γ-Silylboronates in the chiral Brønsted acid-catalysed allylboration of aldehydes†
Abstract
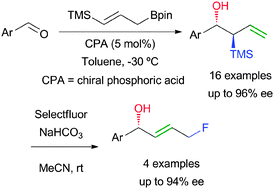
The use of functionalised allylboronic esters in the catalytic enantioselective allylboration of aldehydes is described for the first time. γ-Silylallyl pinacolate derivatives give rise to α-silyl homoallylic alcohols in high yields, with complete diastereoselectivities and high enantioselectivities, in most of the cases. The usefulness of such intermediates is showcased by their transformation into fluorinated allylic alcohols.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...