A novel amino acid analysis method using derivatization of multiple functional groups followed by liquid chromatography/tandem mass spectrometry
Abstract
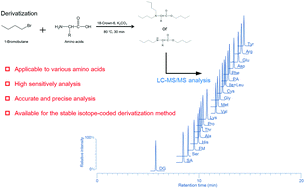
We have developed a novel amino acid analysis method using derivatization of multiple functional groups (amino, carboxyl, and phenolic hydroxyl groups). The amino, carboxyl, and phenolic hydroxyl groups of the amino acids were derivatized with 1-bromobutane so that the hydrophobicities and basicities of the amino acids were improved. The derivatized amino acids, including amino group-modified amino acids, could be detected with high sensitivity using liquid chromatography/tandem mass spectrometry (LC-MS/MS). In this study, 17 amino acids obtained by hydrolyzing proteins and 4 amino group-modified amino acids found in the human body (N,N-dimethylglycine, N-formyl-L-methionine, L-pyroglutamic acid, and sarcosine) were selected as target compounds. The 21 derivatized amino acids could be separated using an octadecyl-silylated silica column within 20 min and simultaneously detected. The detection limits for the 21 amino acids were 5.4–91 fmol, and the calibration curves were linear over the range of 10–100 nmol L−1 (r2 > 0.9984) with good repeatability. A confirmatory experiment showed that our proposed method could be applied to the determination of a protein certified reference material using the analysis of 12 amino acids combined with isotope dilution mass spectrometry. Furthermore, the proposed method was successfully applied to a stable isotope-coded derivatization method using 1-bromobutane and 1-bromobutane-4,4,4-d3 for comparative analysis of amino acids in human serum.

 Please wait while we load your content...
Please wait while we load your content...