Rhodium-catalyzed C–H activation of hydrazines leads to isoquinolones with tunable aggregation-induced emission properties†
Abstract
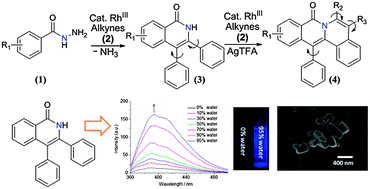
Using an internally oxidizing directing group (DG) strategy, we report a RhIII-catalyzed synthesis of isoquinolones via C–H activation/annulation of benzoylhydrazines and alkynes. Tunable double cascade cyclization of benzoylhydrazines with two equivalents of alkynes led to tetracyclic amides. These N-heterocycles demonstrated adjustable AIE properties.

 Please wait while we load your content...
Please wait while we load your content...