Open Access Article
Open Access ArticleHighly durable carbon-supported Pt catalysts prepared by hydrosilane-assisted nanoparticle deposition and surface functionalization†
Akinori
Saito
a,
Hiromi
Tsuji
b,
Iwao
Shimoyama
c,
Ken-ichi
Shimizu
d and
Yuta
Nishina
*be
aGraduate School of Natural Science and Technology, Okayama University, 3-1-1, Tsushimanaka, Kita-ku, Okayama 700-8530, Japan
bResearch Core for Interdisciplinary Sciences, Okayama University, 3-1-1, Tsushimanaka, Kita-ku, Okayama 700-8530, Japan. E-mail: nisina-y@cc.okayama-u.ac.jp
cJapan Atomic Energy Agency, Quantum Beam Science Directorate, Tokai-mura, Naka-gun, Ibaraki 319-1195, Japan
dCatalysis Research Centre, Hokkaido University, Kita21, Nishi10, Kita-ku, Sapporo, Hokkaido 001-0021, Japan
ePrecursory Research for Embryonic Science and Technology, Japan Science and Technology Agency, 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
First published on 23rd February 2015
Abstract
Hydrosilane enabled the formation of Pt nanoparticles and the silane functionalization of a carbon support material in one pot. The metal/Si-modified carbon composites are highly durable during catalytic methane oxidation.
Carbon-supported noble metal composites are used as catalysts in organic synthesis,1 fuel cells,2 super capacitors,3 solar cells,4 and sensors.5 Support materials composed of sp2 hybridized carbon atoms have advantages of high electron and thermal conductivities, which enhance the performance of the metal species.6 In contrast, carbon reacts readily with oxygen to eliminate gaseous carbon oxides upon heating especially in the presence of metal species, and thus catalytic combustion occurs rapidly. Therefore, the development of thermally durable carbon materials is of great importance.7 The preparation of metal-supported carbon materials covered with silica layers has been reported and they have been used as catalysts for cyclohexane dehydrogenation.8 However, multistep functionalization processes such as (1) the deposition of metal nanoparticles onto carbon, (2) surface treatment with a silane coupling agent, and (3) silica-layer formation by the hydrolysis of an alkoxysilane are required.
We focused on the development of a one-pot synthesis of a metal/Si-modified carbon composite with high thermal durability. To achieve this we used hydrosilane (R3SiH) as the dual-role reagent for the metal nanoparticle formation and for the surface modification of the carbon support. Hydrosilanes can reduce metal salts (Mn+2) to metal nanoparticles (Mn) through transmetallation or by ligand exchange. A successive reductive elimination9 is accompanied by the formation of R3SiX (X = the counter anion of Mn+2 or OH) that functions as a silane coupling agent10 towards OH groups on the carbon support. Among the various carbon-based support materials, we initially focused on graphene oxide (GO) because of its highly oxygenated and two-dimensional nanosheet structure. This allows for uniform nanoparticle distribution and silane functionalization. The thermal resistance of the composites was evaluated by a catalytic methane oxidation reaction. This reaction requires high energy because of the high C–H bond dissociation energy (104 kcal mol−1).11
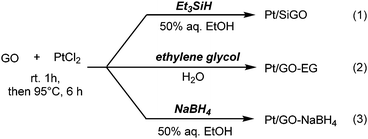
A Pt/GO composite (Pt/SiGO) was prepared from GO and PtCl2 using Et3SiH as a reductant.12 The mixture was stirred at room temperature for 1 h and then heated to 95 °C for 6 h (Scheme 1-1). For comparison, PtCl2 was reduced by ethylene glycol (Pt/GO–EG, Scheme 1-2) or NaBH4 (Pt/GO–NaBH4, Scheme 1-3).
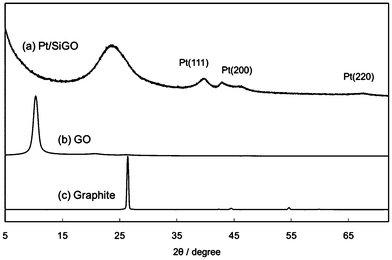
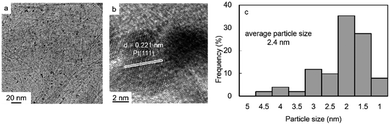
The amount of Pt on Pt/SiGO was found to be 4.2 wt% by energy dispersive X-ray (EDX) spectroscopy (Table 1, entry 1). 1.5 wt% Si was also detected by EDX. The Pt nanoparticles on the surface of GO were characterized by X-ray diffraction (XRD). The XRD patterns of graphite, GO, and Pt/SiGO are shown in Fig. 1. The peak at 2θ = 10.3° in the XRD pattern corresponds to GO (002), and the broad peak at around 2θ = 23° is attributed to the stacked graphene sheets produced by the reduction of GO.13 For Pt/SiGO the peaks are at 2θ = 39.7, 46.2, and 67.6° and these peaks correspond to the (111), (200), and (220) planes of a face-centered cubic (fcc) Pt crystal, respectively.14 The morphology of the Pt nanoparticles was observed using a transmission electron microscope (TEM) (Fig. 2). The average particle size of the Pt nanoparticles was 2.4 nm. The lattice space of the Pt nanoparticles was 0.22 nm, which corresponds to the (111) plane of a Pt crystal.14
The thermal durability of Pt/SiGO was evaluated by the catalytic methane oxidation reaction (Fig. 3), because this reaction requires high temperature. For comparison, commercially available Pt/C and Pt/GO composites prepared using ethylene glycol (Pt/GO–EG) or NaBH4 (Pt/GO–NaBH4)15 were also evaluated.16 Catalysts containing 10 mg of Pt, as determined by EDX (Table 1), were subjected to treatment with a gas containing 7% methane, 33% O2, and 60% He at 400 °C. The flow rate was 30 mL min−1 and the conversion of methane was measured by gas chromatography (Fig. S2, ESI†). For Pt/C (Fig. 3a) the catalytic activity decreased significantly after 40 min while Pt/SiGO maintained its activity (Fig. 3b). The Pt/GO prepared using ethylene glycol (Pt/GO–EG) (Fig. 3c) or NaBH4 (Pt/GO–NaBH4) (Fig. 3d) did not show high activity at any point.
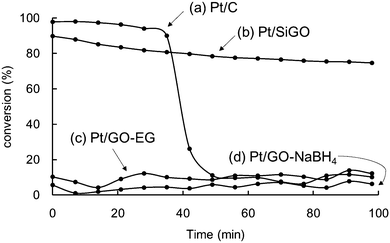 | ||
| Fig. 3 Time course of the catalytic activity of methane conversion under oxygen at 400 °C. (a) Pt/C, (b) Pt/SiGO, (c) Pt/GO–EG, and (d) Pt/GO–NaBH4. | ||
The recovered catalysts were analyzed by TEM (Fig. 4). The original Pt/C contained 3.3 nm Pt nanoparticles (Fig. 4a); however, after methane oxidation almost all the carbon support disappeared and nanoparticle grain growth occurred (Fig. 4b). In contrast, the recovered Pt/GO that was prepared using Et3SiH did not undergo such a drastic structural change. Its particle size increased slightly from 2.4 nm (Fig. 2) to 3.4 nm (Fig. 4c).
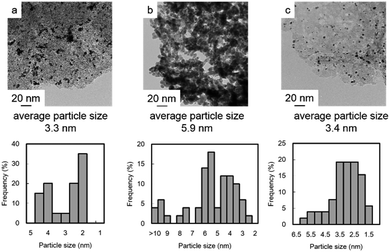 | ||
| Fig. 4 TEM images and particle size distributions of (a) fresh Pt/C, (b) recovered Pt/C, and (c) recovered Pt/SiGO. | ||
XPS was measured before and after the methane oxidation reaction. The intensities of each spectra were normalized to that of the Pt 4f7/2 and 4f5/2 regions (65–76 eV), because we hypothesized that the amount of Pt does not change before and after the reaction. For Pt/SiGO, the intensity of the Si 2p and 2s regions (99 and 151 eV) increased after the reaction at 400 °C (Fig. 5A). This suggests that silyl functional groups were covalently attached to GO because the boiling points of Et3SiH and Et3SiOH are only 107 and 158 °C, respectively. The increase in Si indicates that other elements such as C and O were removed and, therefore, XPS spectra of the C 1s regions were analyzed. As expected, the intensities of the C 1s regions of Pt/SiGO after the methane oxidation reaction decreased; however, the decrease is much smaller than that of Pt/C (Fig. 5B).
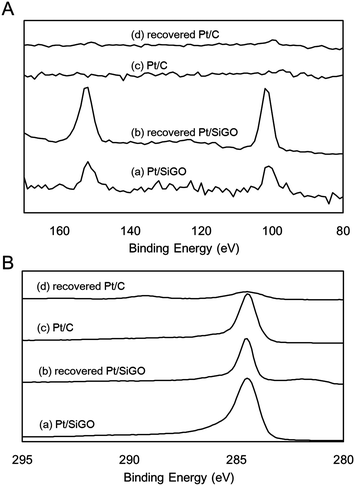 | ||
| Fig. 5 XPS of (A) Si 2p and 2s, and (B) the C 1s regions of Pt/SiGO and Pt/C before and after the methane oxidation reaction. | ||
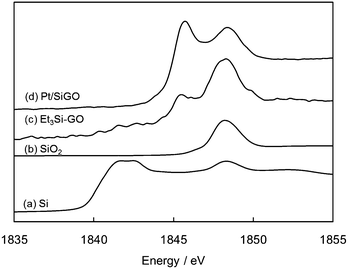
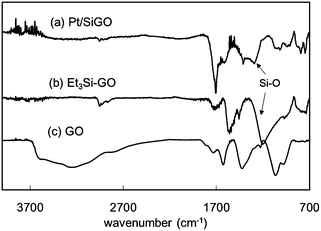
Si atoms were well dispersed over GO as determined by scanning transmission electron microscopy (STEM)-EDX mapping (Fig. S3, ESI†). The hydroxyl groups on GO are known to react with silane coupling reagents (silyl chlorides) to form silylated GO.17 Here, we used Et3SiH to reduce Pt(II) to Pt nanoparticles and, as a result, Et3SiOH is produced.18 Et3SiOH can also function as a silane coupling reagent10 and, therefore, the OH groups on GO are functionalized with Et3Si groups via dehydrative condensation. To determine the chemical state of Si on GO, an X-ray absorption near edge structure (XANES) spectrum of Pt/SiGO was obtained. Si(0) and SiO2 have peaks at 1842 and 1848 eV, respectively (Fig. 6a and b).19 The XANES spectrum of the Et3Si-modified GO (Et3Si–GO), produced using Et3SiCl, had peaks at 1845 eV and 1848 eV (Fig. 6c). For Pt/SiGO a similar spectrum to that of Et3Si–GO was obtained (Fig. 6d). This suggests that the Et3Si groups are attached to Pt/SiGO by silyl ether bonds.20 Fourier transform infrared (FT-IR) spectroscopy of Pt/SiGO also supports the presence of the Et3Si groups; peaks of alkyl chains were observed at 2740–2980 cm−1 (Fig. 7). Si–O bond has a strong IR absorption at 1100–1200 cm−1, which is observed for both Pt/SiGO (Fig. 7a) and Et3Si–GO (Fig. 7b).
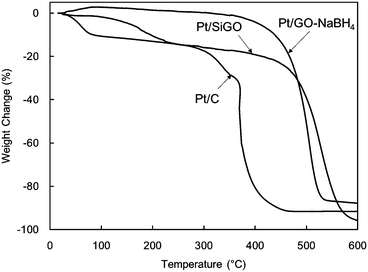
To clarify the durability of Pt/SiGO under heating, thermogravimetric analysis (TGA) was conducted (DTA is shown in Fig. S4, ESI†). A drastic weight loss was observed at 350 °C on Pt/C under air. Pt/SiGO and Pt/GO–NaBH4 showed higher thermal durability; however, the obvious effect of silyl functionalization was not observed (Fig. 8). These results suggest that the silyl group does not affect carbon supports, but stabilizes Pt particles. The weight loss of Pt/SiGO at 50–100 °C would derive from the desorption of water, and the loss at 100–350 °C would derive from the removal of residual oxygen functional groups and alkyl chains, which were also supported by IR analysis (Fig. S5, ESI†).
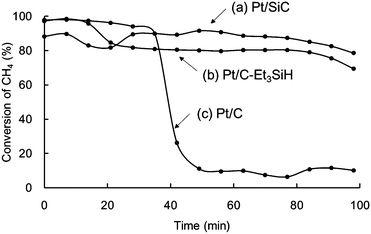
The hydrosilane-assisted deposition of Pt also enhanced the thermal durability of activated carbon (Pt/SiC) (Fig. 9a). Interestingly, treatment of the commercially available Pt/C with Et3SiH (Pt/C–Et3SiH) also showed improved durability (Fig. 9b) compared with the as-obtained compound (Fig. 9c).
We developed a one-pot synthesis of Pt/Si-modified carbon composites. Si atoms were uniformly present on the carbon surface upon dehydrative silyl ether formation between Et3SiOH and the OH group of the carbon. The thermal durability of the composite during methane oxidation improved upon silyl group modification. This work was partly supported by the Nanotechnology Platform Program, JST PRESTO, KAKENHI of the Ministry of Education, Culture, Sports, Science and Technology, and the Cooperative Research Program of Catalysis Research Centre, Hokkaido University. We acknowledge Dr Hideki Hashimoto for STEM-EDS measurements.
Notes and references
- (a) H. Sakurai, T. Tsukuda and T. Hirao, J. Org. Chem., 2002, 67, 2721 CrossRef CAS PubMed; (b) T. Maegawa, Y. Kitamura, S. Sako, T. Udzu, A. Sakurai, A. Tanaka, Y. Kobayashi, K. Endo, U. Bora, T. Kurita, A. Kozaki, Y. Monguchi and H. Sajik, Chem. – Eur. J., 2007, 13, 5937 CrossRef CAS PubMed; (c) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133 CrossRef CAS PubMed; (d) F. X. Felpin, T. Ayad and S. Mitra, Eur. J. Org. Chem., 2006, 2679 CrossRef CAS.
- (a) P. J. Ferreira, G. J. La Ó, Y. Shao-Horn, D. Morgan, R. Makharia, S. Kocha and H. A. Gasteiger, J. Electrochem. Soc., 2005, 152, A2256 CrossRef PubMed; (b) Y. Shao, G. Yin, Y. Gao and P. Shi, J. Electrochem. Soc., 2006, 153, A1093 CrossRef CAS PubMed; (c) Z. Zhou, S. Wang, W. Zhou, G. Wang, L. Jiang, W. Li, S. Song, J. Liu, G. Sun and Q. Xin, Chem. Commun., 2003, 394 RSC; (d) J. R. Varcoe, R. C. T. Slade, G. L. Wright and Y. Chen, J. Phys. Chem. B, 2006, 110, 21041 CrossRef CAS PubMed.
- G. Y. Yu, W. X. Chen, Y. F. Zheng, J. Zhao, X. Li and Z. D. Xu, Mater. Lett., 2006, 60, 2453 CrossRef CAS PubMed.
- Y. H. Ng, I. Lightcap, K. Goodwin, M. Matsumura and P. V. Kamat, J. Phys. Chem. Lett., 2010, 1, 2222 CrossRef CAS.
- I. V. Lightcap, S. Murphy, T. Schumer and P. V. Kamat, J. Phys. Chem. Lett., 2012, 3, 1453 CrossRef CAS.
- (a) A. Kongkanand, K. Vinodgopal, S. Kuwabata and P. V. Kamat, J. Phys. Chem. B, 2006, 110, 16185 CrossRef CAS PubMed; (b) R. Muszynski, B. Seger and P. V. Kamat, J. Phys. Chem. C, 2008, 112, 5263 CrossRef CAS; (c) K.-S. Ha, G. Kwak, K.-W. Jun, J. Hwang and J. Lee, Chem. Commun., 2013, 49, 5141 RSC.
- L. D. Rogatis, M. Cargnello, V. Gombac, B. Lorenzut, T. Montini and P. Fornasiero, ChemSusChem, 2010, 3, 24 CrossRef PubMed.
- (a) K. Nakagawa, T. Okayama, Y. Tanimoto, K. Sotowa, S. Sugiyama, T. Moriga, S. Takenaka and M. Kishida, Appl. Catal., A, 2012, 419, 13 CrossRef PubMed; (b) K. Nakagawa, T. Hoshinoo, K. Sotowa, S. Sugiyama, L. Rkiouak, V. Dubois and S. Hermans, Chem. Lett., 2012, 41, 1308 CrossRef CAS; (c) K. Nakagawa, Y. Tanimoto, T. Okayama, K. Sotowa, S. Sugiyama, S. Takenaka and M. Kishida, Catal. Lett., 2010, 136, 71 CrossRef CAS PubMed; (d) S. Takenaka, T. Iguchi, E. Tanabe, H. Matsune and M. Kishida, Carbon, 2009, 47, 1251 CrossRef CAS PubMed; (e) S. Takenaka, H. Matsumori, K. Nakagawa, H. Matsune, E. Tanabe and M. Kishida, J. Phys. Chem. Lett., 2007, 111, 15133 CrossRef CAS; (f) S. Takenaka, T. Arike, H. Matsune, E. Tanabe and M. Kishida, J. Catal., 2008, 257, 345 CrossRef CAS PubMed.
- (a) L. N. Lewis and N. Lewis, J. Am. Chem. Soc., 1986, 108, 7228 CrossRef CAS; (b) B. Fu, M. N. Missaghi, C. M. Downing, M. C. Kung, H. H. Kung and G. Xiao, Chem. Mater., 2010, 22, 2181 CrossRef CAS.
- Y. Xie, C. A. S. Hill, Z. Xiao, H. Militz and C. Mai, Composites, Part A, 2010, 41, 806 CrossRef PubMed.
- S. J. Blanksby and G. B. Ellison, Acc. Chem. Res., 2003, 36, 255 CrossRef CAS PubMed.
- Pt nanoparticle formation with other hydrosilanes was also investigated. See ESI†.
- P. Cui, J. Lee, E. Hwang and H. Lee, Chem. Commun., 2011, 47, 12370 RSC.
- Powder Diffraction File No. 04-0802.
- (a) B. Seger and P. V. Kamat, J. Phys. Chem. Lett., 2009, 113, 7990 CrossRef CAS; (b) Y. Li, L. Tang and J. Li, Electrochem. Commun., 2009, 11, 846 CrossRef CAS PubMed; (c) J. D. Qiu, G. C. Wang, R. P. Liang, X. H. Xia and H. W. Yu, J. Phys. Chem. C, 2011, 115, 15639 CrossRef CAS; (d) Y. Li, W. Gao, L. Ci, C. Wang and P. M. Ajayan, Carbon, 2010, 48, 1124 CrossRef CAS PubMed.
- The surface area of graphene oxide derivatives determined by the BET method is generally small (<100 m2 g−1). We measured the surface area by methylene blue adsorption. Pt/SiGO: 534 m2 g−1, Pt/GO-NaBH4: 744 m2 g−1, Pt/GO-EG: 921 m2 g−1, Pt/C: 1162 m2 g−1, and GO: 1292 m2 g−1. See ESI† for more details.
- (a) Y. Matsuo, T. Tabata, T. Fukunaga, T. Fukutsuka and Y. Sugie, Carbon, 2005, 43, 2875 CrossRef CAS PubMed; (b) Y. Matsuo, T. Fukunaga, T. Fukutsuka and Y. Sugie, Carbon, 2004, 42, 2113 CrossRef PubMed.
- (a) K. Shimizu, T. Kubo and A. Satsuma, Chem. – Eur. J., 2012, 18, 2226 CrossRef CAS PubMed; (b) B. P. S. Chauhan, A. Sarkar, M. Chauhan and A. Roka, Appl. Organomet. Chem., 2009, 23, 385 CrossRef CAS; (c) M. Jeon, J. Han and J. Park, ACS Catal., 2012, 2, 1539 CrossRef CAS.
- Y. Baba, T. Sekiguchi, I. Shimoyama and N. Hirao, Surf. Sci., 2013, 612, 77 CrossRef CAS PubMed.
- Si K-edge XANES was also studied by the DV-Xα molecular-orbital calculation method. The presence of two peaks in XANES spectra suggested the existence of Si–O and Si–C bonds. See ESI†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc10298c |
| This journal is © The Royal Society of Chemistry 2015 |