Coomassie Brilliant Blue-binding: a simple and effective method for the determination of water-insoluble protein surface hydrophobicity
Abstract
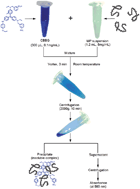
A simple and convenient method for water-insoluble protein surface hydrophobicity determination was developed and validated. The method is based on the non-covalent binding of Coomassie Brilliant Blue G-250 (CBBG) to aromatic and basic amino acid residues on the surface of proteins, generating insoluble protein–CBBG complexes that could be precipitated by centrifugation and cause a reduction in the absorbance at 585 nm in the supernatant. The amount of protein-bound CBBG is applied as an indicator of protein surface hydrophobicity. Thermally treated, water-insoluble myofibrillar proteins (MPs) were chosen to validate the proposed method. The amount of protein-bound CBBG increased in thermally treated protein samples and the results were compared with the surface hydrophobicity (S0) calculated by the commonly accepted fluorescence method using a 1-anilino-8-naphthalenesulfonate (ANS) probe and intrinsic tryptophan fluorescence. Results from the CBBG-binding method linearly correlated with the S0 of the ANS fluorescence method (R = 0.95), the maximum emission wavelength of protein intrinsic fluorescence (R = 0.96) and the intrinsic fluorescence intensity at 337 nm (R = −0.73), strongly suggesting the reliability of the proposed CBBG-binding method.

 Please wait while we load your content...
Please wait while we load your content...