Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A red-shifted photochromic sulfonylurea for the remote control of pancreatic beta cell function†
J.
Broichhagen
a,
J. A.
Frank
a,
N. R.
Johnston
b,
R. K.
Mitchell
b,
K.
Šmid
a,
P.
Marchetti
c,
M.
Bugliani
c,
G. A.
Rutter
b,
D.
Trauner
*a and
D. J.
Hodson
*b
aDepartment of Chemistry and Center for Integrated Protein Science, LMU Munich, Munich, Germany. E-mail: dirk.trauner@lmu.de
bSection of Cell Biology and Functional Genomics, Department of Medicine, Imperial College London, London W12 0NN, UK. E-mail: d.hodson@imperial.ac.uk
cDepartment of Clinical and Experimental Medicine, Islet Cell Laboratory, University of Pisa, Pisa, Italy
First published on 26th February 2015
Abstract
Azobenzene photoresponsive elements can be installed on sulfonylureas, yielding optical control over pancreatic beta cell function and insulin release. An obstacle to such photopharmacological approaches remains the use of ultraviolet-blue illumination. Herein, we synthesize and test a novel yellow light-activated sulfonylurea based on a heterocyclic azobenzene bearing a push–pull system.
Type 2 diabetes (T2D) is a modern pandemic currently affecting ∼6% of the global population. This disease is characterized by diminished insulin secretion from pancreatic beta cells, which together with peripheral resistance to the secreted hormone, leads to defective glucose homeostasis.1 The resulting elevated glucose concentration drives a variety of complications including heart disease, cancer, retinal degeneration, and nerve and vascular problems.2
While current medical treatments work well, they are associated with complications largely due to off-target or persistent actions.3 Moreover, they are unable to recreate pulsatile insulin release, a more effective signal for glucoregulation.4 Thus, T2D is ideally suited to photopharmacology, which harnesses the precision of light to spatiotemporally deliver drug activity.5 We have recently shown that a sulfonylurea possessing an azobenzene photoresponsive element (a.k.a. AzoSulfonylurea) can be used to optically control beta cell function and insulin release via its effects on ATP-sensitive potassium (KATP) channels and Exchange Protein directly Activated by cAMP 2A (Epac2A) signaling.6
However, a significant barrier to the use of such ‘azo-drugs’ for T2D treatment is their ultraviolet-blue absorption spectra, increasing phototoxicity and limiting tissue penetration due to photon scattering.7 By contrast, visible/near infrared wavelengths demonstrate better penetrance in the body.8
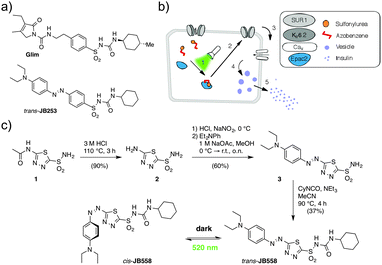
Spurred on by recent studies of ortho- or para-substituted azobenzenes,9–11 we therefore devised a novel approach for the synthesis of wavelength-tuned photopharmaceuticals with red-shifted photochromism. An AzoSulfonylurea based on glimepiride was achieved by installing a heterocyclic aromatic unit, rather than sterically bulky electron-donating halogen or amine moieties (Scheme 1).
Starting with the deacetylation of acetazolamide (1) in refluxing HCl, heterocycle 2 was obtained that could be further diazotized with in situ generated HNO2. Trapping the resulting diazonium salt with N,N-diethylaniline generated sulfonamide azobenzene 3. Finally, reaction with cyclohexyl isocyanate yielded JB558via acylation of the sulfonamide, giving unprecedented access to a sulfonylurea containing a heterocyclic azobenzene. While yields were reduced compared to the previously described JB253 (37% versus 97%),6 this was most likely due to the presence of a less reactive sulfonamide intermediate, as predicted by the lower pKa value for 3 (7.36, Fig. S1, ESI†) and JB558 (2.35, see ESI†).
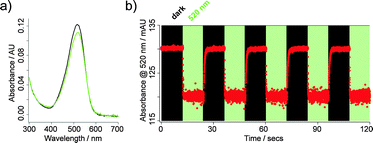
JB558 possessed a red-shifted absorption spectrum (λmax = 526 nm) in DMSO (Fig. 1a), and could be repeatedly photoconverted to its cis-state with green–yellow light (λ = 520 nm) (Fig. 1b). Thermal back relaxation occurred rapidly in the dark and switching kinetics were within the millisecond range (τcis = 64.9 ± 1.5 ms; τtrans = 410.8 ± 12.6 ms), without obvious decomposition (Fig. 1b). JB558 was stable in the presence of Escherichia coli azoreductase, an enzyme expected to limit oral bioavailability through diazene cleavage in the intestine (Fig. S2, ESI†).
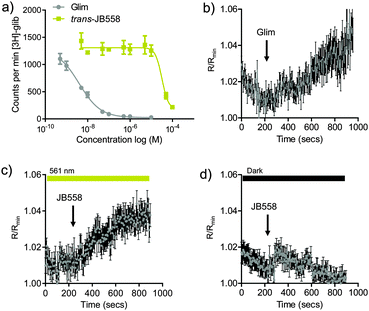
To determine the binding affinity of JB558 to the KATP channel subunit SUR1, as well as Epac2A, [3H]-glibenclamide displacement and FRET assays were performed. While trans-JB558 bound SUR1 with ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold less affinity than glimepiride (IC50(trans-JB558) = 37.3 μM; IC50(Glim) = 1.8 nM) (Fig. 2a), it was able to strongly and light-dependently activate an Epac2-camps biosensor containing the sulfonylurea binding domain (Fig. 2b–d).12
000-fold less affinity than glimepiride (IC50(trans-JB558) = 37.3 μM; IC50(Glim) = 1.8 nM) (Fig. 2a), it was able to strongly and light-dependently activate an Epac2-camps biosensor containing the sulfonylurea binding domain (Fig. 2b–d).12
Electrophysiological recordings of K+ currents in HEK293T-SUR1-Kir6.2 cells revealed partial KATP channel blockade by trans-JB558, presumably due to the momentary stationary state favoring some continued cis-isomerization (Fig. S3, ESI†).
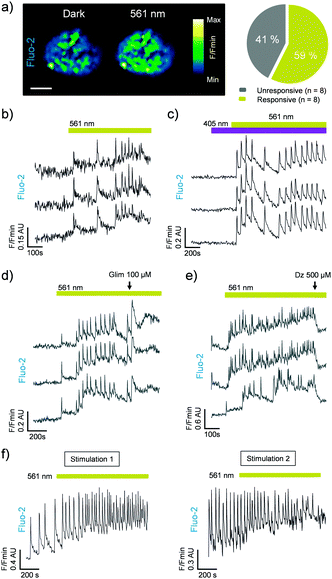
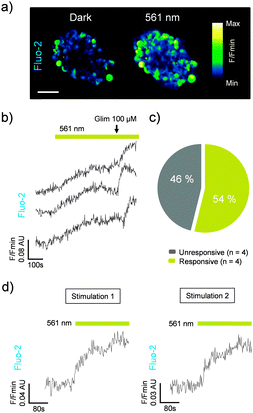
We next assessed the photoswitching properties of JB558 in native beta cells where sulfonylurea-mediated KATP channel–Epac2A signaling is intimately linked to voltage-dependent Ca2+ channel (VDCC) activity and insulin exocytosis.13–16 As expected, JB558 was able to evoke large increases in intracellular Ca2+ concentrations in ∼60% of beta cells following exposure to yellow (λ = 561 ± 5 nm) (Fig. 3a and b), but not violet (λ = 405 ± 5 nm) light (Fig. 3c). These effects were potentiated using a high concentration of glimepiride (Fig. 3d), and abrogated using diazoxide (Fig. 3e) to force open the KATP channel pore. Repeated switching of cytosolic Ca2+ concentrations could be achieved in the same islet following a brief period of dark exposure to induce trans-JB558 accumulation (Fig. 3f).
Similar to the results observed in rodent tissue, cis-JB588 was able to confer light-sensitivity on Ca2+-spiking activity in human pancreatic islets (Fig. 4a–c), and this effect could be reversed following 5 min relaxation in the dark (Fig. 4d).
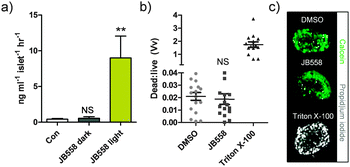
To link photocontrol of Ca2+ levels with insulin secretion, batches of rodent islets were incubated with JB558 and exposed to either dark (no illumination) or light (λ = 560 ± 10 nm). JB558-treated islets kept under dark conditions were no different to controls (5 mM glucose-alone) (Fig. 5a), suggesting that the observed stationary state KATP channel block was insufficient to elicit exocytosis. By contrast, irradiation dramatically stimulated insulin release (Fig. 5a). Finally, cytotoxicity assays demonstrated that JB558 did not adversely affect cell viability, as assessed using the vital stain calcein and the necrosis indicator propidium iodide (Fig. 5b and c).
The data presented here outline a synthetic route for the production of AzoSulfonylureas with red-shifted photochromism. Consistent with its sulfonylurea backbone, JB558 was able to bind SUR1 and activate Epac2A. Formation of cis-JB558 occurred with green–yellow light (λ = 520–561 nm), and thermal back relaxation in the dark yielded trans-JB558. While photoconversion between cis- and trans-forms was rapid in solution, it was slower in the tissue setting, taking minutes for reisomerization. An effect of Fluo-2 excitation on the isomer equilibrium cannot be excluded, although illumination at λ = 491 nm per se was unable to evoke Ca2+ rises in either Fluo-2- or Fura2 (λ = 340 nm/380 nm)-loaded islets (Fig. S4, ESI†).
A more plausible explanation is the inactivation of beta cell Epac2A-signaling, which may lag behind that of JB558 due to persistent mobilization of intracellular Ca2+.1,17 Such tissue effects may be desirable for the development of photopharmaceuticals, since pulsed illumination would reduce phototoxicity, while sustaining compound activity to match long-lasting (dozens of minutes) insulin peaks.4 Indeed, JB558 displayed almost 3-fold more potency than its blue-light activated predecessor JB253,6 most likely due to slower back-relaxation during the light pulses used in the secretion assays.
Neither were we able to detect photoswitching of K+ currents in HEK293T cells overexpressing KATP channels, free from orthogonal wavelengths (Fig. S5, ESI†). This was likely because HEK293T cells do not express sufficient Epac2A to allow JB558 to properly toggle KATP activity,13,15,18 and/or the inability to deliver sufficient illumination using the non-coherent source on our patch-clamp setup (ε520nm(JB558) = 1.14 × 105 mol−1 cm−1; see ESI†).
Nonetheless, we clearly show that JB558 light-dependently binds Epac2A, allowing optical control of cell function and insulin secretion with λ = 560 nm in the most physiologically-relevant testbed, viz. the islets of Langerhans. Thus, JB558 represents a blueprint for red-shifted AzoSulfonylureas based upon heterocyclic azobenzenes. Further studies are now warranted to improve isomerization kinetics in tissue to improve the use of JB558 as a research tool for rapid KATP channel manipulation. Importantly, similar synthetic approaches may also be applicable to other clinically-relevant azobenzene-possessing compounds where steric hindrance may affect molecule motion e.g. neuromodulators,19 neurotransmitters,20,21 enzymes22 and antibiotics.23
J.B. was supported by a European Foundation for the Study of Diabetes (EFSD) Albert Renold Young Scientist Fellowship and a Studienstiftung des deutschen Volkes PhD studentship. N.R.J. was supported by a Diabetes UK RW and JM Collins Studentship (12/0004601). J.A.F. was supported by a Collaborative Research Centre Grant (SFB1032). G.A.R. was supported by Wellcome Trust Senior Investigator (WT098424AIA), MRC Programme (MR/J0003042/1), Diabetes UK Project Grant (11/0004210) and Royal Society Wolfson Research Merit Awards. D.T. was supported by an Advanced Grant from the European Research Commission (268795). D.J.H. was supported by a Diabetes UK R.D. Lawrence Research Fellowship (12/0004431). The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 155005 (IMIDIA), resources of which are composed of a financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies' in kind contribution (G.A.R.).
Notes and references
- G. A. Rutter, Mol. Aspects Med., 2001, 22, 247–284 CrossRef CAS.
- J. M. Forbes and M. E. Cooper, Physiol. Rev., 2013, 93, 137–188 CrossRef CAS PubMed.
- S. Bolen, L. Feldman, J. Vassy, L. Wilson, H. C. Yeh, S. Marinopoulos, C. Wiley, E. Selvin, R. Wilson, E. B. Bass and F. L. Brancati, Ann. Intern. Med., 2007, 147, 386–399 CrossRef.
- S. Seino, T. Shibasaki and K. Minami, J. Clin. Invest., 2011, 121, 2118–2125 CrossRef CAS PubMed.
- W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed.
- J. Broichhagen, M. Schönberger, S. C. Cork, J. A. Frank, P. Marchetti, M. Bugliani, A. M. J. Shapiro, S. Trapp, G. A. Rutter, D. J. Hodson and D. Trauner, Nat. Commun., 2014, 5, 5116 CrossRef CAS PubMed.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS PubMed.
- A. Bakker, B. Smith, P. Ainslie and K. Smith, Applied Aspects of Ultrasonography in Humans, InTech, Near-Infrared Spectroscopy, 2012 Search PubMed.
- M. A. Kienzler, A. Reiner, E. Trautman, S. Yoo, D. Trauner and E. Y. Isacoff, J. Am. Chem. Soc., 2013, 135, 17683–17686 CrossRef CAS PubMed.
- A. Rullo, A. Reiner, A. Reiter, D. Trauner, E. Y. Isacoff and G. A. Woolley, Chem. Commun., 2014, 50, 14613–14615 RSC.
- A. A. Beharry, O. Sadovski and G. A. Woolley, J. Am. Chem. Soc., 2011, 133, 19684–19687 CrossRef CAS PubMed.
- K. J. Herbst, C. Coltharp, L. M. Amzel and J. Zhang, Chem. Biol., 2011, 18, 243–251 CrossRef CAS PubMed.
- C. L. Zhang, M. Katoh, T. Shibasaki, K. Minami, Y. Sunaga, H. Takahashi, N. Yokoi, M. Iwasaki, T. Miki and S. Seino, Science, 2009, 325, 607–610 CrossRef CAS PubMed.
- G. A. Rutter and D. J. Hodson, Cell. Mol. Life Sci., 2014, 72, 453–467 CrossRef PubMed.
- M. Almahariq, F. C. Mei and X. Cheng, Trends Endocrinol. Metab., 2014, 25, 60–71 CrossRef CAS PubMed.
- L. Aguilar-Bryan, J. P. t. Clement, G. Gonzalez, K. Kunjilwar, A. Babenko and J. Bryan, Physiol. Rev., 1998, 78, 227–245 CAS.
- C. A. Leech, O. G. Chepurny and G. G. Holz, Vitam. Horm., 2010, 84, 279–302 CAS.
- T. Tsalkova, F. C. Mei, S. Li, O. G. Chepurny, C. A. Leech, T. Liu, G. G. Holz, V. L. Woods, Jr. and X. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 18613–18618 CrossRef CAS PubMed.
- M. Stein, S. J. Middendorp, V. Carta, E. Pejo, D. E. Raines, S. A. Forman, E. Sigel and D. Trauner, Angew. Chem., Int. Ed. Engl., 2012, 51, 10500–10504 CrossRef CAS PubMed.
- M. Volgraf, P. Gorostiza, R. Numano, R. H. Kramer, E. Y. Isacoff and D. Trauner, Nat. Chem. Biol., 2006, 2, 47–52 CrossRef CAS PubMed.
- I. Tochitsky, M. R. Banghart, A. Mourot, J. Z. Yao, B. Gaub, R. H. Kramer and D. Trauner, Nat. Chem., 2012, 4, 105–111 CrossRef CAS PubMed.
- J. Broichhagen, I. Jurastow, K. Iwan, W. Kummer and D. Trauner, Angew. Chem., Int. Ed. Engl., 2014, 53, 7657–7660 CrossRef CAS PubMed.
- W. A. Velema, J. P. van der Berg, M. J. Hansen, W. Szymanski, A. J. Driessen and B. L. Feringa, Nat. Chem., 2013, 5, 924–928 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc01224d |
| This journal is © The Royal Society of Chemistry 2015 |