Open Access Article
Open Access ArticlePolyhomologation based on in situ generated boron-thexyl-silaboracyclic initiating sites: a novel strategy towards the synthesis of polyethylene-based complex architectures†
Zhen
Zhang
ab,
Hefeng
Zhang
ab,
Yves
Gnanou
a and
Nikos
Hadjichristidis
*ab
aKing Abdullah University of Science and Technology (KAUST), Physical Sciences and Engineering Division, KAUST Catalysis Center, Polymer Synthesis Laboratory, Thuwal 23955, Saudi Arabia. E-mail: nikolaos.hadjichristidis@kaust.edu.sa
bKing Abdullah University of Science and Technology (KAUST), Physical Sciences and Engineering Division, Thuwal 23955, Saudi Arabia
First published on 10th April 2015
Abstract
A novel strategy, based on the in situ generated boron-thexyl-silaboracyclic initiating sites for the polyhomologation of dimethylsulfoxonium methylide, has been developed for the synthesis of complex polyethylene-based architectures. As examples, the synthesis of a 4-arm polyethylene star, three (polystyrene)(polyethylene)2 3-miktoarm stars and a PE-branched double graft copolymer is given.
Polyethylene (PE), the largest volume commodity polymer in the world, is indispensable in many applications ranging from packaging to precision-processed materials.1 The low cost, excellent physical properties, easy processability and recyclability of PE have led to its commercial success.2 However, the poor compatibility of PE with other polymers limits its further applications. To overcome this deficiency, the development of PE-based (co)polymeric materials with novel architectures is needed.3
Recently, Shea developed a living polymerization procedure leading to linear well-defined hydroxyl-terminated polymethylene (polyethylene).4 The general mechanism involves the formation of a complex between an ylide (monomer) and an organoborane (initiator) followed by migration/insertion of –CH2– into the initiator. As a consequence, the methylene groups are randomly inserted one by one (C1 polymerization) into the three arms of the initiator leading to a 3-arm polymethylene star. By oxidizing/hydrolysing the resulted star, an OH-terminated polymethylene (polyethylene) is obtained.
There are four methods for the synthesis of PE-based polymers with different architectures via polyhomologation: (1) direct use of the terminal –OH of PE-OH for ring-opening polymerization (ROP),5 or post-polymerization modification of the –OH appropriate for atom transfer radical (ATRP),6 or reversible addition-fragmentation chain-transfer polymerization (RAFT)7 initiating sites; (2) reaction of PE-OH with functionalized monomers to give macromonomers followed by (co)polymerization to afford graft copolymers or molecular brushes;8 (3) stitching reactions to freeze the resultant polyhomologation star structure by transforming the sensitive to oxidation/hydrolysis boron-junction to stable carbon;9 and (4) design of novel borane initiators.10
For example, ROP of ε-caprolactone initiated directly from the PE-OH macroinitiator with stannous octanoate (Sn(Oct)2) as a catalyst affords polyethylene-b-poly(ε-caprolactone). By transforming the terminal –OH of the diblock copolymer to the ATRP initiating site polyethylene-b-poly(ε-caprolactone)-b-poly(acrylic acid) triblock copolymers have been synthesized.5a Well-defined PE brushes have been synthesized by ring opening metathesis polymerization (ROMP) of norbornyl PE-macromonomers prepared by reaction of PE-OH with 5-norbornene-2-carboxylic acid.8 Stitching reactions have been used to synthesize PE-based linear, macrocarbocyclic and 3-arm star structures.9 Utilizing novel borane initiators is another efficient way to obtain 3-arm star and multiblock copolymers. For example, polyhomologation of ylides with 1-boraadamantane led to a 3-arm PE10 and hydroboration of an allyl-terminated polystyrene oligomer with BH3 gave an organoborane macroinitiator which by polyhomologation led to poly(ethylene-b-styrene) (PE-b-PS) block copolymers.11 Our group synthesized 3-arm-polybutadiene, polystyrene and (polystyrene-b-polydiene)borane stars by reaction of the corresponding living macroanions with BF3·EtO2, which served as macroinitiators for the synthesis of OH-terminated di- and triblock co- and terpolymers.12
In this communication, we propose a novel general strategy based on the in situ formation of B-thexyl-silaboracyclic structure, having two silicon-connected initiating sites and one blocked, for the synthesis of PE-based complex macromolecular architectures.
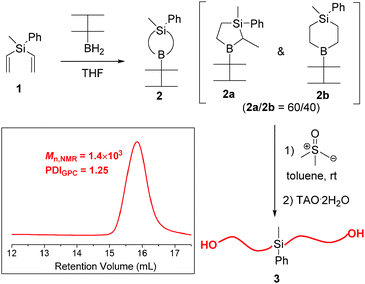
To check the principle of this strategy, B-thexyl-methylphenylsilaborocycle 2 (mixture of 2a and 2b isomers), a small equivalent molecule, was prepared by hydroboration of methylphenyldivinylsilane 1 with thexylborane13 and used in situ to promote the polyhomologation of dimethylsulfoxonium methylide (Scheme 1). Details of the synthesis and characterization are given in the ESI.† After hydrolysis of the polyhomologation product, only α, ω-dihydroxy polymethylene was produced as evidenced by the appearance of only one peak in the GPC chromatogram and the absence of the thexyl group in the NMR spectrum, meaning that the B-thexyl site is inactive to polyhomologation and all other B-initiating sites (in 2a and 2b isomers) are equivalent, in agreement with Shea's work using B-p-methoxyl-phenylethyl-9-BBN as an initiator.9 This is easy to explain since after the first insertion of a –CH2– on the boron-tertiary carbon (B-CH) all sites (B-CH2) become equivalent.
Subsequently, this strategy was employed for the macromolecular design and synthesis of a 4-arm polyethylene star, three (polystyrene)(polyethylene)2 3-miktoarm stars and a PE-branched double graft copolymer.
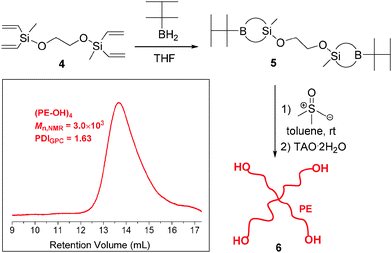
The general reaction scheme for the synthesis of a 4-arm star is given in Scheme 2. The hydroboration of 3,8-dimethyl-3,8-divinyl-4,7-dioxa-3,8-disiladeca-1,9-diene 4 with thexylborane gives bis-B-thexyl-silaboracycles 5. After treating 5 with excess ylide and subsequent oxidation–hydrolysis reaction using TAO·2H2O, 4-arm PE star 6 (Mn,NMR = 3.0 × 103, PDIGPC = 1.63) was obtained (Fig. S2, ESI†). Details are given in the ESI.†
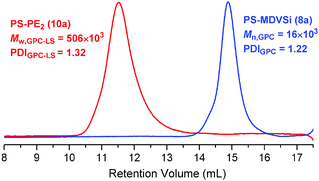
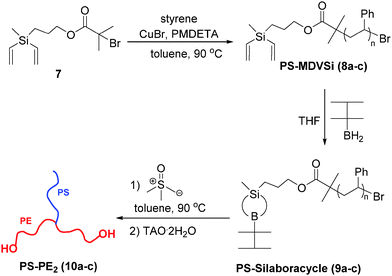
The synthesis of PE-based 3-miktoarm stars was achieved according to Scheme 3 by combining polyhomologation with ATRP through an heterofunctional initiator, 3-(methyldivinylsilyl)propyl-2-bromo-2-methylpropanoate 7 (the synthesis procedure is given in the ESI,† Scheme S4). The 2-bromo-2-methylpropanoate group was used to initiate the ATRP of styrene (CuBr/PMDETA (N,N,N′,N′,N′′,N′′-pentamethyldiethylene-triamine)) and methyldivinylsilyl after transformation to B-thexyl-silaboracycles to initiate polyhomologation. Three samples of divinyl-terminated polystyrene with different molecular weights and low polydispersities have been designed and synthesized (8a: Mn,GPC = 16.0 × 103, PDIGPC = 1.22; 8b: Mn,GPC = 11.0 × 103, PDIGPC = 1.18, Tg = 85 °C; 8c: Mn,GPC = 5.2 × 103, PDIGPC = 1.18) (Fig. 1 and Fig. S3 and S4, ESI†). The terminal methyldivinylsilyl group was readily transformed to B-thexyl-silaboracycles by hydroboration with thexylborane resulting in the corresponding macroinitiators for polyhomologation (9a–c). High hydroboration efficiencies were indicated by quantitative consumption of the terminal vinyl groups (δ = 5.6 to 6.2 ppm) revealed by 1H NMR results. As an example, the NMR spectra of 8a (before hydroboration) and 9a (after hydroboration) are shown in Fig. 2 (spectra a and b). The resultant boron-macroinitiators were used in situ for the polyhomologation of dimethylsulfoxonium methylide leading to the corresponding PE-based 3-miktoarm stars.
 | ||
| Scheme 3 Synthesis of PE-based 3-miktoarm star polymers by combination of ATRP and polyhomologation. | ||
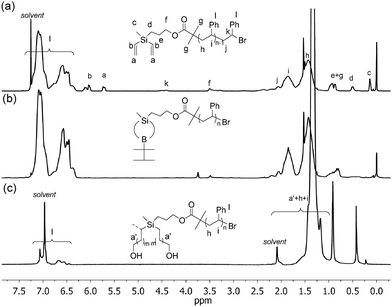 | ||
| Fig. 2 1H NMR spectra of (a) PS-MDVSi (8a) (chloroform-d, 25 °C); (b) PS-Silaboracycle (9a) (chloroform-d, 25 °C); (c) PS-PE2 (10a) (toluene-d8, 80 °C). | ||
The success of this strategy was confirmed by both GPC and NMR results (Fig. 1 and 2). After polyhomologation, the peak shifted to a higher molecular weight range keeping the narrow distribution profiles (10a: Mw,GPC-LS = 506 × 103, PDIGPC-LS = 1.32; 10b: Mw,GPC-LS = 367 × 103, PDIGPC-LS = 1.56, Tm = 116 °C; 10c: Mw,GPC-LS = 423 × 103, PDIGPC-LS = 1.35) (Fig. 1 and Fig. S3 and S4, ESI†). Also, the fingerprints of the PS and PE blocks were found in the 1H NMR spectrum (Fig. 2c).
A “grafting from” strategy shown in Scheme 4 was developed to synthesize a double graft copolymer. First, copolymerization of styrene with 2-((methyldivinylsilyl)oxy)ethyl methacrylate (DVSiOMA, 11) (for preparation see ESI,† Scheme S6) by ATRP initiated by ethyl 2-bromoisobutyrate (EBiB) with the CuBr/PMDETA catalytic system led to PS-co-PDVSiOMA 12. Then, the pendant divinylsilyl groups were transformed to B-thexyl-silaboracycles by hydroboration with thexylborane, followed by polyhomologation. As an example, PS38-co-PDVSiOMA3 (PDIGPC = 1.31) was synthesized (feed ratio 50/4.5).
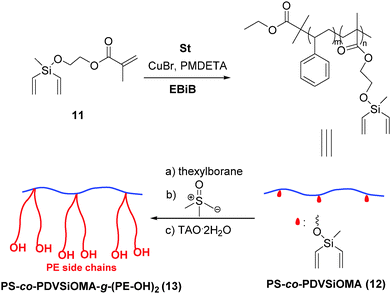 | ||
| Scheme 4 Synthesis of PE-branched double graft copolymer by combination of ATRP and polyhomologation. | ||
Hydroboration of PS38-co-PDVSiOMA3/polyhomologation by the resultant macroinitiator leads to PE-branched double graft copolymer PS-co-PDVSiOMA-g-(PE-OH)213 (Mw,GPC-LS = 218 × 103, PDIGPC-LS = 1.35) (see ESI,† Fig. S5). High hydroboration efficiency was indicated by the quantitative disappearance of the signal at 5.7–6.2 ppm in 1H NMR spectra for vinyl protons. The PS backbone is identifiable in NMR spectra (toluene-d8, 25 °C) and the PE fingerprint is much more evident by increasing the temperature up to 80 °C (see ESI,† Fig. S6).
In conclusion, a novel strategy using the in situ synthesized B-thexyl-silaboracyclic moieties with two silicon-connected initiating and one blocked sites for polyhomologation was successfully developed. This general strategy opens a new horizon for the synthesis of PE-based complex macromolecular architectures. Only a few examples are given in this communication, i.e. a 4-arm polyethylene star, three PS-PE2 3-miktoarm stars and a PE-branched double graft copolymer. Combination of this general strategy with other living and living/controlled polymerization techniques will lead to novel architectures such as multi-arm stars (8, 12, 16-arm), H-shaped, molecular brush copolymers, etc.
Notes and references
- (a) P. Galli and G. Vecellio, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 396–415 CrossRef CAS PubMed; (b) D. B. Malpass, Introduction to Industrial Polyethylene: Properties, Catalysts, and Processes, John Wiley and Sons Ltd, 2010 Search PubMed.
- P. S. Chum and K. W. Swogger, Prog. Polym. Sci., 2008, 33, 797–819 CrossRef CAS PubMed.
- (a) L. Leibler, Prog. Polym. Sci., 2005, 30, 898–914 CrossRef CAS PubMed; (b) C. W. Macosko, H. K. Jeon and T. R. Hoye, Prog. Polym. Sci., 2005, 30, 939–947 CrossRef CAS PubMed; (c) P. J. Phillips, Polym. Cryst. Rep. Prog. Phys., 1990, 53, 549–604 CrossRef CAS.
- (a) J. Luo and K. J. Shea, Acc. Chem. Res., 2010, 43, 1420–1433 CrossRef CAS PubMed; (b) K. J. Shea, J. W. Walker, H. Zhu, M. M. Paz and J. Greaves, J. Am. Chem. Soc., 1997, 119, 9049–9050 CrossRef CAS; (c) K. J. Shea, Chem. – Eur. J., 2000, 6, 1113–1119 CAS.
- (a) C. Yuan, H. Lu, Q. Li, S. Yang, Q. Zhao, J. Huang, L. Wei and Z. Ma, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2398–2405 CrossRef CAS PubMed; (b) Q.-Z. Li, G.-Y. Zhang, J.-Z. Chen, Q.-L. Zhao, H.-C. Lu, J. Huang, L.-H. Wei, F. D'Agosto, C. Boission and Z. Ma, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 511–517 CrossRef CAS PubMed.
- (a) H. Lu, Y. Xue, Q. Zhao, J. Huang, S. Xu, S. Cao and Z. Ma, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3641–3647 CrossRef CAS PubMed; (b) Y. Xue, H. Lu, Q. Zhao, J. Huang, S. Xu, S. Cao and Z. Ma, Polym. Chem., 2013, 4, 307–312 RSC; (c) J. Chen, K. Cui, S. Zhang, P. Xie, Q. Zhao, J. Huang, L. Shi, G. Li and Z. Ma, Macromol. Rapid Commun., 2009, 30, 532–538 CrossRef CAS PubMed; (d) J. Li, Q.-L. Zhao, J.-Z. Chen, L. Li, J. Huang, Z. Ma and Y.-W. Zhong, Polym. Chem., 2010, 1, 164–167 RSC.
- X. Wang, J. Gao, Q. Zhao, J. Huang, G. Mao, W. Wu, Y. Ning and Z. Ma, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2892–2899 CrossRef CAS PubMed.
- H. Zhang, Y. Gnanou and N. Hadjichristidis, Polym. Chem., 2014, 5, 6431–6434 RSC.
- (a) K. J. Shea, S. Y. Lee and B. B. Busch, J. Org. Chem., 1998, 63, 5746–5747 CrossRef CAS; (b) K. J. Shea, B. B. Busch and M. M. Paz, Angew. Chem., Int. Ed., 1998, 37, 1391–1393 CrossRef CAS.
- (a) C. E. Wagner and K. J. Shea, Org. Lett., 2001, 3, 3063–3066 CrossRef CAS PubMed; (b) C. E. Wagner, J.-S. Kim and K. J. Shea, J. Am. Chem. Soc., 2003, 125, 12179–12195 CrossRef CAS PubMed.
- (a) X.-Z. Zhou and K. J. Shea, Macromolecules, 2001, 34, 3111–3114 CrossRef CAS; (b) K. J. Shea, C. L. Staiger and S. Y. Lee, Macromolecules, 1999, 32, 3157–3158 CrossRef CAS.
- (a) H. Zhang, N. Alkayal, Y. Gnanou and N. Hadjichristidis, Chem. Commun., 2013, 49, 8952–8954 RSC; (b) H. Zhang, N. Alkayal, Y. Gnanou and N. Hadjichristidis, Macromol. Rapid Commun., 2014, 35, 378–390 CrossRef PubMed.
- (a) J. A. Soderquist and A. Hassner, J. Org. Chem., 1983, 48, 1801–1810 CrossRef CAS; (b) J. A. Soderquist, F.-Y. Shiau and R. A. Lemesh, J. Org. Chem., 1984, 49, 2565–2569 CrossRef CAS; (c) M. F. Hawthorne, J. Am. Chem. Soc., 1961, 83, 2541–2544 CrossRef CAS; (d) H. C. Brown and E. Negishi, J. Am. Chem. Soc., 1972, 94, 3567–3572 CrossRef CAS; (e) E. Negishi and H. C. Brown, Synthesis, 1974, 77–89 CrossRef CAS; (f) H. C. Brown and A. K. Mandal, J. Org. Chem., 1992, 57, 4970–4976 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details as well as supporting data including NMR and GPC profiles. See DOI: 10.1039/c5cc01579k |
| This journal is © The Royal Society of Chemistry 2015 |