Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereoselective formation of coordination polymers with 1,4-diaminonaphthalene on various Cu substrates†
Marek
Knor
ab,
Hong-Ying
Gao
*ab,
Saeed
Amirjalayer
ab,
Armido
Studer
c,
Hongjun
Gao
d,
Shixuan
Du
d and
Harald
Fuchs
abe
aCenter for Nanotechnology, Heisenbergstraße 11, 48149 Münster, Germany. E-mail: mknor01@uni-muenster.de; gaoh@uni-muenster.de; fuchsh@uni-muenster.de
bPhysikalisches Institut, Westfälische Wilhelms-Universität, Wilhelm-Klemm-Straße 10, 48149 Münster, Germany
cOrganisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstraße 40, 48149 Münster, Germany
dInstitute of Physics, Chinese Academy of Sciences, Beijing 100190, China
eInstitute for Nanotechnology, Karlsruhe Institute of Technology, 76344 Karlsruhe, Germany
First published on 1st June 2015
Abstract
Polymerization of 1,4-diaminonaphthalene on various Cu substrates resulting in stereoselectively well-defined metal–organic coordination polymers is reported. By using different crystallographic planes (111), (110) and (100) of a Cu substrate the structure of the resulting coordination polymer was controlled.
On-surface polymerization has recently been used as an efficient method for the preparation of well defined, novel types of polymers directly at a surface. Many contributions in this area deal with on-surface covalent carbon–carbon or carbon–nitrogen bond formation.1 In addition, this approach has also been successfully applied to the formation of metal–organic polymers containing carbon-metal2 or nitrogen–metal coordination bonds.3 In the latter cases, a metal atom is either added to or extracted from the surface during polymerization. It is well known that the surface is not static (especially above room temperature) and some of the metal atoms can be readily pulled out from the surface and be ligated by the organic adsorbate.4 Metal–organic coordination polymers are highly interesting because they show unique properties. Considering structural properties, either 3D or 2D coordination frameworks can be constructed at the surface. Moreover, such surface adsorbed coordination networks reveal interesting catalytic,5 magnetic6 and electronic7 properties. It is obvious that all these properties are influenced and controlled by the structure and geometry of the coordination polymer. Another aspect is that the electronic configuration of the coordination center (metal atom) in such networks is not readily predictable. The metal can have different oxidation states and charges because the surface works as an electron reservoir which can interact with the organometallic complex at the surface.8 In specific cases the electronic configuration of the complex at the surface is different as compared to its electronic state in solution and in the solid state. For example, a Cu adatom on a Cu(111) substrate can be negatively charged when it is bonded to a cyano group.4d It is obvious that complexes interacting with a substrate might show different catalytic activity. Therefore, understanding the geometry of coordination systems at a surface within the 2D network and also its interaction with the substrate is of great importance for future application of these systems, for example in catalysis. Here, we report the effect of the substrate reconstitution on the final geometry of the coordination network and show that different crystallographic structures of the same metal (in this case Cu) control the stereochemistry of polymerization leading to coordination polymers with different relative configurations.
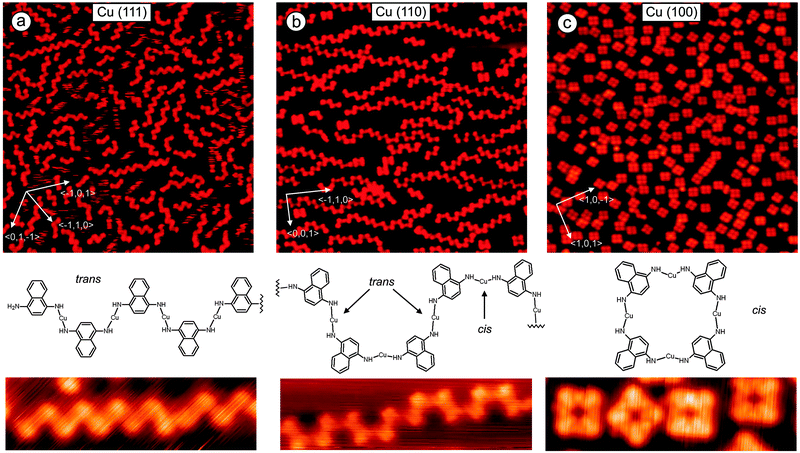
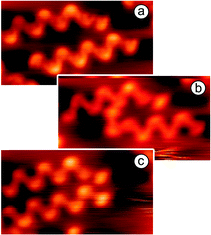
Polymerization of 1,4-diaminonaphthalene at Cu(111): evaporation of 1,4-diaminonaphthalene (DAN) at the Cu(111) substrate at room temperature leads to formation of monolayers where the organic molecules are randomly distributed showing different orientations on the surface. The random orientation of DANs indicates that the interaction between the organic molecules is weaker than the one between DANs and the Cu-substrate. After heating the sample to 390 K most of the molecules just desorbed but we also observed the formation of some dimers which were found to be very mobile at the surface under applied conditions. These dimers and remaining monomers upon further heating of the surface to 400 K started to form longer oligomers. This process was slow and not very efficient due to low concentration of monomers and dimers caused by the competing thermal desorption. Better results were obtained by depositing DANs directly at a hot Cu(111) surface. This process allowed the desorbed diaminonaphthalenes to be continuously recharged to the surface which can diffuse and react with oligomers leading to a further growth of the polymers (Fig. 1a). By using this set up, oligomers with a chain length of up to 20 nm were formed. It is clearly visible from the STM images that most polymers grew along one of the three main crystallographic axes of the substrate. The Cu–N bond in the Cu-complex was found to be very stable. The amino ligands are trans-oriented to each other as a naphthyl–NH–Cu–NH–naphthyl complex. Note that dehydrogenation in amines is known to occur at Cu(111) and at Au(111) substrates.9 Importantly, while in solution bisamino–Cu bonds can rotate around the Cu–N-bond, due to van der Waals interactions with the substrate free rotation is suppressed at the Cu surface. Therefore, Cu–amino complexes can be formed as different stereoisomers at Cu. Guided by the cis/trans-nomenclature to describe alkene geometry, we herein also use the trans and cis terminology to specify the surface adsorbed biasamino–Cu-complexes. In the trans-isomer, the protons of the amino ligands are trans-oriented to each other whereas in the cis-complex the protons of the amino ligands are oriented in cis configuration to each other. As the major oligomeric structure formed on a hot Cu(111) surface we found an all trans-configured coordination polymer revealing that oligmerization occurred with high trans-selectivity. The stability of the coordination polymers is remarkably high. Chains could be mechanically manipulated by the STM tip without losing their shape (Fig. 2a–c). The interaction of a Cu built-in atom of the oligomer with the N atom is much stronger than that with the metal substrate. For example, an oligomer containing 7Cu atoms was readily manipulated by pushing from one edge without destroying the polymeric structure. This means that the weakest bond in the system (likely the Cu–N bond) is much stronger than the interaction of the all oligomer with its 7Cu atoms with the substrate. A strong interaction within the oligomers and weak interaction of the oligomer with the substrate gives also the possibility to elastically bend the coordination bonds (Fig. 2b). It was interesting that after manipulation the oligomer returned to its original lowest energy structure revealing shape memory behavior (Fig. 2c).
Polymerization of 1,4-diaminonaphthalene at Cu(110): when DAN molecules were deposited on Cu(110) at room temperature in vacuum they form well-ordered structures where the organic molecules interact via hydrogen bonding. Heating of the substrate to 420 K leads to desorption of most DANs, but some of them formed short oligomers. As for the Cu(111) case discussed above, better results were achieved when the organic molecules were directly deposited on a hot Cu(110) surface. Polymer chains were formed, but interestingly, the shape of most oligomers differs significantly from the shape obtained for DAN-polymerization at Cu(111) where most of the polymers were generated with trans-configuration leading to a zig-zag type shape of the chain. However, at Cu(110) the largest part of the monomers is connected with alternating trans–cis–trans–cis relative configuration providing a U-shape type structure (Fig. 1b). One can clearly identify a minor fraction which polymerized with trans-configuration leading to a zig-zag-type oligomer.
Polymerization of 1,4-diaminonaphthalene at Cu(100): DAN monomers were deposited at room temperature at Cu(100) providing a submonolayer with random distribution of the molecules. DAN did not form ordered or well packed islands. Differences in self-assembled monolayers between Cu(111), Cu(110) and Cu(100) are caused by different periodicity and symmetry of these surfaces. Heating of the substrate containing physisorbed DAN molecules to 390 K provided tetramers with rectangular shape at high yield (Fig. 1c). The build-up of cyclic tetramers is a result of a highly diastereoselective all-cis-amino–Cu complex formation. The suggested chemical structure is shown below. For comparison with the Cu(111) and Cu(110) cases we also deposited DANs directly at a hot Cu(100) surface. For this substrate the hot and cold deposition procedures afforded similar results. Only a small amount of the molecules was desorbed during heating likely due to faster reaction and also due to stronger interaction of the monomer with the Cu substrate. To a small extent, the formation of cyclic tetramers was also observed at the previous Cu(110) substrate (Fig. 1b). To get a better picture of the selectivity of DAN oligomerization as a function of the substrate, we analyzed the number of trans and cis configured naphthylNH–Cu–NHnapthyl complexes extracted from various STM pictures which allowed us to gain statistical information on the trans/cis-selectivity as a function of the Cu substrate. On the Cu(111) surface trans-configured bisamino–Cu-complexes occurred with 85% abundance A near complete cis-selectivity was observed for Cu(100) where almost all complexes (99%) were formed as cis-isomers. Cu(110) results in an intermediate state with the trans to cis complexes in a 66% to 34% ratio.
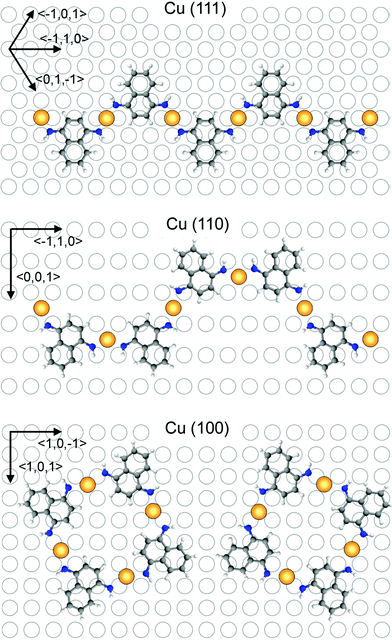
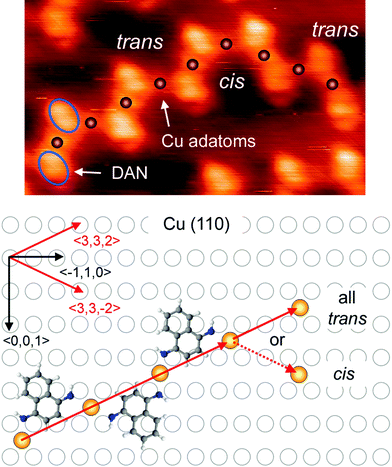
Theoretical investigations: based on STM data we proposed possible configurations of the various Cu-DAN-oligomers (Fig. 3), which were in good agreement with the experimentally observed structures. In the all-trans-configured oligomer identified as a zig/zag-shaped system on Cu(111), the Cu-adatoms are lined up with a calculated distance of 0.75 nm which is in very good agreement with the experimentally determined value (0.75 nm). Metal atom chains are parallel to the three main symmetry axes (〈0,1,−1〉, 〈−101〉, and 〈−110〉) of the Cu(111) substrate which forms the polymer mostly parallel to these three directions (see main chains in Fig. 1c). This also fits in to the theoretical model because structure can be easily rotated by 60° to get the same adsorption geometry. For the Cu(110) substrate the experimentally observed U-shape oligomer occurred with some zig/zag shape oligomer similar to previous structure. In this case, the Cu-adatoms appear to be highly regular with a distance of 0.79 nm from each other and form a zig/zag relative arrangement. In some cases all metal adatoms can lie in line along 〈3,3,2〉 axes or its mirror direction 〈3,3,−2〉 marked by red arrows (Fig. 4). When all Cu adatoms are parallel to this axis it effects only the trans substitution of the DAN molecules. When adsorption of the adatoms is not linear and it changes between these axes (dotted arrow) it causes the substitution of next DAN molecule in cis configuration. This substitution and growth mechanism are random which are clearly visible in the large area (Fig. 1b) and it fits well to the proposed model. Cis substitution can only occur parallel to the 〈−1,1,0〉 direction when two DAN molecules are mirrored in 〈0,0,1〉 axes. Interestingly, whereas for the trans-configuration the Cu-adatom lies in line in the middle of the two N-atoms of the amino ligand (angle N–Cu–N = 180°), in the cis-configured complex the N–Cu–N angle is smaller (120°). This bending of the complex leads to an oligomeric structure where all distances between the Cu-adatoms are very similar (theory: 0.79 nm, experiment: 0.81 nm) with main trans–cis–trans–cis alternating configurations. A smaller angle (150°) was also observed for the cis-complexes on Cu(100) where theory predicts the rectangular tetrameric structures. In this case there are two theoretical configurations on the surface which are their mirror reflection (mirror plane is parallel to one of the symmetry axes of the substrate 〈1,0,−1〉 or 〈1,0,1〉). Counting both of these configurations on several pictures gives a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio and information that the energy of adsorption and geometry at the substrate are exactly the same is in very good agreement with the proposed structure. Also the distance between Cu adatoms is in good agreement with the theoretical model (theory: 0.85 nm, experiment: 0.9 nm). Comparing optimal temperatures for the reaction, we discovered that reaction can easily occur at Cu(111) and Cu(100) substrates (390–400 K). Creation of the polymers at the Cu(110) surface needs 20 K higher temperature. Probably it is caused by higher stability of the Cu atom in the subsurface region and higher energy of its diffusion which is necessary to create longer polymer chains.
50 ratio and information that the energy of adsorption and geometry at the substrate are exactly the same is in very good agreement with the proposed structure. Also the distance between Cu adatoms is in good agreement with the theoretical model (theory: 0.85 nm, experiment: 0.9 nm). Comparing optimal temperatures for the reaction, we discovered that reaction can easily occur at Cu(111) and Cu(100) substrates (390–400 K). Creation of the polymers at the Cu(110) surface needs 20 K higher temperature. Probably it is caused by higher stability of the Cu atom in the subsurface region and higher energy of its diffusion which is necessary to create longer polymer chains.
In summary we observed that different crystallographic planes of the same substrate [Cu(111), Cu(110) and Cu(100)] can influence the nature of polymers. Growth of the polymer at the Cu(111) substrate provides mostly trans conformation while Cu(100) gives only cis conformation. Cu(110) has both of these properties and trans and cis conformation can exist on the surface with random distribution or also regular trans–cis–trans–cis order. This strong selectivity of the crystallographic plane of Cu- surface orientations with respect to the final product structures can be helpful to optimize catalytic processes and isomerization at surfaces. We also showed by manipulation of the polymer chains that coordination bonded systems N–Cu–N are very stable when metal atoms are extracted from the surface while their interaction with the metal substrate is weak (compared to the interaction in the polymer chain).
We would like to thank the Deutsche Forschungsgemeinschaft (TRR 61, project B3) for financial support.
References
- (a) J. Cai, P. Ruffieux, R. Jaafar, M. Bieri, T. Braun, S. Blankenburg, M. Muoth, A. P. Seitsonen, M. Saleh, X. Feng, K. Mullen and R. Fasel, Nature, 2010, 466, 470–473 CrossRef CAS PubMed; (b) D. F. Perepichka and F. Rosei, Science, 2009, 323, 216–217 CrossRef CAS PubMed; (c) M. Matena, T. Riehm, M. Stähr, T. A. Jung and L. H. Gade, Angew. Chem., Int. Ed., 2008, 47, 2414–2417 CrossRef CAS PubMed; (d) A. C. Marele, R. Mas-Ballesté, L. Terracciano, J. Rodríguez-Fernández, I. Berlanga, S. S. Alexandre, R. Otero, J. M. Gallego, F. Zamora and J. M. Gómez-Rodríguez, Chem. Commun., 2012, 48, 6779–6781 RSC; (e) M. Treier, N. V. Richardson and R. Fasel, J. Am. Chem. Soc., 2008, 130, 14054–14055 CrossRef CAS PubMed; (f) I. Q. Zhang, N. Kepcija, M. Kleinschrodt, K. Diller, S. Fischer, A. C. Papageorgiou, F. Allegretti, J. Bjork, S. Klyatskaya, F. Klappenberger, M. Ruben and J. V. Barth, Nat. Commun., 2012, 3, 1286 CrossRef PubMed; (g) D. Zhong, J. H. Franke, S. K. Podiyanachari, T. Blomker, H. Zhang, G. Kehr, G. Erker, H. Fuchs and L. Chi, Science, 2011, 334(6053), 213–216 CrossRef CAS PubMed; (h) J. A. Lipton-Duffin, O. Ivasenko, D. F. Perepichka and F. Rosei, Small, 2009, 5(5), 592–597 CrossRef CAS PubMed; (i) A. Ciesielski, M. El Garah, S. Haar, P. Kovaricek, J. M. Lehn and P. Samori, Nat. Chem., 2014, 6, 1017–1023 CrossRef CAS PubMed.
- (a) S. Haq, F. Franke, M. S. Dyer, M. Persson, P. Iavicoli, D. B. Amabilino and R. Raval, J. Am. Chem. Soc., 2011, 133, 12031–12039 CrossRef CAS PubMed; (b) M. Bieri, S. Blankenburg, M. Kivala, C. A. Pignedoli, P. Ruffieux, K. Mulen and R. Fasel, Chem. Commun., 2011, 47, 10239–10241 RSC; (c) Q. Li, J. R. Owens, C. Han, B. G. Sumpter, W. Lu, J. Bernholc, V. Meunier, P. Maksymovych, M. Fuentes-Cabrera and M. Pan, Sci. Rep., 2013, 3(2102), 1–6 Search PubMed; (d) H. Zhang, J. H. Franke, D. Zhong, Y. Li, A. Timmer, O. D. Arado, H. Monig, H. Wang, L. Chi, Z. Wang, K. Mullen and H. Fuchs, Small, 2014, 10(7), 1361–1368 CrossRef CAS PubMed; (e) J. Eichhorn, T. Strunskus, A. Rastgoo-Lahrood, D. Samanta, M. Schmittel and M. Lackinger, Chem. Commun., 2014, 50, 7680–7682 RSC.
- (a) D. Kuhne, F. Klappenberger, R. Decker, U. Schlickum, H. Brune, S. Klyatskaya, M. Ruben and J. V. Barth, J. Am. Chem. Soc., 2009, 131, 3881–3883 CrossRef PubMed; (b) M. Marschall, J. Reichert, A. Weber-Bargioni, K. Seufert, W. Auwarter, S. Klyatskaya, G. Zoppellaro, M. Ruben and J. V. Barth, Nat. Chem., 2010, 2, 131–137 CrossRef CAS PubMed; (c) J. Liu, T. Lin, Z. Shi, F. Xia, L. Dong, P. N. Liu and N. Lin, J. Am. Chem. Soc., 2011, 133, 18760–18766 CrossRef CAS PubMed; (d) D. Heim, D. Ecija, K. Seufert, W. Auwarter, C. Aurisicchio, C. Fabbro, D. Bonifazi and J. V. Barth, J. Am. Chem. Soc., 2010, 132, 6783–6790 CrossRef CAS PubMed; (e) U. Schlickum, R. Decker, F. Klappenberger, G. Zoppellaro, S. Klyatskaya, M. Ruben, I. Silanes, A. Arnau, K. Kern, H. Brune and J. V. Barth, Nano Lett., 2007, 7(12), 3813–3817 CrossRef CAS PubMed; (f) A. Langner, S. L. Tait, N. Lin, C. Rajaduari, M. Ruben and K. Kern, Proc. Natl. Acad. Sci. U.S.A., 2007, 104(46), 17927–17930 CrossRef CAS PubMed.
- (a) V. Barth, J. Weckesser, N. Lin, A. Dmitriev and K. Kern, Appl. Phys. A: Mater. Sci. Process., 2003, 76, 645–652 CrossRef PubMed; (b) N. A. Kautz and S. A. Kandel, J. Am. Chem. Soc., 2008, 130(22), 6908–6909 CrossRef CAS PubMed; (c) F. Li, L. Tang, W. Zhou and Q. Guo, J. Am. Chem. Soc., 2010, 132, 13059–13063 CrossRef CAS PubMed; (d) G. Pawlin, K. L. Wong, D. Kim, D. Sun, L. Bartels, S. Hong, T. S. Rahman, R. Carp and M. Marsella, Angew. Chem., Int. Ed., 2008, 47, 8442–8445 CrossRef PubMed.
- D. Grumell, B. Wurster, S. Stepanow and K. Kern, Nat. Commun., 2013, 4, 2904 Search PubMed.
- (a) N. Abdurakhmanova, T. C. Tseng, A. Langner, C. S. Kley, V. Sessi, S. Stepanov and K. Kern, Phys. Rev. Lett., 2013, 110, 027202 CrossRef CAS; (b) P. Gambardella, S. Stepanow, A. Dimitriev, J. Honolka, F. M. F. de Groot, M. Lingenfelder, S. S. Gupta, D. D. Sarma, P. Bencok, S. Stanescu, S. Clair, S. Pons, N. Lin, A. P. Seitsonen, H. Brune, J. V. Barth and K. Kern, Nat. Mater., 2009, 8, 189–193 CrossRef CAS PubMed.
- L. Bartels, Nat. Chem., 2010, 2, 87–95 CrossRef CAS PubMed.
- L. Vitali, G. Levita, R. Ohmann, A. Comisso, A. De Vita and K. Kern, Nat. Mater., 2010, 9, 320–323 CrossRef CAS PubMed.
- (a) M. Stohr, M. Wahl, C. H. Galka, T. Riehm, T. A. Jung and L. H. Gade, Angew. Chem., Int. Ed., 2005, 44, 7394–7398 CrossRef CAS PubMed; (b) H. Kong, Q. Sun, L. Wang, Q. Tan, C. Zhang, H. Sheng and W. Xu, ACS Nano, 2014, 8(2), 1804–1808 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc03130c |
| This journal is © The Royal Society of Chemistry 2015 |