Open Access Article
Open Access ArticleMicroelemental characterisation of Aboriginal Australian natural Fe oxide pigments
R. S.
Popelka-Filcoff
*a,
C. E.
Lenehan
a,
E.
Lombi
b,
E.
Donner
b,
D. L.
Howard
 c,
M. D.
de Jonge
c,
D.
Paterson
c,
K.
Walshe
d and
A.
Pring
ad
c,
M. D.
de Jonge
c,
D.
Paterson
c,
K.
Walshe
d and
A.
Pring
ad
aSchool of Chemical and Physical Sciences, Flinders University, Adelaide, SA, Australia. E-mail: rachel.popelkafilcoff@flinders.edu.au
bCentre for Environmental Risk Assessment and Remediation, University of South Australia, Adelaide, SA, Australia
cAustralian Synchrotron, Clayton, VIC, Australia
dSouth Australian Museum, Adelaide, SA, Australia
First published on 30th July 2015
Abstract
This manuscript presents the first comprehensive microcharacterisation of Fe oxide minerals used in Aboriginal Australian mineral pigments. The combination of X-ray fluorescence microscopy (XFM) and light microscopy provides a broad characterisation as well as the ability to spatially match visual observation with elemental composition. A novel method for casting pigment samples in a pattern on a slide was used for consistent elemental mapping. Semiquantitative bulk data was also collected and compared to the microscopic and microelemental data. These analyses demonstrate the ability to document the variability in ochre pigments in Australia, as well as which elements drive the variation within and between ochre source locations. The methods developed provide a more comprehensive understanding of other complex natural mineral pigments worldwide.
Introduction
Ochre and related natural Fe oxide pigments are a significant material in Indigenous cultures worldwide. Ochre pigments are utilised for their durability and widespread availability as observed in rock art from thousands of years ago, as well as their cultural symbolism in colour in modern times. In Aboriginal Australia, Fe-based mineral pigments are found in nearly every cultural context from a diverse array of cultural objects (shields, spears, boomerangs, bark paintings and others), in archaeological remains and rock art. Ochre continues to have strong cultural traditions in the present day. Examples of this include the use of natural mineral pigments in contemporary art centres such as those in the Kimberley region of Western Australia, and the use of ochre pigments on the body.1,2To visual inspection (approximately 10 cm distance) natural ochre pigments can appear to be homogeneous with a solid coverage of the substrate at a uniform thickness and particle size and shape. However under magnification, it can be observed that the pigments are composed of a range of particle shapes and sizes, and in many cases a range of colours. This distribution of natural pigmented particles, often caused by accessory minerals has not been well characterised.
In addition, the pigments adhere to the substrate with a degree of variation depending on the method of application, type of substrate, type of binder and any variability in the substrate itself. Variation in these factors include whether the pigment is applied by hand or by brush or other implement. Typical substrates for Aboriginal Australian objects include bark, wood and plant fibres, or in the case of rock art, natural rock substrates. Various types of binders were also used for mixing pigments, which include water and various plant and animal materials.3,4
Ochre is a complex mixture of Fe oxides and other minerals that can include calcite, gypsum, clays and feldspars. Iron oxides occur in varied geological formations, including red beds, banded iron formations, gossans and sediments. Ochre formations are also prevalent in soils that can contain several Fe-oxide minerals simultaneously.5 Each ochre carries a chemical and mineralogical “signature” that reflect the digenetic processes of the source. Sampling across a deposit provides information on the local variability of the geochemical signature. It has been demonstrated that a single sample from a site is not necessarily characteristic of a single geochemical composition of the site, due to geological variability within the mine or quarry.6,7 Characterisation of multiple samples from within a site is essential to give a more complete assessment of the diversity within the site. For these samples, neutron activation analysis (NAA) provides elemental data on variability between bulk samples on the order of 50–100 mg as single concentration values for 30+ elements per sample. Previous studies have demonstrated that NAA data used with multivariate statistical techniques can characterise individual sources geochemistry on the bulk scale as well as differentiate ochre sites from each other. While these data are valuable for understanding the source geochemical variability on a large scale, the variability at the micron scale of the ochre cannot be determined by NAA. The Fe oxides in ochre are known to be admixed with a range of accessory minerals such as clays, calcites and others, however the contribution of each mineral to the characteristic geochemical signatures is unknown.8 Therefore an elemental examination of the ochre on the micron level is essential to understand not only the elemental composition of the ochre material and minerals, but also the inherent variability of micron-sized particles of minerals that compose the complex ochre mixture. An analysis of this scale provides not only information on the elemental distribution of the sample, but also spatial and morphological information on the elemental distributions. While recent advances in scanning electron microscopy (SEM) coupled with elemental analysis (EDAX), have allowed elemental raster mapping of samples, the resolution is generally on the order of 100 microns. In addition, the maps produced are low resolution and not representative of the fine structure of the surface. Samples introduced to the SEM are limited to those that are small enough (<2 mm diameter stub) to fit into the chamber and that can tolerate high vacuum conditions, and potentially coated with carbon or gold for imaging purposes. None of the above conditions are acceptable for artefacts or ochre from cultural heritage materials.
X-ray fluorescence microscopy (XFM) as a form of micro-XRF analysis performed at the Australian Synchrotron (AS) provides the necessary analysis on the elemental level to understand the original source variability of complex natural mineral ochre pigments. Using the Maia detector provides high resolution mapping of individual particles inherent in the original sourced or archaeological ochre sample. In addition, unlike NAA, XFM requires only micrograms of material and is non destructive. XFM can analyse larger areas (on the order of cm) on many sample types with much higher sensitivity than SEM-EDAX. These capabilities are ideal for cases where trace elements can be diagnostic in terms of fingerprinting and where these trace elemental distributions may be smaller than SEM resolution of 100 micron or where the presence of the element was unknown. XFM can further be used to analyse cultural heritage artefacts as well as individual pigment samples, which is not possible using SEM-EDAX or NAA. A comparison of the individual pigment samples to the artefact provides a valuable advantage of a direct data comparison and technique from sample to artifact. A few prior studies have demonstrated successful application of XFM to cultural pigments.9–13
Recently there have been several studies that have explored a range of techniques with the aim of developing methods for establishing the provenance of ochre and pigments with archaeological and ethnographic associations including possible trade routes.1,6,7,14–16 However, many of these studies are based on the analysis of the bulk of the material, with such techniques as neutron activation analysis (NAA) and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS).6,7,17 However there is one recent study on the characterisation of Australian ochre using synchrotron XRD and PIXE.18 There are a few studies utilising microscopy of Fe oxides, however these concern the mineral properties and not the cultural aspects.5
This manuscript will focus on the multiple samples taken from five well known and documented ochre sources from Australia: Bookartoo (also spelled Bukartu and Pukatoo) (South Australia), Karrku (Northern Territory), Moana (South Australia), Pine Point (South Australia) and Wilgie Mia (Western Australia). Samples from these sites have been collected over several years by researchers including M. Nobbs, P. Jones, M. Smith and others associated with the South Australian Museum and the Western Australian Museum.6,19–26
Here we describe the first systematic microanalysis and documentation of ochre samples towards a more complete understanding and micro-characterisation of Aboriginal Australian ochre pigments. These data provide a foundation data set for feasibility studies for the in-beam analysis of Aboriginal Australian objects from several of the most significant ochre quarries in Australia and enhances knowledge of this inherently complex material. This study also expands the capability of the method towards understanding complex geological systems and formations, which may also inform environmental influence on Fe-oxide deposits.
Experimental
Sample selection
A suite of ochre samples has been assembled from individual researchers as well as from the South Australian Museum Archaeology and Ethnographic collections.6,19–26 The majority of these samples have also been analysed by complementary techniques such as NAA.6,24 From this larger combined collection, 165 samples were chosen for analysis by X-ray fluorescence microscopy (XFM). These 165 samples represented source (site) samples as well as samples from archaeological context. Sources in this project are defined as the original “quarry” or geological formation of the ochre material; therefore these samples were collected directly from the source in the field or presumed to be. On the other hand, archaeological samples may or may not be from the original quarry as they may have been exchanged through cultural networks.Located in the Flinders Ranges, South Australia, Bookartoo samples have been collected from this extensive site over different time periods and therefore likely different locations within the site.16,23,27 OCH050 and OCH24 were collected by Margaret Nobbs, whereas the OCH075-76-79A and OCH255 series are from Mike Smith. Karrku, located in the Northern Territory, is the local name for “ochre”.28 The samples were collected by Mike Smith and Barry Fankhauser as part of ochre characterisation studies completed in the 1990s. These samples were collected over a period of two years from the site. Moana is an exposed coastal site near Adelaide (South Australia) and therefore is subject to more intense weathering and effects of the ocean. Geologically, it is a sedimentary site as opposed to Karrku and Bookartoo, which are weathered banded iron formations. Similarly to Moana, Pine Point is an exposed and therefore weathered site on the coast of Yorke Peninsula (South Australia). Wilgie Mia (Western Australia), also a weathered banded iron formation is perhaps the best-known site due to the quality of the ochre and the large scale of the site.29
Sample preparation
In order to minimise the variation in the original state of the material, a consistent method was implemented to standardise the preparation of the ochre minerals for microscopy and XFM analysis.All samples were hand ground in a Brazilian agate mortar and pestle to a consistent particle size powder. Samples were dried overnight in a 100 °C oven and stored in glass vials. All samples were sieved to <250 micron using individually cut disposable plastic mesh screens to minimise cross contamination between samples. This step was performed to remove potentially large particles as well as organic material such as grasses and plant fibres. Additionally, the particle size of 250 micron was chosen as a likely maximum size for the use of Indigenous pigments on objects. However, depending on the origins of the pigment and the particular characteristics of the mineral, a variation in particle size and distribution is expected.
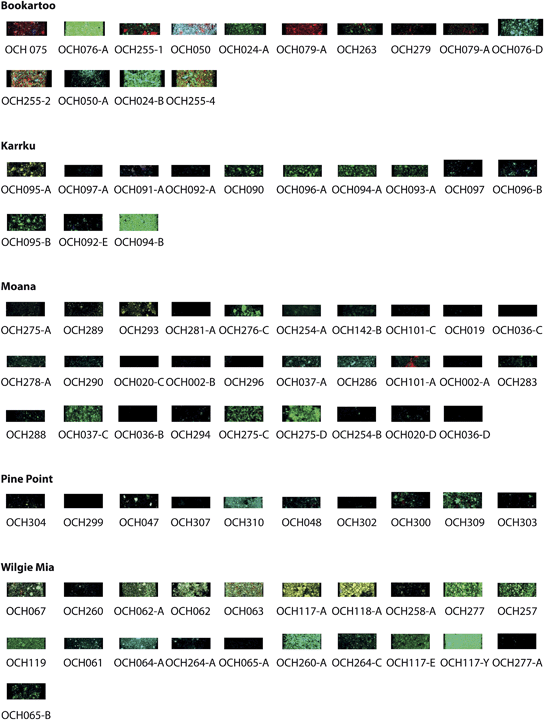
A standard microscopy quartz slide (2 × 1 inches, 5.08 × 2.54 cm) was prepared by sanding it with fine grit sandpaper to aid in the attachment of the epoxy cast mineral to the slide. A grid was laid out on the slide with space for 45 samples per slide, in 15 columns and 3 rows (Fig. 1). The samples were cast individually in epoxy on a quartz slide in even distribution for ease of analysis by XFM. Duplicates of each slide were also made.
 | ||
| Fig. 1 A demonstration slide displaying the sticker mask (light blue, right), with ochre squares cast in resin remaining on the slide after sticker removal (left). | ||
A grid mask was designed to affix to the slide to aid the casting of the samples, to maintain the pattern of 2 × 2 mm squares for each individual sample, separated by 0.5 mm. The grid mask was designed by CAD design and produced in a polymer mask “sticker”. The individual blocks were cut by a blade and removed individually, and each individual sticker was applied to the front face of each slide.
In order to affix the samples permanently to the slides, approximately 5–10 milligrams of each sample were mixed individually with Petropoxy 154 epoxy (Burnham Petrographics) in a glass vial with a glass stirrer in a 50/50 volume. This epoxy was chosen for its known capabilities in mineral slide preparation, fast set time as well as its relatively contaminant-free substrate that would not introduce further elemental impurities. After allowing the epoxy to cure for approximately 60 minutes in an 80 °C oven, the samples were applied manually, with the aid of a binocular microscope to each individual well, filling each well with a layer of sample. After the first applications had solidified a second layer for individual samples was applied to each well. After a complete curing of the epoxy to hardness in an 80 °C oven for 30 min, the entire slide was allowed to cool and the epoxy to set for several hours until completely cured. The “sticker” mask was then removed with a blade. The entire slide was then cured on a 130 °C hotplate for a few minutes. The slides were then polished to approximately 80–100 micron thickness to achieve a consistent thickness of epoxy samples. Fig. 2 is and example of one completed slide set with 45 ochre samples. It is expected that the samples will be heterogeneous across the individual sampling squares, but this sampling method will be more representative of a cultural application of a natural ochre pigment to an object. Although there is slight variation in the shape and coverage of each sample, the area in the centre of the square is representative of each sample.
 | ||
| Fig. 2 An example of completed slide with the 45 individual ochre samples cast in Petropoxy resin. Each square is approximately 2 mm square, with 0.5 mm between the squares. | ||
XFM beam line parameters
The experiment utilised the standard set up of the XFM experimental parameters with the Maia detector (a 384-element array of 1 × 1 mm Si detectors, oriented axially in backscatter geometry, which is 180° to beam).11,30 X-ray focusing was achieved with Kirkpatrick-Baez mirrors, which provide longer working distance and high sensitivity requirements as compared to other X-ray analysis setups.30 The Australian Synchrotron operated at 3 GeV with a stored current of 150–200 mA. During these experiments, the XFM beam had an incident energy of 18.5 keV and a flux of approximately 8 × 108 photons per second.For the experimental set-up, the individual polished slides were attached to the plexiglass sample holder frame used for XFM measurements. This setup is regularly used for the experiments and also allows for a set distance from the slides to the beam and detector.
Since the area of each slide is relatively small, a smaller step size was chosen to maximise the characterisation of the sub-micron areas of pigment. During the experiments the beam size was 2 micron. The samples were analysed at a rate of 0.768 mm per second with a step size of 2 microns. A sampling area of approximately 0.7 mm high strip through the centre of each row of the samples was chosen for a consistent measurement area of each row and to minimise variation. An area of 36.9 × 0.7 mm was analysed for each row of the slides, producing a total of 11 rows of 15 samples (165 samples total). The slides were analyzed in three separate experiments, but with similar conditions. Data analysis was accomplished using the GeoPIXE software package.11,30
The elements measured in this study included As, Ca, Cr, Cu, Fe, Ga, Mn, Pb (L line), Rb, Sc, Se, Sr, Ti, V, and Zn. For each pixel, the full XRF spectrum is collected. GeoPIXE is used to deconvolute these individual spectra into elemental concentration maps. Relative concentrations of each element or combinations of elements are indicated by colour and intensity of the colour. For each point in the image as well as for areas in the image, GeoPIXE can determine semiquantitative values of the elements analysed. A rectangular area of 1.664 mm wide × 0.608 mm high was used to calculate the semiquantitative areas and limits of detection for each element for each pigment square. Semiquantitative data were used to compare pigments to each other as well as to compositional data from other techniques.
Light microscopy
The slides used for XFM analyses were also studied in the light microscopy analysis to provide a direct comparison between the elemental maps and microscopy images. The slides were viewed through an Olympus CX41 transmitted light microscope at 100×. Digital images were captured using an Olympus DP21 camera.Results and discussion
XFM images
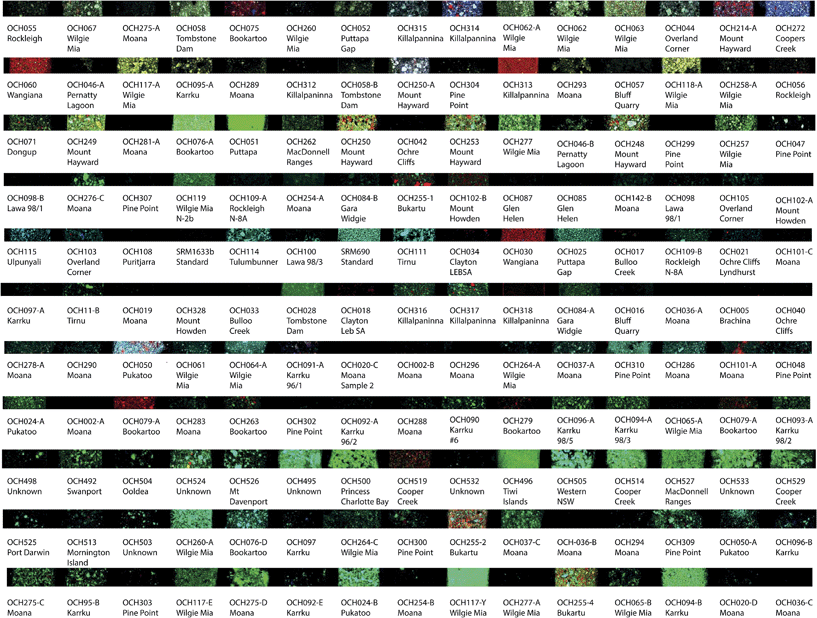
Images from the XFM analysis demonstrated the inherent variability of the ochre samples across sources and between sources. While 165 samples were analysed, individual examples from each highlighted site are selected for discussion (Fig. 3–6).Data were acquired for all 15 elements, however, some elements demonstrated very low concentrations and therefore very little data in the maps. These results indicate that the elements in the ochre pigment sample are below detection limits of the technique. Of the elements examined, data for the maps were consistently found for As, Ca, Cr Fe, Mn, Ti, and V. Elemental concentrations for Cu, Ga, Pb, Sc, Se, Sr and Zn were generally close to or below detection limits for the sites highlighted in this study; therefore images are not presented. Prior to this study data for Cu and Pb were unknown for ochre samples, as NAA is not able to analyse for these two elements. These data reveal that Cu and Pb are not diagnostic for identifying or characterising the ochre origin and these elements will not be discussed further. Table 1 presents the semiquantitative data for the elements analysed and for the sites discussed. The range of values generally agrees with other bulk methods such as NAA within 10–20%.6,7 However there is not a perfect agreement with the bulk methods. NAA is a traceable method that uses certified and reference standards for calculation of values and uncertainties. GeoPIXE utilises a user-supplied model of the specimen matrix, in which the matrix composition (Fe2O3), its density (5.24 g cm−3), and uniform thickness (100 μm) are used to model the X-ray fluorescence yields. Deviations of the specimen from the assumed matrix parameters will lead to a less accurate determination of sample concentration. The matrix and its thickness will vary between samples, thus we regard the results as semiquantitative. We note that assuming hematite or goethite as a matrix makes negligible difference to the determined concentration, but a grossly different matrix such as silicate will be significant. The sampling size between the two methods is quite different, on the order of mg for XFM as compared to at least 50 mg for NAA, which will influence the samples representation to the bulk. In particular for XFM, the sample is further diluted approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume in the epoxy resin, therefore affecting the calculations by GeoPIXE. In general, this will reduce the concentrations by approximately one half for iron and heavier elements, reduce by 30% for V–Cr and increase Ca, Ti by about 30%.
1 by volume in the epoxy resin, therefore affecting the calculations by GeoPIXE. In general, this will reduce the concentrations by approximately one half for iron and heavier elements, reduce by 30% for V–Cr and increase Ca, Ti by about 30%.
| ID | Site | Ca | Ca LOD | Sc | Sc LOD | Ti | Ti LOD | V | V LOD | Cr | Cr LOD | Mn | Mn LOD | Fe | Fe LOD | Cu | Cu LOD | Zn | Zn LOD | As | As LOD | Rb | Rb LOD | Sr | Sr LOD | PbL | Pb LOD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OCH075 | Bookartoo | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 081 |
35 | <LOD | 9 | 232 | 4 | <LOD | 2 | <LOD | 1 | 1325 | 3 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
10 | <LOD | 1 | <LOD | 1 | 5.8 | 0.7 | <LOD | 0.3 | 18.6 | 0.4 | <LOD | 0.8 |
| OCH076-A | Bookartoo | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 454 454 |
43 | <LOD | 10 | 1299 | 6 | 551 | 4 | 299 | 2 | 7989 | 8 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0716 0716 |
13 | <LOD | 2 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.3 | <LOD | 0.4 | 71 | 1 |
| OCH078-A | Bookartoo | 9315 | 86 | 4 | 22 | 1703 | 5 | 1373 | 2 | 374 | 1 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758 758 |
4 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1427 1427 |
8 | <LOD | 2 | 60.8 | 1 | <LOD | 0.9 | 26.8 | 0.4 | 36.6 | 0.5 | 385 | 1 |
| OCH263 | Bookartoo | <LOD | 39 | 263 | 11 | 810 | 5 | 529 | 3 | 213 | 2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 044 044 |
7 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5920 5920 |
14 | <LOD | 2 | <LOD | 2 | <LOD | 0.9 | 25.2 | 0.4 | 16.9 | 0.5 | 233 | 1 |
| OCH279 | Bookartoo | <LOD | 37 | <LOD | 10 | 566 | 4 | <LOD | 2 | <LOD | 1 | 2688 | 4 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 931 931 |
10 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 22.9 | 0.4 | 30.2 | 0.5 | 31.6 | 1 |
| OCH079-A | Bookartoo | 1800 | 51 | <LOD | 14 | 829 | 5 | <LOD | 2 | <LOD | 2 | 5040 | 5 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 047 047 |
14 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 66.3 | 0.4 | 39.7 | 0.6 | 44.5 | 1 |
| OCH076-D | Bookartoo | <LOD | 8 | <LOD | 4 | 101 | 3 | 209 | 2 | 128 | 1 | 1745 | 3 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 089 089 |
11 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.5 | 0.87 | 0.2 | <LOD | 0.3 | 59.8 | 0.7 |
| OCH255-1 | Bukartu | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 638 |
60 | <LOD | 17 | 1625 | 7 | 633 | 4 | 187 | 3 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 226 |
10 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9012 9012 |
14 | <LOD | 2 | 738 | 3 | <LOD | 1 | 11.3 | 0.5 | 240 | 0.7 | 418 | 2 |
| OCH255-4 | Bukartu | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 719 719 |
23 | <LOD | 7 | 222 | 3 | 267 | 2 | 142 | 1 | 2076 | 4 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
11 | <LOD | 1 | 436 | 2 | 35.4 | 0.6 | <LOD | 0.2 | 54.7 | 0.3 | 54.9 | 0.7 |
| OCH255-2 | Bukartu | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185 |
26 | <LOD | 7 | 130 | 3 | 146 | 2 | 95 | 1 | 1515 | 3 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
10 | <LOD | 1 | 189 | 1 | <LOD | 0.5 | <LOD | 0.2 | 41.2 | 0.3 | 27.7 | 0.7 |
| OCH095-A | Karrku | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 693 |
24 | <LOD | 7 | 701 | 4 | <LOD | 2 | <LOD | 1 | 4937 | 5 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 427 |
12 | <LOD | 1 | <LOD | 1 | <LOD | 0.7 | 132 | 0.5 | 290 | 0.6 | 22.7 | 0.9 |
| OCH095-B | Karrku | <LOD | 8 | <LOD | 3 | 84.4 | 2 | <LOD | 1 | 51.4 | 0.9 | 937 | 3 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 647 647 |
9 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.5 | 13.3 | 0.3 | 14 | 0.3 | 12.5 | 0.6 |
| OCH092-E | Karrku | <LOD | 6 | <LOD | 3 | <LOD | 2 | <LOD | 1 | <LOD | 0.7 | 112 | 1 | 7100 | 4 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | 9.49 | 0.2 | 13.7 | 0.3 | <LOD | 0.6 |
| OCH094-B | Karrku | 789 | 11 | 99 | 6 | 695 | 4 | 533 | 3 | 433 | 2 | 4730 | 5 | 161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845 845 |
11 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.6 | 17.6 | 0.3 | <LOD | 0.3 | 212 | 0.9 |
| OCH097 | Karrku | <LOD | 6 | <LOD | 3 | <LOD | 2 | <LOD | 0.9 | <LOD | 0.6 | 99.3 | 1 | 6676 | 4 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | 3.96 | 0.2 | 16.9 | 0.3 | <LOD | 0.6 |
| OCH096-B | Karrku | 355 | 8 | <LOD | 3 | 121 | 2 | <LOD | 1 | <LOD | 0.8 | 312 | 2 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 431 431 |
6 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | 35.6 | 0.3 | 73.3 | 0.4 | 5.8 | 0.6 |
| OCH090 | Karrku #6 | <LOD | 16 | <LOD | 8 | <LOD | 6 | <LOD | 3 | <LOD | 2 | 374 | 8 | 4847 | 14 | <LOD | 2 | <LOD | 1 | <LOD | 0.9 | 30.3 | 0.5 | 34 | 0.6 | 75.8 | 1 |
| OCH091-A | Karrku 96/1 | <LOD | 16 | <LOD | 5 | 295 | 3 | <LOD | 2 | <LOD | 1 | 2948 | 3 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343 343 |
7 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.6 | 121 | 0.4 | 297 | 0.5 | 75.2 | 0.8 |
| OCH092-A | Karrku 96/2 | <LOD | 13 | <LOD | 5 | 841 | 4 | <LOD | 2 | <LOD | 1 | 418 | 4 | 5849 | 9 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 25.4 | 0.5 | 65.7 | 0.5 | 41.2 | 1 |
| OCH093-A | Karrku 98/2 | <LOD | 15 | 398 | 8 | 1336 | 6 | <LOD | 3 | <LOD | 2 | 3671 | 8 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
14 | <LOD | 2 | <LOD | 1 | 24.1 | 0.8 | 26.2 | 0.4 | 30 | 0.5 | 21.8 | 1 |
| OCH094-A | Karrku 98/3 | 159 | 16 | 49 | 8 | 904 | 6 | 363 | 4 | 253 | 3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 463 463 |
9 | 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 188 188 |
14 | <LOD | 2 | <LOD | 1 | <LOD | 0.9 | 61.4 | 0.4 | 20.9 | 0.5 | 74.2 | 1 |
| OCH096-A | Karrku 98/5 | <LOD | 16 | 189 | 8 | 1252 | 6 | 45 | 4 | 28.9 | 3 | 7168 | 9 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166 166 |
14 | <LOD | 2 | <LOD | 1 | <LOD | 0.9 | 75.1 | 0.4 | 45.2 | 0.5 | 64.5 | 1 |
| OCH097-A | Karrku JW | <LOD | 14 | <LOD | 8 | 1098 | 5 | <LOD | 3 | <LOD | 2 | 4603 | 5 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
12 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 212 | 0.6 | 368 | 0.8 | 81.6 | 1 |
| OCH062-A | Little Wilgie Mia | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 545 545 |
35 | <LOD | 8 | 820 | 5 | 80 | 3 | 247 | 2 | 6945 | 7 | 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 272 272 |
13 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | <LOD | 0.3 | <LOD | 0.3 | 58.6 | 1 |
| OCH062 | Little Wilgie Mia | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 302 302 |
39 | <LOD | 9 | 1125 | 6 | 157 | 3 | 337 | 2 | 8134 | 7 | 191![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 268 268 |
13 | <LOD | 2 | <LOD | 2 | <LOD | 0.9 | <LOD | 0.3 | <LOD | 0.3 | 96.8 | 1 |
| OCH275-A | Moana | 8852 | 21 | <LOD | 6 | 664 | 4 | <LOD | 2 | <LOD | 1 | 1298 | 3 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 322 322 |
10 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 15.5 | 0.3 | <LOD | 0.3 | <LOD | 0.8 |
| OCH289 | Moana | 3324 | 15 | <LOD | 5 | <LOD | 3 | <LOD | 1 | <LOD | 1 | 1839 | 3 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
8 | <LOD | 2 | <LOD | 1 | <LOD | 0.5 | <LOD | 0.3 | <LOD | 0.3 | <LOD | 0.7 |
| OCH293 | Moana | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 554 554 |
22 | <LOD | 6 | 575 | 4 | <LOD | 2 | <LOD | 1 | 4071 | 5 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 829 829 |
12 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 10 | 0.3 | <LOD | 0.3 | <LOD | 0.8 |
| OCH281-A | Moana | 2134 | 14 | <LOD | 5 | 379 | 3 | <LOD | 1 | <LOD | 0.9 | 220 | 2 | 8618 | 5 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 47 | 0.4 | 6.7 | 0.4 | <LOD | 0.8 |
| OCH278-A | Moana | <LOD | 20 | <LOD | 6 | 910 | 4 | <LOD | 2 | 9.8 | 1 | 6152 | 4 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005 005 |
10 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 75.1 | 0.4 | 33.1 | 0.3 | 48.3 | 0.8 |
| OCH290 | Moana | <LOD | 15 | <LOD | 5 | 632 | 3 | <LOD | 1 | <LOD | 1 | 3701 | 3 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
8 | 19.3 | 2 | 35.8 | 1 | <LOD | 0.6 | 27.5 | 0.3 | 32.9 | 0.3 | 48.7 | 0.8 |
| OCH002-B | Moana | <LOD | 13 | <LOD | 5 | 554 | 3 | <LOD | 1 | <LOD | 0.9 | 1444 | 2 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 622 |
5 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 97.3 | 0.4 | 42.8 | 0.3 | 55.9 | 0.8 |
| OCH296 | Moana | <LOD | 9 | <LOD | 3 | <LOD | 2 | <LOD | 1 | <LOD | 0.7 | 374 | 1 | 4847 | 2 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 30.3 | 0.3 | 34 | 0.3 | 75.8 | 0.8 |
| OCH037-A | Moana | <LOD | 20 | 189 | 6 | 1252 | 4 | 45 | 2 | 28.9 | 1 | 7168 | 4 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166 166 |
10 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 75.1 | 0.3 | 45.2 | 0.3 | 64.5 | 0.8 |
| OCH286 | Moana | <LOD | 23 | <LOD | 6 | 453 | 4 | 214 | 2 | 88 | 1 | 9979 | 5 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019 019 |
12 | <LOD | 1 | <LOD | 1 | 19.7 | 0.7 | 25.4 | 0.3 | 27 | 0.3 | 72.9 | 0.8 |
| OCH101-A | Moana | 1800 | 17 | <LOD | 5 | 829 | 3 | <LOD | 2 | <LOD | 1 | 5040 | 3 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 047 047 |
8 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 66.3 | 0.3 | 39.7 | 0.3 | 44.5 | 0.8 |
| OCH002-A | Moana | <LOD | 14 | <LOD | 6 | 632 | 5 | <LOD | 2 | <LOD | 1 | 3701 | 3 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
8 | 19.3 | 2 | 35.8 | 1 | <LOD | 0.8 | 27.5 | 0.5 | 32.9 | 0.5 | 48.7 | 1 |
| OCH283 | Moana | <LOD | 13 | <LOD | 7 | 50 | 5 | 72.3 | 2 | 19.4 | 2 | 6935 | 5 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894 894 |
14 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 19.9 | 0.4 | 21.9 | 0.5 | 74.9 | 1 |
| OCH288 | Moana | <LOD | 12 | <LOD | 6 | 554 | 4 | <LOD | 2 | <LOD | 1 | 1444 | 5 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 622 |
12 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 97.3 | 0.4 | 42.8 | 0.5 | 55.9 | 1 |
| OCH276-C | Moana | 1229 | 19 | 848 | 10 | 1964 | 8 | 1031 | 4 | 524 | 3 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 945 945 |
13 | 329![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 089 089 |
14 | <LOD | 2 | 539 | 2 | <LOD | 1 | 71.2 | 0.5 | 16.2 | 0.5 | 886 | 2 |
| OCH254-A | Moana | 3214 | 19 | <LOD | 8 | 860 | 6 | 459 | 3 | 174 | 2 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159 159 |
10 | 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 377 377 |
14 | <LOD | 2 | <LOD | 1 | 413 | 1 | 37.3 | 0.4 | 890 | 1 | 84.7 | 1 |
| OCH142-B | Moana | 1031 | 17 | 494 | 9 | 2169 | 7 | 293 | 3 | 148 | 2 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 048 |
8 | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230 |
14 | <LOD | 2 | <LOD | 1 | <LOD | 1 | 134 | 0.6 | 70.5 | 0.5 | 118 | 1 |
| OCH019 | Moana | <LOD | 11 | <LOD | 6 | 129 | 4 | <LOD | 2 | <LOD | 1 | 2400 | 4 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 190 |
9 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 50 | 0.5 | 32.2 | 0.5 | 29.8 | 1 |
| OCH036-A | Moana | <LOD | 10 | 4 | 7 | 665 | 5 | <LOD | 2 | <LOD | 1 | 491 | 3 | 5879 | 5 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | 28.7 | 0.4 | 51.6 | 0.5 | 42.4 | 1 |
| OCH275-C | Moana | 228 | 9 | <LOD | 4 | 54.2 | 2 | 0.8 | 1 | 53.9 | 0.9 | 982 | 3 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 503 503 |
9 | <LOD | 1 | <LOD | 0.7 | 125 | 0.6 | 9.74 | 0.2 | <LOD | 0.3 | 11.3 | 0.6 |
| OCH275-D | Moana | 596 | 9 | <LOD | 4 | 386 | 3 | 119 | 2 | 163 | 1 | 2118 | 4 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 331 |
11 | <LOD | 1 | 61.2 | 0.9 | <LOD | 0.5 | <LOD | 0.2 | <LOD | 0.3 | 37.7 | 0.7 |
| OCH254-B | Moana | <LOD | 6 | <LOD | 2 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.6 | 81.7 | 1 | 6242 | 4 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | <LOD | 0.2 | <LOD | 0.3 | <LOD | 0.6 |
| OCH020-D | Moana | <LOD | 7 | <LOD | 4 | 1066 | 4 | <LOD | 1 | <LOD | 0.7 | 153 | 2 | 8949 | 5 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.5 | 2.34 | 0.2 | <LOD | 0.3 | 1.6 | 0.6 |
| OCH036-C | Moana | <LOD | 6 | <LOD | 4 | 759 | 3 | <LOD | 1 | <LOD | 0.6 | <LOD | 1 | 3461 | 3 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.5 | 5.6 | 0.2 | 3.89 | 0.3 | 2.2 | 0.6 |
| OCH037-C | Moana | 371 | 8 | <LOD | 4 | 394 | 3 | <LOD | 1 | 50.2 | 0.9 | 853 | 3 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 171 171 |
8 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | 25.9 | 0.3 | <LOD | 0.3 | 2.9 | 0.6 |
| OCH036-B | Moana | <LOD | 5 | <LOD | 2 | <LOD | 2 | <LOD | 0.8 | <LOD | 0.5 | <LOD | 0.9 | 827 | 2 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | 0.11 | 0.2 | <LOD | 0.3 | <LOD | 0.6 |
| OCH294 | Moana | <LOD | 6 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | <LOD | 0.6 | <LOD | 1 | 2928 | 3 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | 2.83 | 0.2 | <LOD | 0.3 | <LOD | 0.6 |
| OCH020-C | Moana (sample 2) | <LOD | 11 | <LOD | 5 | 841 | 4 | <LOD | 1 | <LOD | 0.8 | 418 | 2 | 5849 | 3 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.6 | 25.4 | 0.3 | 65.7 | 0.3 | 41.2 | 0.7 |
| OCH101-C | Moana N-6 | <LOD | 12 | <LOD | 12 | 302 | 4 | <LOD | 2 | <LOD | 1 | 2063 | 4 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 309 309 |
9 | <LOD | 2 | <LOD | 1 | 72.6 | 1 | 138 | 0.6 | 64.9 | 0.5 | 56.5 | 1 |
| OCH050 | Pukatoo | 9315 | 35 | 4 | 9 | 1703 | 5 | 1373 | 3 | 374 | 2 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758 758 |
8 | 251![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 427 |
13 | <LOD | 2 | 60.8 | 2 | <LOD | 0.9 | 26.8 | 0.3 | 36.6 | 0.4 | 385 | 1 |
| OCH024-A | Pukatoo | <LOD | 17 | <LOD | 8 | 910 | 6 | <LOD | 4 | 9.8 | 3 | 6152 | 10 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005 005 |
14 | <LOD | 2 | <LOD | 1 | <LOD | 1 | 75.1 | 0.4 | 33.1 | 0.5 | 48.3 | 1 |
| OCH024-B | Pukatoo | 618 | 10 | 45 | 5 | 467 | 4 | 646 | 2 | 321 | 2 | 3690 | 5 | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
11 | <LOD | 1 | <LOD | 0.8 | <LOD | 0.6 | 3.13 | 0.2 | <LOD | 0.3 | 132 | 0.8 |
| OCH050-A | Pukatoo | <LOD | 7 | <LOD | 3 | <LOD | 2 | <LOD | 1 | 3.3 | 0.8 | 456 | 2 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 219 219 |
6 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.5 | <LOD | 0.2 | <LOD | 0.3 | 6 | 0.6 |
| OCH067 | Wilgie Mia | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 552 |
34 | <LOD | 9 | 1011 | 5 | 76.4 | 3 | 215 | 2 | 5909 | 6 | 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 746 |
13 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | <LOD | 0.3 | <LOD | 0.3 | 45.6 | 1 |
| OCH260 | Wilgie Mia | 4157 | 16 | <LOD | 4 | <LOD | 3 | <LOD | 1 | <LOD | 1 | 880 | 3 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843 843 |
8 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.6 | <LOD | 0.3 | <LOD | 0.3 | <LOD | 0.8 |
| OCH063 | Wilgie Mia | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 403 403 |
44 | <LOD | 11 | 1448 | 6 | 202 | 3 | 375 | 2 | 8838 | 8 | 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 901 901 |
13 | <LOD | 2 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.3 | <LOD | 0.3 | 107 | 1 |
| OCH117-A | Wilgie Mia | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 397 397 |
33 | <LOD | 9 | 1021 | 5 | 46.8 | 3 | 131 | 2 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
8 | 183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 097 097 |
12 | <LOD | 2 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.3 | <LOD | 0.3 | 94.2 | 1 |
| OCH118-A | Wilgie Mia | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 576 576 |
37 | <LOD | 10 | 1547 | 6 | 234 | 3 | 261 | 2 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 918 918 |
9 | 233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 004 004 |
12 | <LOD | 2 | <LOD | 1 | <LOD | 1 | <LOD | 0.3 | <LOD | 0.4 | 173 | 1 |
| OCH258-A | Wilgie Mia | 4258 | 17 | <LOD | 6 | 1048 | 4 | <LOD | 2 | <LOD | 1 | 1919 | 3 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 701 701 |
9 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | <LOD | 0.3 | <LOD | 0.3 | <LOD | 0.7 |
| OCH277 | Wilgie Mia | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 329 329 |
33 | <LOD | 8 | 803 | 5 | 97.7 | 3 | 213 | 2 | 5945 | 7 | 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713 713 |
13 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | <LOD | 0.3 | <LOD | 0.3 | 44.3 | 1 |
| OCH257 | Wilgie Mia | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 611 611 |
30 | <LOD | 7 | 566 | 4 | 17 | 2 | 139 | 2 | 4424 | 6 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080 |
13 | <LOD | 2 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.3 | <LOD | 0.3 | 16.3 | 1 |
| OCH061 | Wilgie Mia | <LOD | 17 | <LOD | 5 | 50 | 3 | 72.3 | 2 | 19.4 | 1 | 6935 | 4 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894 894 |
10 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 19.9 | 0.3 | 21.9 | 0.3 | 74.9 | 0.8 |
| OCH064-A | Wilgie Mia | <LOD | 22 | 263 | 6 | 810 | 3 | 529 | 2 | 213 | 1 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 044 044 |
5 | 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 920 |
13 | <LOD | 1 | <LOD | 1 | <LOD | 0.6 | 25.2 | 0.3 | 16.9 | 0.3 | 233 | 0.9 |
| OCH264-A | Wilgie Mia | <LOD | 13 | <LOD | 5 | 566 | 3 | <LOD | 1 | <LOD | 0.9 | 2688 | 2 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 931 931 |
6 | <LOD | 1 | <LOD | 0.9 | <LOD | 0.6 | 22.9 | 0.3 | 30.2 | 0.3 | 31.6 | 0.7 |
| OCH065-A | Wilgie Mia | <LOD | 12 | <LOD | 6 | 453 | 5 | 214 | 2 | 88 | 1 | 9979 | 3 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019 019 |
9 | <LOD | 2 | <LOD | 1 | 19.7 | 0.8 | 25.4 | 0.4 | 27 | 0.5 | 72.9 | 1 |
| OCH117-E | Wilgie Mia | <LOD | 8 | <LOD | 4 | 620 | 3 | 1.8 | 1 | 64.5 | 1 | 947 | 3 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 025 025 |
9 | <LOD | 1 | <LOD | 0.8 | 3 | 0.5 | <LOD | 0.2 | <LOD | 0.3 | <LOD | 0.6 |
| OCH117-Y | Wilgie Mia | 2708 | 14 | 675 | 7 | 1360 | 6 | 983 | 3 | 913 | 2 | 9350 | 8 | 330![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997 997 |
11 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.8 | 40 | 0.3 | <LOD | 0.2 | 669 | 1 |
| OCH277-A | Wilgie Mia | <LOD | 5 | <LOD | 2 | <LOD | 1 | <LOD | 0.8 | <LOD | 0.6 | <LOD | 1 | 2816 | 3 | <LOD | 0.9 | <LOD | 0.7 | <LOD | 0.4 | <LOD | 0.2 | <LOD | 0.3 | <LOD | 0.6 |
| OCH065-B | Wilgie Mia | 243 | 9 | <LOD | 4 | 711 | 3 | <LOD | 1 | 11.8 | 0.8 | 404 | 2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 692 692 |
6 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.4 | <LOD | 0.2 | <LOD | 0.3 | <LOD | 0.6 |
| OCH260-A | Wilgie Mia | 174 | 9 | <LOD | 4 | 309 | 3 | 218 | 2 | 234 | 1 | 2516 | 4 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 304 304 |
11 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.5 | 1.2 | 0.2 | <LOD | 0.2 | 52.7 | 0.7 |
| OCH264-C | Wilgie Mia | <LOD | 7 | <LOD | 3 | 293 | 3 | <LOD | 1 | 7.5 | 0.8 | 402 | 2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 422 |
6 | <LOD | 1 | <LOD | 0.7 | <LOD | 0.4 | 1.35 | 0.2 | <LOD | 0.3 | <LOD | 0.6 |
| OCH119 | Wilgie Mia N-2b | 3732 | 21 | 1330 | 12 | 2460 | 9 | 1525 | 5 | 853 | 4 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 757 757 |
15 | 449![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933 933 |
14 | <LOD | 2 | <LOD | 1 | <LOD | 1 | 60.5 | 0.5 | <LOD | 0.4 | 886 | 2 |
Discussion of the elemental maps will follow by element. A further discussion of the sites and their archaeology is discussed elsewhere in the literature.
Fe
As Fe is the main constituent of Fe-oxide ochre pigments, the elemental maps of Fe were studied in more detail to identify trends in the data. Concentrations ranged from 26 ppm to 45%.Bookartoo
Variations can be observed in the overall intensity of the false colour maps but this is likely an effect of the variability in the scaling of the image brightness relative to other samples rather than a reflection of the actual concentration of Fe in each sample (see below). The samples exhibit a dense coverage of small iron oxide particles with some larger platy iron oxide particles of higher concentration. These data support earlier suggestions of “micaceous” ochre observed from the site.19,23,27 This ochre is visually a deep purple red, however yellow ochre is also found at this site. OCH079-A is an example of yellow ochre, and its lower Fe concentration indicated is composition of goethite [α-FeO(OH)] rather than hematite (Fe2O3), or admixture of yellow iron pigments with other lower-Fe minerals such as siderite (FeCO3) or jarosite-group minerals (XFe3(SO4)2(OH)6) with X = K, Na, Pb etc.Karrku
Samples OCH 092-A and OCH 090, OC096 and OCH 094-A display variation in particle size and shape. A plausible reason for this is that the samples were collected during two different campaigns in 1996 and 1998 by Mike Smith et al.; therefore the variation likely reflects different sampling locations. OCH090 and OCH 092 also display more voids as compared to OCH-96-A and OCH094-A, which are more densely covered in Fe concentration. The site is known to contain soft earthy specular hematite between quartzite and is mined in a cave location.26Moana
Samples from the sites were obtained from the South Australian Museum, Mike Smith and the Margaret Nobbs collection. OCH020, OCH036, OCH037, OCH019, and OCH278A are from the Nobbs surveys. Remarkably, they demonstrate very low, if any, Fe signal in the elemental maps except for OCH037-A and OCH037-C, which are sub samples from a larger sample. OCH101-A and OCH 101-A were collected by Mike Smith, and demonstrate similar Fe elemental maps to the Nobbs samples. More recent samples OCH283-293 have a similar Fe distribution and profile except OCH 289 however samples 283–293 are similar to OCH-037. Samples OCH 275 and OCH 281 and OCH 254 are also listed as being from Moana, however they are from the Archaeology and Ethnography collections rather than from a specific researcher survey. Care needs to be taken in the interpretations of these samples as they may not be true source samples, rather examples of ochre collected from an archaeological excavation. Therefore these samples are not representative of the site but perhaps artefact ochre found at the site that had been imported from elsewhere. These samples provide examples as potential “unknowns” for attribution to determined source locations established in this study.Pine Point
Similarly to Moana, this site is a coastal weathered site and the relatively low Fe concentrations reflect this. As samples OCH299-309 were sampled directly from the site, they represent the variability within the areas sampled. OCH047 and 048 are from the Nobbs collection and bear more similarity to each other than the other samples, reflecting differing sampling campaigns.Wilgie Mia
Wilgie Mia and Little Wilgie Mia are both banded iron formations located in Western Australia. A recent paper characterised these materials in the bulk using LA-ICP-MS.17 The samples from the site again reflect at least three different sampling trips, from Nobbs, Smith and the Western Australian Museum, in order of oldest to most recent. In general the samples are relatively rich in Fe, especially samples such as OCH 117-Y, 260-A and 119, which are composed of high Fe concentration with tightly packed small particles. In contrast OCH 277 and 257, collected by Nobbs, and from the collections of the South Australian Museum, respectively are very low Fe and perhaps not representative of the site.In general across the five sites discussed, the distribution of the Fe in the samples allows insight into the relationship of the Fe to particular particle sizes and shapes, rather than a bulk analysis. In previous analyses, a distribution in the bulk composition was also observed, with the concentration of Fe ranging from 10–60% iron oxides, depending on the site and mixture.6,7 The large range of Fe composition in the samples as well as several samples having a very low concentration of Fe calls into question the definition of ochre as far as the majority composition of Fe oxides of the material. As ochre is an admixture of Fe-oxides and other minerals, the varying ratios of these materials and types of materials affect the distribution. Elemental analysis cannot provide direct information on the mineralogical state of the material; therefore some Fe-oxides may be hematite or goethite-based.
Ca
Ca was an element of interest as at least one site (Bookartoo), the ochre material is found within a dolomite [CaMg(CO3)2] matrix.27 Semiquantitative values of Ca ranged from below detection limits up to 9%. Previous archaeometric studies had identified Ca as a discriminatory element for this site.From previous analyses, Bookartoo is known for its relatively high Fe, Ca and V concentrations.27 The microelemental analysis supports these conclusions from the bulk chemical analyses, however the concentration and distribution across the samples is not consistent. Karrku, Moana and Pine Point have very little Ca, however Wilgie Mia has Ca associated with both small rounded and large angular particles. There is a direct correlation between the Fe and Ca in both Bookartoo and Wilgie Mia samples suggesting that during deep weathering of the sites the Fe oxides may have reacted with the dolomite or calcite to form ankerite CaFe(CO3)2, the Fe analogue of dolomite.16,31,32
While not exhaustive of every site in Australia these results suggest that Ca is indicative with banded iron formations within covered deposits or related formations, and not as associated with weathered, exposed sites. For this sample set, Ca is one of a few clear discriminatory elements for Bookartoo and Wilgie Mia and is characteristic of these sites in higher concentrations.
Rb
Rb was an element that ranged from below detection limits to 212 ppm in the sites examined, and was detected in most of the samples analysed (Table 1).However, essentially very little Rb was identified in the Bookartoo or Wilgie Mia ochre samples, and therefore it is not associated with Fe or other Fe-bearing minerals from the site.
Moana has variability with the samples in the concentration of Rb. The higher concentrations of Rb appear to be associated with larger, angular particles rather than smaller rounded particles. Similarly to Moana, Karrku also has Rb associated with the larger angular particles, but this is not universally found across all samples from the site.
Pine Point ochre samples have some distribution of Rb, particularly in the larger particles in samples OCH302, 303 and 304, which represent a particular sampling location within the site. OCH 309 and 310, 299 and 048 do not have the same distribution of Rb, reflecting the diversity of the ochre material within the site.
Other elements
Images of elemental maps of the slides with elements that were less consistently observed will not be discussed as extensively as the above elements. These include As, Ti and Mn.For As, most samples are devoid of even low concentrations of As, with the exception of OCH254-A, OCH101-C and OCH275-C from Moana. As discussed earlier, these samples may be related to the archaeology of the site rather than being examples of source samples.
Ti is not in high concentration or distribution for the sites with the exception of Wilgie Mia. The amount of Ti in the sample is not necessarily related to the sampling campaign for these. Of these samples, OCH062, 062-A and 063, 067, 065 (Western Australia Museum) and 119, 117-Y, 117-A and 119 (Mike Smith) have relatively high concentrations of Ti. However, OCH 260 (Mike Smith) and 065-A (WA Museum) do not. As discussed earlier, this reflects the large scale of the site and the possibilities of the sampling locations. Titanium can occur at low levels in hematite, but can also can be present as one of the TiO2 polymorphs of are ilmenite (FeTiO3), which are widespread detrital minerals in sediments.
Both Cr and Mn have similar distributions patterns to Fe in the ochre samples, with similar trends in concentration and distribution. This indicates they are present substituting into hematite and magnetite. The distributions of these elements will not be discussed in depth.
Tri-elemental images
One of the strengths of the XFM technique combined with GeoPIXE is the ability to generate elemental maps of three elements simultaneously. This “overlaying” of elemental maps allows clear differentiation of correlation and anti-correlation of elements as well as the relationship of elemental concentration to a particle or particles or features in the sample. As with the single element plots, the individual elements are falsely coloured to indicate concentration intensity. Using the three colours of red, green and blue, the elements can be easily identified as well as mixtures (e.g. equal amounts of red and blue generate purple regions). As there were many combinations of elements, the combination of Ca (red), Fe, (green) and V (blue) was a representative example.Visualising the samples in this context allows more interpretation of the elemental distribution for each sample type. In this combination, Karrku, Moana, Pine Point and Wilgie Mia generally are composed of varying concentrations of Fe. However, Bookartoo is revealed to be a complex mixture of Ca, Fe and V, especially in cases such as OCH 255-2 and 255-4 where elemental concentrations can be attributed to individual particles. Moana samples OCH 254, 281 and 275 as attribution case study examples provide a revealing story. The tri-elemental plots allow for characterisation using three discriminatory elements. Moana does not necessarily have a clear elemental profile or definition; rather three to four patterns are seen in the particle size and elemental concentrations. As seen at the site itself, Moana is a large distribution of red and yellow formations with differing characteristics. Unlike Bookartoo, the diversity of the Moana site makes it more difficult to attribute an archaeological “unknown” to the site, whereas this attribution would be easier with a sample from Bookartoo or Wilgie Mia.
Fig. 7 displays the tri-elemental plot of Ca, Fe and V for all 165 elements analysed. These data represent a large variety of deposits and sites from across Australia. This figure demonstrates the great diversity of deposit chemistry and particle distribution and sizes.
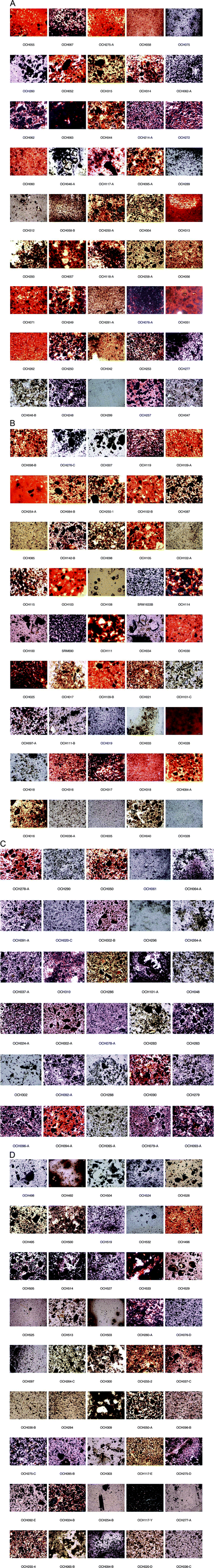
Light microscopy
With visual inspection of the slides it can be observed that for several samples there is a very low amount of ochre sample within the epoxy resin (Fig. 8). Therefore the elemental maps with low concentrations of Fe are more representative of the relatively few particles of ochre rather than a low concentration of a particular element in the particles. This relatively low concentration may be a reflection of the properties of mixing of the material with the resin; therefore mixing of the same material with another traditional paint binder such as water, natural resin, or proteinaceous binder may have a different effect on the number of particles in suspension.4 In addition, as all samples were pre-sieved to less than 250 micron, it may be the case that the ochre samples from these sites have more particles larger than 250 micron.The colour of the individual ochre sample can also be observed for each sample. Most examples are in the traditional palette of reds, oranges, yellows and browns and even some purple samples. The colour and particle shape variability can also be observed within the individual samples and between samples from the same site. For instance, OCH024 and 255-A (B1) demonstrate the deep red colour and mixture of intense fine particles (5–10 micron) along with the larger “platy” dark particles (200 micron) characteristic of the site. However, OCH 101-C (A2, Moana) contains a collection of yellow spherical particles on the order of 50–100 micron. The particle colour as well as the shape influence the overall colour when viewed by eye, and the elemental composition in combination with lighter elements such as Ca, O, Si, K and others form the original mineralogy of the material. Viewing this microscopic palette of the 165 samples analysed in this study gives a comprehensive view into the diversity of minerals and mineral mixtures that compose Australian ochre.
Conclusions
This study demonstrates the first successful use of X-ray fluorescence microanalysis towards the characterisation of multiple Australian Aboriginal ochre samples from across Australia. Samples from cultural areas including both sources and archaeological contexts provide a wide-ranging data set to examine the variability within and between site locations. The micron-scale elemental maps produced in this work provide compositional and spatial information on the distribution of minerals in complex pigment materials that are not available in bulk techniques. The results from this study support earlier bulk technique data on the elemental characterisation of ochre samples, and supports the need for sensitive multielemental techniques to fully understand this complex and variable material. Semiquantitative data from GeoPIXE also aids in understanding elemental trends in the samples for a standardised area on a sample slide.As each sample is representative of a small area of an ochre source with inherent variability due to genesis and site history, one sample from each site cannot be considered representative of a large site location. As demonstrated in this study, some sites such as Bookartoo the deposits are relatively consistent, whereas for a site such as Moana where digenetic processes have altered the site has a more diverse elemental and mineral profile. Rather, the combination of source samples using multiple elements allows characterisation down to the association of elemental profile with single particles. In addition, the presence or absence of a single element is not indicative of a particular source location; rather a combination of elements and concentrations as well as elements relative to a particular particle pattern aid in the characterisation of the material from a particular site. The addition of light microscopy of the same areas elementally analysed provides a fuller picture of the particle colours and distribution. This study assists in the attribution of ochre to original sites, aiding in the understanding of the cultural uses and potential exchange of ochre, however assignments of samples to particular cultural locations must be made with care and with an understanding of both the chemistry and cultural context. Future studies include expansion of the database of deposits and archaeological sites and application to artefacts.
Acknowledgements
We gratefully acknowledge the South Australian Museum Board and South Australian Museum Aboriginal Advisory Group for support and permission to access and analyse the collections. We also thank the staff at the South Australian Museum Aboriginal Australian Collections for access to the collections. We are also grateful to Mike Smith and other collaborators for access to their collections for analysis. We acknowledge the preparation of the epoxy cast samples by Ms Caroline Watson and Mr Owen Osborne. The staff at Pontifex, Kensington, South Australia are gratefully acknowledged for their careful final preparation of the slides. We acknowledge Professor Paul Kirkbride, Flinders University for the use of the microscope. The project has approval number 4670 from the Social and Behavioural Research Ethics Committee of Flinders University. Funding is gratefully acknowledged from Australian Institute of Nuclear Science and Engineering (AINSE) Research Fellowship (Popelka-Filcoff), and Australian Research Council Grant #LP0882597 (Lenehan, Pring and Popelka-Filcoff). Part of this research was undertaken on the XFM beam line at the Australian Synchrotron, Victoria, Australia.Notes and references
- P. Nel, P. A. Lynch, J. S. Laird, H. M. Casey, L. J. Goodall, C. G. Ryan and R. J. Sloggett, Nucl. Instrum. Methods Phys. Res., Sect. A, 2010, 619, 306–310 CrossRef CAS PubMed.
- P. S. C. Tacon, in Soils, Stones and Symbols: Cultural Perceptions of the Mineral World, ed. N. O. M. A. Boivin, UCL Press, London, 2004, pp. 31–42 Search PubMed.
- A. J. Blee, K. Walshe, A. Pring, J. S. Quinton and C. E. Lenehan, Talanta, 2010, 82, 745–750 CrossRef CAS PubMed.
- T. Reeves, R. S. Popelka-Filcoff and C. E. Lenehan, Anal. Chim. Acta, 2013, 803, 194–203 CrossRef CAS PubMed.
- R. M. Cornell and U. Schewertmann, The Iron Oxides, Wiley-VCH Verlag GmbH &Co., Weinheim, 2003 Search PubMed.
- R. S. Popelka-Filcoff, C. Lenehan, K. Walshe, J. W. Bennett, A. Stopic, P. Jones, A. Pring, J. S. Quinton and A. Durham, Journal of the Anthropological Society of South Australia, 2012, 35, 81–90 Search PubMed.
- R. S. Popelka-Filcoff, C. E. Lenehan, M. D. Glascock, J. W. Bennett, A. Stopic, J. S. Quinton, A. Pring and K. Walshe, J. Radioanal. Nucl. Chem., 2012, 291, 19–24 CrossRef CAS.
- R. S. Popelka-Filcoff, A. Mauger, C. E. Lenehan, K. Walshe and A. Pring, Anal. Methods, 2014, 6, 1309–1316 RSC.
- U. Bergmann, P. L. Manning and R. A. Wogelius, in Annual Review of Analytical Chemistry, ed. R. G. Cooks and E. S. Yeung, Annual Reviews, Palo Alto, 2012, vol. 5, pp. 361–389 Search PubMed.
- L. Bertrand, L. Robinet, M. Thoury, K. Janssens, S. Cohen and S. Schöder, Appl. Phys. A, 2012, 106, 377–396 CrossRef CAS.
- D. L. Howard, M. D. de Jonge, D. Lau, D. Hay, M. Varcoe-Cocks, C. G. Ryan, R. Kirkham, G. Moorhead, D. Paterson and D. Thurrowgood, Anal. Chem., 2012, 84, 3278–3286 CrossRef CAS PubMed.
- K. Janssens, J. Dik, M. Cotte and J. Susini, Acc. Chem. Res., 2010, 43, 814–825 CrossRef CAS PubMed.
- L. Monico, G. van der Snickt, K. Janssens, W. de Nolf, C. Miliani, J. Dik, M. Radepont, E. Hendriks, M. Geldof and M. Cotte, Anal. Chem., 2011, 83, 1224–1231 CrossRef CAS PubMed.
- R. L. Green and R. J. Watling, J. Forensic Sci., 2007, 52, 851–859 CrossRef CAS PubMed.
- M. Jercher, A. Pring, P. G. Jones and M. D. Raven, Archaeometry, 1998, 40, 383–401 CrossRef CAS PubMed.
- M. Smith and B. Fankhauser, Geochemistry and identification of Australian red ochre deposits National Museum of Australia and Centre for Archaeological Research, Canberra, 2009 Search PubMed.
- R. Scadding, V. Winton and V. Brown, J. Archaeol. Sci., 2015, 54, 300–312 CrossRef CAS PubMed.
- D. C. Creagh, M. E. Kubik and M. Sterns, Nucl. Instrum. Methods Phys. Res., Sect. A, 2007, 580, 721–724 CrossRef CAS PubMed.
- P. Jones, Journal of the Anthropological Society of South Australia, 1984, 22, 3–10 Search PubMed.
- P. Jones, Journal of the Anthropological Society of South Australia, 1984, 22, 10–19 Search PubMed.
- P. Jones, Ochre and Rust: Artefacts and Encounters on Australian Frontiers, Wakefield Press Adelaide, 2007 Search PubMed.
- P. Jones and P. Sutton, Art and Land: Aboriginal Sculptures of The Lake Eyre Region, South Australian Museum and Wakefield Press, Adelaide, Australia, 1986 Search PubMed.
- M. F. Nobbs, Aboriginal painters of the Olary District of South Australia an ethnoecological study of the Lake Frome Plains and the adjoining uplands, with particular reference to the granite area of the Olary Uplands/By Margaret Nobbs, History, 1995, 1996.
- R. Popelka-Filcoff, C. Lenehan, M. Glascock, J. Bennett, A. Stopic, J. Quinton, A. Pring and K. Walshe, J. Radioanal. Nucl. Chem., 2012, 291, 19–24 CrossRef CAS.
- M. A. Smith and B. Fankhauser, An archaeological perspective on the geochemistry of Australian red ochre deposits: Prospects for fingerprinting major sources, Australian Institute of Aboriginal and Torres Strait Islander Studies, Canberra, 1996 Search PubMed.
- M. A. Smith and S. Pell, J. Archaeol. Sci., 1997, 24, 773–778 CrossRef.
- J. L. Keeling, Geological Survey: Bookartoo Ochre Deposit Sec. 85 HD. Parachilna 831, Department of Mines and Energy, South Australia, 1984 Search PubMed.
- N. Paterson and R. Lampert, Rec. Aust. Mus., 1985, 37, 1–9 CrossRef.
- J. Clarke, Stud. Conserv., 1976, 21, 134–142 CAS.
- C. G. Ryan, R. Kirkham, R. M. Hough, G. Moorhead, D. P. Siddons, M. D. de Jonge, D. J. Paterson, G. de Geronimo, D. L. Howard and J. S. Cleverley, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 619, 37–43 CrossRef PubMed.
- J. Clarke and N. North, in Rock Art and Posterity: Conserving, Managing and Recording Rock Art, ed. C. Pearson and B. K. Swartz Jr., Australian Rock Art Research Association, Melbourne, 1991, pp. 80–87 Search PubMed.
- J. Clarke and N. North, in Rock Art and Posterity: Conserving, Managing and Recording Rock Art, ed. C. Pearson and B. K. Swartz Jr., Australian Rock Art Research Association, Melbourne, 1991, pp. 88–92 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |