Open Access Article
Open Access ArticleSolution processable, cross-linked sulfur polymers as solid electrolytes in dye-sensitized solar cells†
Peng
Liu
,
James M.
Gardner
* and
Lars
Kloo
*
Applied Physical Chemistry, Department of Chemistry, KTH Royal Institute of Technology, Teknikringen 30, SE-10044 Stockholm, Sweden. E-mail: jgardner@kth.se; larsa@kth.se
First published on 14th August 2015
Abstract
Inverse-vulcanized polymeric sulfur has been prepared and utilized for solid-state dye sensitized solar cells. A power conversion efficiency of 1.5% was recorded with a short-circuit current of 4.1 mA cm−2 and an open-circuit voltage of 0.75 V under standard AM 1.5G illumination (1000 W m−2). The results in the present study qualify the new polymeric sulfur material as a future candidate as low-cost, hole-transport material for solid-state dye-sensitized solar cells.
It is the overall cost of making, installing, and using photovoltaics that will determine if they become the future, primary renewable energy source. The important factors for reducing cost involve an increase in conversion efficiency, an increase in usable lifetime, as well as a decrease of inherent material costs for the production of the solar cells. The identification of inexpensive and abundant materials that are easily processed represents a major challenge. Exactly this challenge is the main objective of the present work. Dye-sensitized solar cells (DSSCs) have emerged as a promising alternative to traditional solar technologies.1,2 The DSSC devices consist of two electrodes in electrical contact through a solid, or more commonly liquid, electrolyte system. In the DSSC system, the redox electrolyte or hole-transport material (HTM) plays the essential role in regenerating the sensitizing dye molecules. Due to the possible leakage and corrosion problems associated with liquid electrolytes, much effort has been devoted to the development of new solid-state, dye-sensitized solar cells (ssDSSCs).3–6 To permit large-scale applications, one of the most important challenges for ssDSSCs is to develop low-cost, environmentally-friendly hole-transport materials based on facile, straightforward syntheses. The most expensive component in the present high-performance ssDSSCs is the hole-transport materials. Most commonly, the molecular hole-conductor material Sprio-OMeTAD is used, and a typical commercial price of Spiro-OMeTAD is more than $450
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per kilogram. On the other hand, the element of sulfur is known since ancient times as an abundant and inexpensive material, about four orders of magnitude cheaper than Spiro-OMeTAD. Sulfur is widely utilized for the production of sulfuric acid. The low price of sulfur can partly be traced to the strategic position of sulfuric acid in chemistry and materials industry. In addition, sulfur is a byproduct in the refinement of fossil fuels and is produced in megatons each year. Because of its unique electrochemical properties and high natural abundance, sulfur or polysulfides have become widely utilized in efficient lithium batteries7–10 and in thin-film photovoltaic devices.11–13 The chalcogens (sulfur, selenium, and tellurium) are well known semiconductors and have been used in various charge-transfer compounds.14–16 Although the use of polymeric sulfur in Li–S batteries has been solely based on their electron-conducting properties, the chalcogens are characterized by relatively high hole mobilities with respect to their electron mobilities, much like the Group 14 semiconductors.17,18 The expected hole mobilities suggest that they may have applications also as HTMs. However, the major drawbacks for sulfur-containing materials applications are associated with the limited solubility in the vast majority of organic solvents, generating production problems (i.e. costs), as well as the poor stability of polymeric sulfur when not cross-linked. Although sulfur shows relatively good solubility in carbon disulfide,19 some aromatic solvents and certain ionic liquids,20 the solubility under ambient conditions is still low. Hence, the development of novel process methods is required for efficient sulfur exploitation in, for instance, electrochemical applications.21–23 Recently, Pyun and co-workers explored co-polymerization strategies to make solution-processable, polymeric sulfur materials by co-polymerization with divinylic co-monomers.24 Following the synthetic strategies outlined in this paper, we can demonstrate the use of polymeric sulfur in a stable, cross-linked material, obtained by inverse vulcanization, as a hole-transport material in ssDSSCs. To the best of our knowledge, this is the first example of polymeric sulfur in a solar cell.
000 per kilogram. On the other hand, the element of sulfur is known since ancient times as an abundant and inexpensive material, about four orders of magnitude cheaper than Spiro-OMeTAD. Sulfur is widely utilized for the production of sulfuric acid. The low price of sulfur can partly be traced to the strategic position of sulfuric acid in chemistry and materials industry. In addition, sulfur is a byproduct in the refinement of fossil fuels and is produced in megatons each year. Because of its unique electrochemical properties and high natural abundance, sulfur or polysulfides have become widely utilized in efficient lithium batteries7–10 and in thin-film photovoltaic devices.11–13 The chalcogens (sulfur, selenium, and tellurium) are well known semiconductors and have been used in various charge-transfer compounds.14–16 Although the use of polymeric sulfur in Li–S batteries has been solely based on their electron-conducting properties, the chalcogens are characterized by relatively high hole mobilities with respect to their electron mobilities, much like the Group 14 semiconductors.17,18 The expected hole mobilities suggest that they may have applications also as HTMs. However, the major drawbacks for sulfur-containing materials applications are associated with the limited solubility in the vast majority of organic solvents, generating production problems (i.e. costs), as well as the poor stability of polymeric sulfur when not cross-linked. Although sulfur shows relatively good solubility in carbon disulfide,19 some aromatic solvents and certain ionic liquids,20 the solubility under ambient conditions is still low. Hence, the development of novel process methods is required for efficient sulfur exploitation in, for instance, electrochemical applications.21–23 Recently, Pyun and co-workers explored co-polymerization strategies to make solution-processable, polymeric sulfur materials by co-polymerization with divinylic co-monomers.24 Following the synthetic strategies outlined in this paper, we can demonstrate the use of polymeric sulfur in a stable, cross-linked material, obtained by inverse vulcanization, as a hole-transport material in ssDSSCs. To the best of our knowledge, this is the first example of polymeric sulfur in a solar cell.
The synthetic procedure used follows the previously published process.24 In this study, we used 1,3-diisopropenylbenzene (DIB) as the cross-linker with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by weight. The cross-linked polymeric sulfur material shows excellent solubility in organic solvents.24 The UV-vis absorption spectra of cross-linked polymeric sulfur in chlorobenzene solution and adsorbed to a mesoporous TiO2 film are shown in Fig. 1. In solution, an intense absorption peak at 430 nm and a weak peak at 644 nm can be observed. The former peak is similar to the absorption band observed for high-order polymeric sulfur anions,25 and the latter peak is more akin to that observed for low-order polymeric sulfur radical anions.26,27 These results are consistent with previous studies indicating that polymeric sulfur anions tend to form solutions containing several species in equilibrium.20 When deposited onto a TiO2 film, the intensity of the longer wavelength absorption peaks from the polymeric sulfur material decreases in intensity, whereas the absorption peak attributed to high-order polymeric sulfur remains unchanged. The results indicate that it is primarily higher-order polymeric sulfur materials that deposit onto TiO2.
1 ratio by weight. The cross-linked polymeric sulfur material shows excellent solubility in organic solvents.24 The UV-vis absorption spectra of cross-linked polymeric sulfur in chlorobenzene solution and adsorbed to a mesoporous TiO2 film are shown in Fig. 1. In solution, an intense absorption peak at 430 nm and a weak peak at 644 nm can be observed. The former peak is similar to the absorption band observed for high-order polymeric sulfur anions,25 and the latter peak is more akin to that observed for low-order polymeric sulfur radical anions.26,27 These results are consistent with previous studies indicating that polymeric sulfur anions tend to form solutions containing several species in equilibrium.20 When deposited onto a TiO2 film, the intensity of the longer wavelength absorption peaks from the polymeric sulfur material decreases in intensity, whereas the absorption peak attributed to high-order polymeric sulfur remains unchanged. The results indicate that it is primarily higher-order polymeric sulfur materials that deposit onto TiO2.
 | ||
| Fig. 1 Normalized absorption spectra of crosslinked polymeric sulfur in solution and adsorbed onto the surface of a mesoporous TiO2 film. | ||
Fig. 2 shows the Raman spectra of the polymeric sulfur material and elemental sulfur (S8 rings in orthorhombic sulfur). Although fluorescence from the material is problematic, broad bands between 600 and 800 cm−1, which can be assigned to the C–S stretch modes of the cross-linker-sulfur interaction, are observed.28,29 This observation shows that sulfur has been co-polymerized with the assistance of the cross-linker. Also, the peaks located between 2800 and 3100 cm−1 can be assigned to the C–H stretch modes of the cross-linker.30,31 All of the above shows that, as intended, the cross-linker has been incorporated into the polymeric sulfur material.
 | ||
| Fig. 2 Raman spectra of polymeric sulfur and elemental sulfur. Inset: Magnification of the range from 100 to 1000 cm−1. | ||
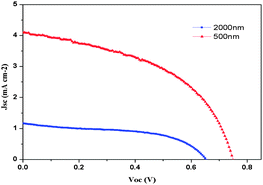
Fig. 3 shows J–V curves of ssDSSCs based on the LEG4 organic sensitizer and the polymeric sulfur hole-transport material for differing film thicknesses. The photovoltaic parameters of the ssDSSCs are summarized in Table 1 and statistical data have been collected in the ESI.† Upon illumination at standard 1 sun light intensity the devices with 2000 nm thickness of TiO2 films yield an efficiency of 0.4% with a Voc of 655 mV, a Jsc of 1.17 mA cm−2, and a fill factor (FF) of 0.51. When using thinner TiO2 films, the devices show an improved efficiency of 1.5% with a Voc of 700 mV a Jsc of 4.1 mA cm−2, and a fill factor of 0.48. This increased efficiency may be attributed to better pore filling, and thus a potentially larger light-harvesting volume, for the thinner mesoporous substrates.32 In order to investigate the monochromatic quantum efficiencies, incident photon-to-current conversion efficiency (IPCE) measurements were performed (Fig. 4). A maximum monochromatic conversion efficiencies of 20% (2 μm TiO2 film) and 40% (500 nm TiO2 film) are located at around 500 nm, which is in good agreement with the maximum in UV-vis absorption spectra of the LEG4 dye.
| LEG4/Polymeric sulfura | J sc (mA cm−2) | V oc (mV) | FFd | PECd (%) |
|---|---|---|---|---|
| a Electrolyte for spin-coating contains 5 mg mL−1 polymeric sulfur, 200 mM 4-tert-butylpyridine and 90 mM Li-TFSI. b 2 μm thickness of TiO2 film. c 500 nm thickness of TiO2 film. d The devices were investigated using a metal mask with an aperture area of 0.126 cm2 and photovoltaic data were recorded under full sun AM 1.5G illumination. The statistical data from different cells are given in the ESI. | ||||
| TiO2 filmb | 1.17 | 655 | 0.51 | 0.4 |
| TiO2 filmc | 4.11 | 750 | 0.48 | 1.5 |
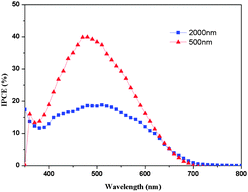 | ||
| Fig. 4 The IPCE spectra of solid-state dye-sensitized solar cells based on LEG4 as sensitizer and polymeric sulfur as hole-conductor material. | ||
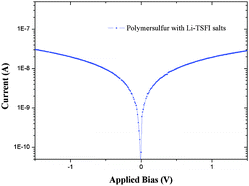
Most likely, the important limiting factors with respect to efficient conversion of solar light can be traced to inefficient pore filling of the mesoporous semiconductor substrate in combination with a low charge-carrier mobility of the hole-transport material itself.33,34Fig. 5 shows the Tafel plot of the polymeric sulfur material doped with 90 mM Li-TFSI; a common dopant used in organic hole-conducting materials. The details of fabrication process are included in the ESI.† The conductivity was determined to 6.13 × 10−5 S cm−1, obtained using a standard 4-probe technique. This conductivity is a factor 10–100 lower than for the so far best performing hole-transport material, doped Spiro-OMeTAD.3 The focus of future research on the improvement of conversion efficiency of ssDSSCs based on polymeric sulfur should be on the enhancement of charge-carrier mobility and density of the hole-transport material and more efficient deposition techniques.
In conclusion, we have provided a fundamental proof-of-function of an entirely new class of hole-transport materials based on the abundant element sulfur in solid-state DSSCs. The devices based on polymeric sulfur as hole-conductor material show surprisingly good efficiencies, even without optimization. However, the low photocurrents can be traced to the intrinsic low conductivity of the sulfur-based materials and poor pore filling of the mesoporous titania substrate. Nevertheless, the current results offer a new and promising line of research aimed at efficient and low-cost materials that through simplicity of generation – the method of synthesis is close to trivial – offers possibilities for large-scale production. Just as for all conceptually new materials introduced, the future offers significant opportunities for improvement.
The authors would like to thank the Swedish Research Council, the Swedish Energy Agency and the Knut & Alice Wallenberg Foundation, as well as the China Scholar Council for financial support. J. G. would like to kindly acknowledge the support of the Swedish Government through the research initiative “STandUP for ENERGY”.
Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629–6634 CrossRef CAS PubMed.
- J. Burschka, A. Dualeh, F. Kessler, E. Baranoff, N.-L. L. Cevey-Ha, C. Yi, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2011, 133, 18042–18045 CrossRef CAS PubMed.
- I. Chung, B. Lee, J. He, R. P. Chang and M. G. Kanatzidis, Nature, 2012, 485, 486–489 CrossRef CAS PubMed.
- P. Liu, B. Xu, K. M. Karlsson, J. Zhang, N. Vlachopoulos, G. Boschloo, L. Sun and L. Kloo, J. Mater. Chem. A, 2015, 3, 4420–4427 CAS.
- J. Wang, J. Yang, C. Wan, K. Du, J. Xie and N. Xu, Adv. Funct. Mater., 2003, 13, 487–492 CrossRef CAS PubMed.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- D.-W. Wang, Q. Zeng, G. Zhou, L. Yin, F. Li, H.-M. Cheng, I. R. Gentle and G. Q. M. Lu, J. Mater. Chem. A, 2013, 1, 9382–9394 CAS.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- Y.-L. Lee and C.-H. Chang, J. Power Sources, 2008, 185, 584–588 CrossRef CAS PubMed.
- Z. Yang, C.-Y. Chen, C.-W. Liu and H.-T. Chang, Chem. Commun., 2010, 46, 5485–5487 RSC.
- V. Chakrapani, D. Baker and P. V. Kamat, J. Am. Chem. Soc., 2011, 133, 9607–9615 CrossRef CAS PubMed.
- E. Ahmed, J. Beck, J. Daniels, T. Doert, S. J. Eck, A. Heerwig, A. Isaeva, S. Lidin, M. Ruck, W. Schnelle and A. Stankowski, Angew. Chem., Int. Ed., 2012, 51, 8106–8109 CrossRef CAS PubMed.
- Z. Tang, Y. Wang, K. Sun and N. A. Kotov, Adv. Mater., 2005, 17, 358–363 CrossRef CAS PubMed.
- J. Beck, Angew. Chem., Int. Ed. Engl., 1994, 33, 163–172 CrossRef PubMed.
- K. Ueda, S. Inoue, S. Hirose, H. Kawazoe and H. Hosono, Appl. Phys. Lett., 2000, 77, 2701–2703 CrossRef CAS PubMed.
- J. Tate, P. F. Newhouse, R. Kykyneshi, P. A. Hersh, J. Kinney, D. H. McIntyre and D. A. Keszler, Thin Solid Films, 2008, 516, 5795–5799 CrossRef CAS PubMed.
- B. Meyer, J. M. Austin and D. Jensen, J. Chem. Eng. Data, 1971, 16, 364–366 CrossRef CAS.
- E. Boros, M. J. Earle, M. A. Gîlea, A. Metlen, A.-V. V. Mudring, F. Rieger, A. J. Robertson, K. R. Seddon, A. A. Tomaszowska, L. Trusov and J. S. Vyle, Chem. Commun., 2010, 46, 716–718 RSC.
- S. Penczek, R. Slazak and A. Duda, Nature, 1978, 273, 738–739 CrossRef CAS PubMed.
- W. J. Chung, A. G. Simmonds, J. J. Griebel, E. T. Kim, H. S. Suh, I.-B. Shim, R. S. Glass, D. A. Loy, P. Theato, Y.-E. Sung, K. Char and J. Pyun, Angew. Chem., Int. Ed., 2011, 50, 11409–11412 CrossRef CAS PubMed.
- Y. Yang, G. Zheng and Y. Cui, Chem. Soc. Rev., 2013, 42, 3018–3032 RSC.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- R. P. Martin, W. H. Doub, J. L. Roberts and D. T. Sawyer, Inorg. Chem., 1973, 12, 1921–1925 CrossRef CAS.
- N. S. Manan, L. Aldous, Y. Alias, P. Murray, L. J. Yellowlees, M. C. Lagunas and C. Hardacre, J. Phys. Chem. B, 2011, 115, 13873–13879 CrossRef CAS PubMed.
- T. Chivers and I. Drummond, Inorg. Chem., 1972, 11, 2525–2527 CrossRef CAS.
- H. E. Van Wart, F. Cardinaux and H. A. Scheraga, J. Phys. Chem., 1976, 80, 625–630 CrossRef CAS.
- E. J. Bastian and R. B. Martin, J. Phys. Chem., 1973, 77, 1129–1133 CrossRef CAS.
- M. Gniadecka, H. C. Wulf, O. F. Nielsen, D. H. Christensen and J. Hercogova, Photochem. Photobiol., 1997, 66, 418–423 CrossRef CAS PubMed.
- H. Knobloch, C. Duschl and W. Knoll, J. Phys. Chem., 1989, 91, 3810–3814 CrossRef CAS PubMed.
- C. T. Weisspfenning, D. J. Hollman, C. Menelaou, S. D. Stranks, H. J. Joyce, M. B. Johnston, H. J. Snaith and L. M. Herz, Adv. Funct. Mater., 2014, 24, 668–677 CrossRef PubMed.
- F. Fabregat-Santiago, J. Bisquert, L. Cevey, P. Chen, M. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2009, 131, 558–562 CrossRef CAS PubMed.
- H. J. Snaith, R. Humphry-Baker, P. Chen, I. Cesar, S. M. Zakeeruddin and M. Grätzel, Nanotechnology, 2008, 19, 1–12 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc04822b |
| This journal is © The Royal Society of Chemistry 2015 |