mediators in dye or quantum dot sensitised solar cells. A close inspection of the experimental sections in these published articles unveils a considerable lack of analytical data of these ILs. The previous synthetic procedures start from water and halide containing reagents and the crude preparation was typically not accompanied by thorough purification.4 These conditions are far from ideal, to say the least – especially in view of the targeted electrochemical applications. This class of compounds is also relevant to lithium sulphur batteries, where they occur as a soluble, capacity-limiting shuttle system5 and to the fabrication of chalcogenide semiconductor materials.6 Also, the general redox and dissolution behaviour of sulphur and related elements in ILs is of continuing general interest.7 In view of the high relevance of these substances, we set out to develop an access to high purity hydrosulphide ILs. It was aspired to exclude water, halides and metals and to establish reliable purification allowing the detailed characterisation of all substances. This could be accomplished by the proton induced decarboxylation of methylcarbonate anions8 employing H2S as the acidic reagent (Scheme 1). The elegant reaction is easily expanded to the hydroselenide salts and a range of different cations. It is characterised by yielding only volatile, easily separated by-products and comprising no equilibria due to the irreversible decay of the methylcarbonate anion to methanol and carbon dioxide.
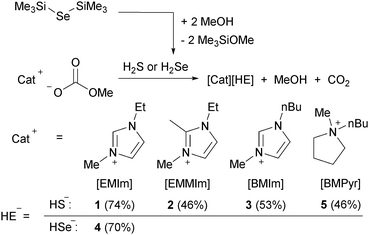 | ||
| Scheme 1 Synthesis of imidazolium and pyrrolidinium hydrochalcogenides, yields after recrystallisation in parentheses. | ||
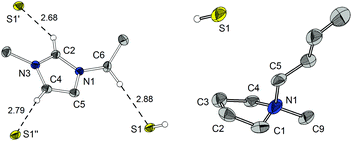
Recrystallisation proved to be an effective purification method. Also [BMPyr][HS] (5), which was formerly described as a yellow oil,4a could be isolated in a crystalline form (Fig. 1).9 Upon attempting to reproduce the synthesis of bisimidazolium sulphides from the corresponding hydrosulphides under vacuum conditions,4b we noticed an unforeseen volatility of the ILs 1 and 3. Subsequently, we were able to implement sublimation at 10−3 mbar and temperatures significantly below 100 °C as an elaborate, high end purification method. Increasing the pressure to 1 mbar still yields pure [EMIm][HS] by sublimation, but the mass transfer rate is significantly decreased. Application of higher temperatures leads to partial thermolysis of the ILs, with 1-alkylimidazole and dialkylimidazole-2-thione as the main decomposition products. In the case of [EMIm][HS] (1), even single crystals suitable for the X-ray structure determination could be obtained by sublimation (Fig. 1).10 The structure is characterised by hydrogen bonds between the C2 proton and the hydrosulphide anion. Weaker C–H⋯S contacts are formed from C4, and the aliphatic CH2 group of C6 (for a H-bond table and graphics of the crystal packing please refer to the ESI‡).
While aprotic ILs have long been understood to exhibit negligible vapour pressure, the last decade has shown that not only do aprotic ILs have a measurable vapour pressure but can also in fact be distilled in a vacuum at elevated temperatures.11 Nevertheless, the very high degree of volatility observed here is, to the best of our knowledge, unprecedented for aprotic ILs. Typical conditions for the vaporisation of aprotic ILs either make use of UHV chambers with a nominal pressure of 1 × 10−7 mbar with temperatures still ranging mostly above 100 °C or employ highly elevated temperatures.12 Detailed studies of the vapour phase have proven that under distillation conditions neutral contact ion pairs are the dominant species.13 In the case of protic ILs, volatility can be ascribed to the acid base equilibrium in the substance being directly correlated with the difference in pKa values of the cation and the anion's conjugate acid. Here the parent acid and base form the volatile species, while the vapour pressure depends on the acid–base equilibrium.14 However, the differentiation between protic and aprotic ILs is not as straightforward as it was assumed for some time. Recent investigations have proven that, on the one hand, several protic ILs distil as neutral ion pairs, if the respective pKa difference is very large.15 On the other hand, if the IL anion is sufficiently basic, e.g. acetate, some 1,3-dialkylimidazolium salts distil as the formal parent acid and base, e.g. acetic acid and the 1,3-dialkyl-NHC.16 The volatile components still form a strongly hydrogen bonded complex though.
These discrepancies motivated us to investigate the phenomenon in more detail. In contrast to 1 and 3, [BMPyr][HS] (5) does not vaporise undecomposed. In this case, decomposition occurs in the form of a ring opening SN2-type attack. We were able to extend the series of volatile hydrosulphide salts to the C2-methylated [EMMIm][HS] (2), though. This proves that the unusual volatility cannot be solely based upon strong hydrogen bonds from the apical proton. Also, the heavier homologue [EMIm][HSe] (4) shows a comparable volatility. Subsequently, we conducted isothermal thermo-gravimetric analysis (TGA) experiments to investigate the vaporisation enthalpies and to relate our substances to literature known values.17Fig. 2 shows the temperature dependence of the molar loss rates per unit area of compounds 1, 2 and 5 in comparison with [BMIm][TFSI]; Table 1 lists the values for ΔHvap, at the average measurement temperature (Tav) and extrapolated to 298 K. The values determined for ferrocene and [BMIm][TFSI] are included for comparison.
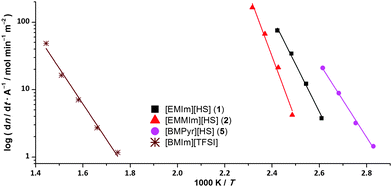 | ||
| Fig. 2 Arrhenius plots of the molar loss rates per unit area for compounds 1, 2 and 5 in comparison with [BMIm][TFSI]. | ||
The vaporisation enthalpies of the hydrochalcogenide salts span a very broad range. [EMMIm][HS] shows the highest value of 192 kJ mol−1 while [BMPyr][HS] exhibits the lowest ΔHvap, at only 112 kJ mol−1. While the determined vaporisation enthalpies of the hydrochalcogenide salt are mostly higher than that of [BMIm][TFSI], the temperature at which the compounds exhibit a significant mass loss rate is strikingly low. In contrast to [BMIm][TFSI], which has to be exposed to an average temperature of 632 K, the imidazolium hydrochalcogenides showed comparable volatility at average temperatures below 417 K. This difference of more than 200 K is in accordance with the very high volatility under sublimation conditions in vacuo. Note that, whereas under vacuum conditions a true sublimation occurs, in the TGA experiments at ambient pressure the elevated temperatures cause a vaporisation from the molten phase. The surprisingly low sublimation enthalpy of [BMPyr][HS] has to be attributed to the decomposition, although Tav lies significantly below Tm. As the small sample volume and the instability of the hydrochalcogenide salts under ambient conditions prevented an examination of the remainder in the TGA crucibles, we conducted vaporisation experiments for salts 1 and 5 at 1 bar and 100 °C under inert conditions. Here the condensate of [BMPyr][HS] consists only of decomposition products as well. In the case of [EMIm][HS], a mixture of 61% IL and 39% decomposition products was observed. For both compounds, the residual substance showed only a minor degree of thermolysis, which confirms that the decomposition products are significantly more volatile than the IL. Thus, the determined vaporisation enthalpies do not represent the pure compound's inherent value, as also for the imidazolium salts, a partial decomposition has to also be anticipated. To determine the vaporisation enthalpies of these salts experimentally, studies in a vacuum using the Langmuir or Knudsen evaporation method are mandatory. We calculated the cohesive energies of the imidazolium ILs 1 to 4 within the density functional theory (DFT, please refer to the ESI‡ for details) in order to estimate the ΔHvap values (Table 1). The results are in reasonable agreement with the TGA experiments and, with the exception of the value for salt 2, are slightly larger by about 10 kJ mol−1.
In view of the recent observation that imidazolium ILs with sufficiently basic anions act as pseudo-protic ILs during vaporisation16 and the unusual volatility of the present imidazolium hydrochalcogenides, we applied the DFT as well as EI mass spectrometry to identify the gas phase species. As has to be expected, the EI mass spectra do not show any single ion pairs (SIPs). Instead, the respective carbene, its fragments and hydrogen sulphide as the conjugate acid are observed. This agrees well with the results of Hollóczki et al., who observed the same phenomenon with imidazolium acetate ILs.16 Also, the respective dialkylimidazole-2-chalcogenone, which was identified as a thermal decomposition product, could be found in the EI mass spectra. DFT calculations concerning the most stable single ion pairs of [EMIm][HS] in the gas phase were conducted on the BP86/def2-TZVP level. An ion pair, where the HS anion is positioned above the plane of the imidazolium cation, was found to be the most stable configuration (Fig. 3). This structure is not solely based on electrostatic interaction but can partially be attributed to a weak π-type orbital interaction between the HOMO of the anion and the LUMO of the cation. Similar interactions were already observed for imidazolium ILs with several anions.18 Ion pairs, in which the cation forms a hydrogen bond to the hydrosulphide via the C2 proton, and which have to be regarded as a pre-complex to the carbene formation, are on the DFT level predicted to be at least 16.9 kJ mol−1 higher in energy (Fig. 3). The complete dissociation to a free carbene and hydrogen sulphide is energetically disfavoured, but only to a small extent, thus allowing its occurrence (ΔH0 = +48.1 kJ mol−1 for a singlet carbene). On the basis of these calculations, several SIP structures are viable for the selected system. Together with the results from EI MS experiments, the vaporisation of H2S and the corresponding carbene appears likely. This is, however, strongly contradicted, by the quantitative sublimation in a dynamic vacuum. That the carbene is the major component detected in the EI mass spectra, leads us to agree with Hollóczki et al. that the very low pressure of the mass spectrometric experiments may lead to the dissociated molecules being favoured due to entropic reasons.16 During the actual sublimation, the π-complex and H-bonded structures have to dominate.
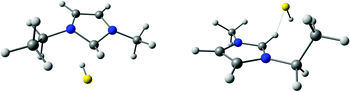 | ||
| Fig. 3 Most stable SIP of [EMIm][HS] with a π-interaction (left, Erel = 0.0 kJ mol−1) and the H-bonded SIP with lowest energy (right, Erel = 16.9 kJ mol−1). | ||
A further non-negligible observation is the presence of 1,3-dialkyl-imidazole-2-chalcogenones among the decomposition products and in the EI mass spectra. The preferred decomposition pathway for imidazolium cations in the presence of highly nucleophilic reaction partners is demethylation.19 This is in accordance with the alkylimidazoles being the major component of the decomposition mixture, as investigated by NMR spectroscopy and also certified by DFT calculations (see the ESI‡ for details). The stability of imidazolium cations towards related but solvated HO− ions is strongly dependent on the substitution pattern.20 In several literature reports, the formation of imidazole-2-chalcogenones results from the reaction of the respective NHC with elemental chalcogen or polychalco-genides.21 While the presence of carbenes is absolutely feasible in view of the previous results, elemental chalcogen or poly-chalcogenides can be excluded in the sublimed and colourless samples. To the best of our knowledge, and in contrast to the heavier homologues, neither homolytic cleavage to elemental sulphur and hydrogen nor equilibria including polysulphide anions are known for pure hydrosulphide salts. While the latter reaction pathway cannot be fully excluded due to the so far unknown influence of the organic cation, we have investigated an alternative formation pathway by computational methods.
As already noted by Hollóczki et al., a neutral thiol species A, resulting from a nucleophilic attack of the hydrosulphide at the C2 position cannot be stabilised.16 This reaction becomes energetically favoured though, if a concerted deprotonation of the respective hydrosulphide is considered (Scheme 2). The resulting thiolate B has to be regarded as a strong hydride donor that will react with the strongest acid present, which is again the hydrosulphide anion. This leads to the formation of imidazole-2-thione, molecular hydrogen and formally a sulphide dianion, which will immediately react with the next imidazolium cation initiating an autocatalytic cycle. The reaction sequence is, according to DFT calculations for the gas phase, exothermic by −6.3 kJ mol−1 in the first and −51.0 kJ mol−1 in the second step. Owing to the complexity of the multimolecular reactions, no transition states could be calculated. Nevertheless, this pathway appears to be a viable alternative for the formation of imidazole-2-chalcogenones in the absence of polychalcogenides.
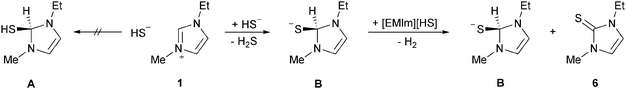 | ||
| Scheme 2 Formation of 1-ethyl-3-methylimidazole-2-thione (6) from the hydrosulphide IL [EMIm][HS] (1). | ||
In conclusion, we presented a new and exceedingly convenient access to pure hydrosulphide and hydroselenide organic salts by reaction of methylcarbonate ILs with H2E (E = S; Se). The title compounds are promising reagents, e.g. for the low temperature synthesis of metal chalcogenide clusters and semiconductor materials under ionothermal flux conditions, or as weakly solvated super nucleophiles in organic and inorganic syntheses. In contrast to earlier experiments on dissolving sulphur in ILs,4a,c,7 these salts may allow the preparation of pure polysulphides, used e.g. as redox mediators in quantum dot sensitised solar cells. Imidazolium hydrochalcogenides exhibit remarkably high volatility, which allows their sublimation under moderate vacuum and at temperatures below 100 °C. DFT calculations were employed to calculate the most stable gas phase structures and the sublimation enthalpies of the respective salts. At elevated temperatures, decomposition occurs, the pathways of which have been backed by quantum chemical calculations.
EIG and RB gratefully acknowledge support from the DFG (SPP 1191). LHF and JS thank the VCI (FCI grant for LHF) and the DFG (GRK 1782) for financial support.
Notes and references
- (a) J. D. Cotton and T. C. Waddington, J. Chem. Soc. A, 1966, 785 RSC; (b) D. H. McDaniel and W. G. Evans, Inorg. Chem., 1966, 5, 2180 CrossRef CAS.
- (a) J. S. Anderson and J. C. Peters, Angew. Chem., Int. Ed., 2014, 53, 5978 CrossRef CAS PubMed; (b) H. Sugimoto, K. Hatakeda, K. Toyota, S. Tatemoto, M. Kubo, T. Ogura and S. Itoh, Dalton Trans., 2013, 42, 3059 RSC; (c) H. Sugimoto, S. Tatemoto, K. Toyota, K. Ashikari, M. Kubo, T. Ogura and S. Itoh, Chem. Commun., 2013, 49, 4358 RSC; (d) E. Galardon, T. Roger, P. Deschamps, P. Roussel, A. Tomas and I. Artaud, Inorg. Chem., 2012, 51, 10068 CrossRef CAS PubMed.
- (a) X.-D. Chen, W. Zhang, J. S. Duncan and S. C. Lee, Inorg. Chem., 2012, 51, 12891 CrossRef CAS PubMed; (b) X.-D. Chen, J. S. Duncan, A. K. Verma and S. C. Lee, J. Am. Chem. Soc., 2010, 132, 15884 CrossRef CAS PubMed; (c) C. P. Berlinguette and R. H. Holm, J. Am. Chem. Soc., 2006, 128, 11993 CrossRef CAS PubMed.
- (a) V. Jovanovski, V. Gonzalez-Pedro, S. Gimenez, E. Azaceta, G. Cabanero, H. Grande, R. Tena-Zaera, I. Mora-Sero and J. Bisquert, J. Am. Chem. Soc., 2011, 133, 20156 CrossRef CAS PubMed; (b) J. Bisquert Mascarell, I. Mora Sero', V. Jovanovski, R. Marcilla Garcia, R. Tena-Zaera, D. Mecerreyes Molero and G. Cabanero Sevillano, EP 2 388 853, 2011; (c) J. Liu, X. Yang, J. Cong, L. Kloo and L. Sun, Phys. Chem. Chem. Phys., 2012, 14, 11592 RSC; (d) J. Cong, X. Yang, Y. Hao, L. Kloo and L. Sun, RSC Adv., 2012, 2, 3625 RSC.
- (a) I. Bauer, M. Kohl, H. Althues and S. Kaskel, Chem. Commun., 2014, 50, 3208 RSC; (b) D. Bresser, S. Passerini and B. Scrosati, Chem. Commun., 2013, 49, 10545 RSC; (c) R. Chen, T. Zhao and F. Wu, Chem. Commun., 2015, 51, 18 RSC; (d) Y. Diao, K. Xie, S. Xiong and X. Hong, J. Power Sources, 2013, 235, 181 CrossRef CAS PubMed; (e) C. J. Hart, M. Cuisinier, X. Liang, D. Kundu, A. Garsuch and L. F. Nazar, Chem. Commun., 2015, 51, 2308 RSC; (f) E. S. Shin, K. Kim, S. H. Oh and W. I. Cho, Chem. Commun., 2013, 49, 2004 RSC.
- (a) Z. Deng, D. Cao, J. He, S. Lin, S. M. Lindsay and Y. Liu, ACS Nano, 2012, 6, 6197 CrossRef CAS PubMed; (b) A. Nag, M. V. Kovalenko, J.-S. Lee, W. Liu, B. Spokoyny and D. V. Talapin, J. Am. Chem. Soc., 2011, 133, 10612 CrossRef CAS PubMed.
- (a) È. Boros, M. J. Earle, M. A. Gîlea, A. Metlen, A.-V. Mudring, F. Rieger, A. J. Robertson, K. R. Seddon, A. A. Tomaszowska, L. Trusov and J. S. Vyle, Chem. Commun., 2010, 46, 716 RSC; (b) N. S. A. Manan, L. Aldous, Y. Alias, P. Murray, L. J. Yellowlees, M. C. Lagunas and C. Hardacre, J. Phys. Chem. B, 2011, 115, 13873 CrossRef CAS PubMed.
- (a) R. Kalb (PROIONIC), WO 2008 052 861, 2008; (b) G. Degen and C. Stock (BASF), WO 2009 040 242, 2009.
- Crystal data for 5: C9H21N1S1, M = 175.33, tetragonal, a = 15.6635(6) Å, b = 15.6635 Å, c = 8.9408(5) Å, α = 90°, β = 90°, γ = 90°, V = 2193.6(2) Å3, T = 100(2) K, P42/mbc, Z = 8, R1 = 0.0601, wR2 = 0.1680. GOOF = 1.056, CCDC 1414154.
- Crystal data for 1: C6H12N2S1, M = 144.24, monoclinic, a = 8.6023(3) Å, b = 7.6710(2) Å, c = 12.7600(4) Å, α = 90°, β = 107.8620(10)°, γ = 90°, V = 801.42(4) Å3, T = 100(2) K, P21/n, Z = 4, R1 = 0.0318, wR2 = 0.0876. GOOF = 1.066, CCDC 1414150.
- (a) L. P. N. Rebelo, J. N. C. Lopes, J. M. S. S. Esperanca and E. Filipe, J. Phys. Chem. B, 2005, 109, 6040 CrossRef CAS PubMed; (b) M. J. Earle, J. M. S. S. Esperanca, M. A. Gilea, J. N. Canongia Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831 CrossRef CAS PubMed; (c) Y. U. Paulechka, D. H. Zaitsau, G. J. Kabo and A. A. Strechan, Thermochim. Acta, 2005, 439, 158 CrossRef CAS PubMed; (d) D. H. Zaitsau, G. J. Kabo, A. A. Strechan, Y. U. Paulechka, A. Tschersich, S. P. Verevkin and A. Heintz, J. Phys. Chem. A, 2006, 110, 7303 CrossRef CAS PubMed; (e) P. Wasserscheid, Nature, 2006, 439, 797 CrossRef CAS PubMed.
- A. W. Taylor, K. R. J. Lovelock, A. Deyko, P. Licence and R. G. Jones, Phys. Chem. Chem. Phys., 2010, 12, 1772 RSC.
- (a) J. P. Leal, J. M. S. S. Esperança, M. E. Minas da Piedade, J. N. Canongia Lopes, L. P. N. Rebelo and K. R. Seddon, J. Phys. Chem. A, 2007, 111, 6176 CrossRef CAS PubMed; (b) J. P. Armstrong, C. Hurst, R. G. Jones, P. Licence, K. R. J. Lovelock, C. J. Satterley and I. J. Villar-Garcia, Phys. Chem. Chem. Phys., 2007, 9, 982 RSC; (c) B. A. D. Neto, E. C. Meurer, R. Galaverna, B. J. Bythell, J. Dupont, R. G. Cooks and M. N. Eberlin, J. Phys. Chem. Lett., 2012, 3, 3435 CrossRef CAS PubMed; (d) J. M. S. S. Esperanca, J. N. Canongia Lopes, M. Tariq, L. M. N. B. F. Santos, J. W. Magee and L. P. N. Rebelo, J. Chem. Eng. Data, 2010, 55, 3 CrossRef CAS.
- (a) R. W. Berg, J. N. Canongia Lopes, R. Ferreira, L. P. N. Rebelo, K. R. Seddon and A. A. Tomaszowska, J. Phys. Chem. A, 2010, 114, 10834 CrossRef CAS PubMed; (b) M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411 CrossRef CAS PubMed; (c) J. Vitorino, J. P. Leal, M. E. Minas da Piedade, J. N. Canongia Lopes, J. M. S. S. Esperanca and L. P. N. Rebelo, J. Phys. Chem. B, 2010, 114, 8905 CrossRef CAS PubMed; (d) J. Vitorino, C. E. S. Bernardes and M. E. Minas da Piedade, Phys. Chem. Chem. Phys., 2012, 14, 4440 RSC.
- M. Horikawa, N. Akai, A. Kawai and K. Shibuya, J. Phys. Chem. A, 2014, 118, 3280 CrossRef CAS PubMed.
- O. Hollóczki, D. Gerhard, K. Massone, L. Szarvas, B. Nemeth, T. Veszpremi and L. Nyulaszi, New J. Chem., 2010, 34, 3004 RSC.
- (a) S. P. Verevkin, R. V. Ralys, D. H. Zaitsau, V. N. Emel'yanenko and C. Schick, Thermochim. Acta, 2012, 538, 55 CrossRef CAS PubMed; (b) S. P. Verevkin, D. H. Zaitsau, V. N. Emelyanenko, A. V. Yermalayeu, C. Schick, H. Liu, E. J. Maginn, S. Bulut, I. Krossing and R. Kalb, J. Phys. Chem. B, 2013, 117, 6473 CrossRef CAS PubMed.
- (a) R. P. Matthews, T. Welton and P. A. Hunt, Phys. Chem. Chem. Phys., 2014, 16, 3238 RSC; (b) P. M. Richard, A. Claire, W. Tom and A. H. Patricia, J. Phys.: Condens. Matter, 2014, 26, 284112 CrossRef PubMed.
- M. T. Clough, K. Geyer, P. A. Hunt, J. Mertes and T. Welton, Phys. Chem. Chem. Phys., 2013, 15, 20480 RSC.
- K. M. Hugar, H. A. Kostalik and G. W. Coates, J. Am. Chem. Soc., 2015, 137, 8730 CrossRef CAS PubMed.
- (a) H. Rodriguez, G. Gurau, J. D. Holbrey and R. D. Rogers, Chem. Commun., 2011, 47, 3222 RSC; (b) S. T. Manjare, S. Sharma, H. B. Singh and R. J. Butcher, J. Organomet. Chem., 2012, 717, 61 CrossRef CAS PubMed; (c) S. Sauerbrey, P. K. Majhi, G. Schnakenburg, A. J. Arduengo III and R. Streubel, Dalton Trans., 2012, 41, 5368 RSC; (d) Y.-F. Han, L. Zhang, L.-H. Weng and G.-X. Jin, J. Am. Chem. Soc., 2014, 136, 14608 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Professor Bernd Harbrecht on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, analytical data, additional information regarding sublimation, isothermal TGA and DFT calculations. CCDC 1414150 and 1414154. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc06224a |
| This journal is © The Royal Society of Chemistry 2015 |