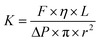Preparation of polyhedral oligomeric silsesquioxane based hybrid monoliths by thiol-ene click chemistry for capillary liquid chromatography
Shufen
Shen
,
Fanggui
Ye
*,
Cong
Zhang
,
Yuhao
Xiong
,
Linjing
Su
and
Shulin
Zhao
Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (Ministry of Education of China), College of Chemistry and Pharmaceutical Science of Guangxi Normal University, Guilin 541004, P. R. China. E-mail: fangguiye@163.com; Fax: +86-773-5832294; Tel: +86-773-5856104
First published on 29th September 2014
Abstract
A facile organic–silica hybrid monolith was prepared by a thiol-ene click reaction of polyhedral oligomeric silsesquioxane methacryl substituted (POSS-MA) with 1,4-bis(mercaptoacetoxy) butane (BMAB) using toluene and dodecanol as a porogenic system. The effects of the ratio of POSS-MA–BMAB and porogenic solvents and click reaction temperature on the morphology, permeability and column performance of the resulting POSS–BMAB hybrid monoliths were studied in detail. A uniform monolithic network with a high porosity was obtained. The POSS–BMAB hybrid monolith exhibited good permeability and high thermal and mechanical stability. A series of test compounds, including alkylbenzenes, polycyclic aromatic hydrocarbon, phenols and, anilines were used to evaluate the retention behaviors of the POSS–BMAB hybrid monolith in capillary liquid chromatography. The prepared POSS–BMAB hybrid monolith exhibited typical reversed-phase chromatographic behavior toward neutral solutes. The minimum plate height of this hybrid monolith was determined as 12.6 and 13.7 μm for thiourea and benzene, respectively. These results demonstrate that thiol-ene click chemistry can provide a facile and robust approach for the preparation of a POSS-based hybrid monolith.
1. Introduction
Monolithic columns have been developed as an alternative to classic particle packed columns for chromatographic separations in high performance liquid chromatography (HPLC), capillary liquid chromatography (cLC) and capillary electrochromatography (CEC).1–3 In comparison with traditional packed columns, monolithic columns possess a continuous porous structure, high porosity and a large through-pore size with small-size skeletons, resulting in good column permeability and high mass transfer efficiency. Based on the nature of the matrix, monolithic columns can be mainly categorized into three types: organic polymer-based, silica-based and organic–silica hybrid monolithic columns.4 In recent years, the organic–silica hybrid monolithic column has received more and more attention as it exhibits higher mechanical stability, better resistance to organic solvents and a wide-pH-range tolerance.5Over the last decade, click chemistry has attracted considerable attention for the preparation of chromatographic materials due to its advantages such as simplicity, compatibility with aqueous media, high reaction yields and specificity.6 The copper(I)-catalyzed alkyne–azide cycloaddition (CuAAC) was the first example of click chemistry, which is currently the most common click reaction used. Chu et al.7 have extensively studied the CuAAc reaction for the preparation of separation materials for HPLC and have reported the benefits of this reaction. However, the CuAAc reaction has some drawbacks such as leaving heavy metal ions in the stationary phase and the explosive nature of the azides used.8,9 Thiol-ene click reaction is a novel click chemistry reaction, which is a potential choice for copper-free click chemistry to complement the CuAAC reaction.10 The thiolether linkage formed serves as a strong and stable covalent bond, which is able to withstand harsh conditions. Thiol-ene click chemistry has been widely employed for the preparation of stationary phases in separation science. The most commonly used approach for elaboration of functionalized columns consists of a traditional two-step process in which a bare silica or polymer support is synthesized and subsequently post-modified via thiol-ene click chemistry.11–22 However, the process of post-modification is always time-consuming and boring, although diverse functional groups can be introduced onto the surface of the stationary phase with this strategy. The more straightforward approach is the so-called “one-pot” sol–gel process, where tetramethoxysilane and 3-mercaptopropyltrimethoxysilane are adopted as co-precursors and vinyl-containing organic monomers are used as functional moieties.23–26 Recently, Zou's group successfully developed a photoinduced thiol-ene polymerization reaction for the fast preparation of macroporous hybrid monoliths and their application in cLC.27
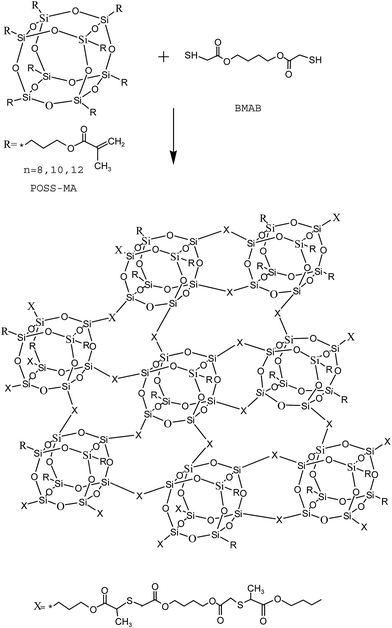
Cubic polyhedral oligomeric silsesquioxanes (POSS) with the unique cage-like structures and nanoscale dimensions has been used as a new cross-linker and has emerged as a candidate for the preparation of hybrid-silica monoliths.28 A POSS-based silica hybrid monolith has become one of the hottest topics in the field of hybrid organic–silica monoliths due to its unique properties, including facile polymerization, good rigidity, and abundant functional moieties for the flexible modification.29,30 With a simple in situ polymerization, many functionalized POSS-based silica hybrid monoliths have been fabricated conveniently with good robustness, high column efficiency and the absence of residual silanol groups, which can be used for cLC or CEC.31–34 Alves et al.35 have demonstrated the preparation of hybrid organic–inorganic monolithic materials employing vinylPOSS and multifunctional thiols by the step-growth mechanism of thiol-ene click chemistry. Polyhedral oligomeric silsesquioxane methacryl substituted (POSS-MA) is one kind of derivative of POSS with multiple vinyl groups, which can be used for thiol-ene click chemistry. However, to the best of our knowledge, no studies on thiol-ene click chemistry for preparation of POSS-based monolithic column for cLC application have been reported so far.
Herein, we developed a novel POSS-based hybrid monolith for cLC by thiol-ene click reaction of POSS-MA with BMAB. The synthetic procedure was simple and efficient without any special handling. The influences of the ratio of POSS-MA–BMAB and porogenic solvents and click reaction temperature on the morphology, permeability and column performances of the hybrid monoliths were investigated in detail. The obtained POSS–BMAB monolithic capillary columns exhibited good mechanical strength as well as solvent and thermal stability. The chromatographic performance of POSS–BMAB hybrid monolith for the separation of alkylbenzenes (ABs), polycyclic aromatic hydrocarbon (PAHs), phenols and anilines were evaluated in detail.
2. Experimental
2.1 Reagents and materials
Polyhedral oligomeric silsesquioxane methacryl substituted (cage mixture, n = 8, 10, 12, POSS-MA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). γ-Methacryloxypropyltrimethoxysilane (γ-MAPS) and dodecanol were purchased from Alfa Aesar (Ward Hill, MA, USA). 1,4-Bis(mercaptoacetoxy) butane (BMAB) and alkylbenzenes (ABs) were purchased from TCI (Shanghai, China). Azobisisobutyronitrile (AIBN) was obtained from Tianjin Chemistry Reagent Factory (Tianjin, China) and recrystallized in ethanol prior to use. Thiourea, polycyclic aromatic hydrocarbons (PAHs), phenols, anilines and HPLC-grade methanol (MeOH) and acetonitrile (ACN) were obtained from Shanghai Chemical Reagents Corporation (Shanghai, China). All other reagents were of analytical grade. Ultrapure water used for the preparation of solutions was produced by a Milli-Q water system (Millipore, Bedford, MA, USA). Fused-silica capillary of 75 μm i.d. and 375 μm o.d. was obtained from Hebei Yongnian Optical Fiber Factory (Hebei, China).2.2 Instrumentation
All chromatographic experiments were performed on a TriSep-2100 pressurized CEC system (this instrument can also be utilized as cLC system, Unimicro Technologies, Pleasanton, CA, USA). Fourier transform infrared (FT-IR) spectra (4000–400 cm−1) in KBr were recorded using a PE Spectrum One FT-IR spectrometer (PE, USA). Scanning electron microscopy (SEM) was carried out using a FEI Quanta 200 FEG SEM (Philips, Netherlands). Thermo gravimetric analysis (TGA) was performed using a Pyris Daimond TG (PE, USA). Micromeritics ASAP 2020M (Micromeritics, USA) was used to determine the Brunauer–Emmett–Teller (BET) surface areas of POSS–BMAB hybrid monoliths.2.3 Preparation of POSS–BMAB hybrid monolith
Prior to surface modification by thiol-ene click chemistry, a 50% γ-MAPS methanol (v/v) solution was used to introduce the vinyl groups onto the inner surface of capillary according to a previously described procedure.36The POSS-based hybrid monolith was prepared by using an in situ radical polymerization method. POSS-MA, BMAB, dodecanol, toluene and AIBN with different compositions (as listed in Table 1) were mixed under ultrasonication to form a homogeneous solution. Then the mixture was manually injected into the pretreated capillary to an appropriate length with a syringe. With both the ends sealed with rubber stoppers, the capillary was submerged into a thermostatic bath (50–60 °C) for 12 h. Finally, the obtained monolithic capillary columns were flushed with MeOH to remove the residual monomers and porogens.
| Column | POSS-MA–BMAB (mol/mol)a | Toluene (mg) | Dodecanol (mg) | T (°C) | Back pressure (MPa) | Permeability (×10−14 m2) | K (benzene) | BET surface area (m2 g−1) |
|---|---|---|---|---|---|---|---|---|
| a Other preparation conditions: POSS-MA, 50 mg; AIBN, 2 mg. | ||||||||
| A | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
110 | 200 | 55 | >25 (too hard to pump) | — | — | — |
| B | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
110 | 200 | 55 | 16.1 | 0.47 | 0.39 | 268.5 |
| C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
110 | 200 | 55 | 12.2 | 0.63 | 0.28 | 221.3 |
| D | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
110 | 200 | 55 | 1.3 | 6.16 | 0.17 | 130.5 |
| E | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 3.0 |
110 | 200 | 55 | 0.3 | 30.80 | 0.35 | 42.0 |
| F | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
110 | 200 | 50 | 0.8 | 9.33 | 0.38 | — |
| G | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
110 | 200 | 60 | 1.0 | 7.59 | 0.21 | — |
| H | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
70 | 240 | 55 | <0.2 (slack, detached) | — | — | — |
| I | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
90 | 220 | 55 | 0.4 | 22.73 | 0.36 | — |
| J | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
130 | 180 | 55 | >25 (too hard to pump) | — | — | — |
2.4 Calculations
The permeability (K) is calculated by the following formula:37where F is the volume flow rate of the eluent, η is the dynamic viscosity of the mobile phase, L is the column length, and ΔP is the pressure drop across the column. In this work, MeOH was used as the mobile phase and the corresponding value of dynamic viscosity was 0.580 × 10−3 kg (ms)−1 at 25 °C.38
3. Results and discussion
3.1 Preparation of POSS–BMAB hybrid monolith
The POSS–BMAB hybrid monolith was prepared by a simple single-step thiol-ene click reaction of POSS-MA with BMAB. Since the morphology and permeability of hybrid monoliths were significantly affected by the composition of the prepolymerization solution and reaction temperature, several parameters such as the ratio of POSS-MA–BMAB and porogenic solvents, and the click reaction temperature were systematically optimized as shown in Table 1 (Fig. 1).Herein, the prepolymerization solutions with the different molar ratios of POSS-MA–BMAB from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
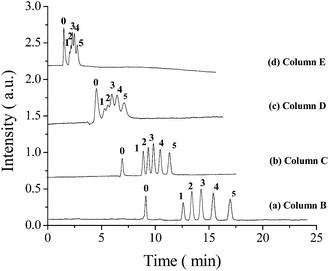
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were examined (column A–E, Table 1). It was observed that the back pressure and BET surface area of the hybrid monoliths gradually decreased with the decrease of the ratio of POSS-MA–BMAB, and the permeability of corresponding monoliths increased from 0.47 × 10−14 to 30.80 × 10−14 m2. The SEM images of column B–E were also obtained and are in Fig. 2. It could be seen that these hybrid monoliths have the morphology of a continuous skeleton and macro through-pores and good attachment to the inner wall of the capillary. It is interesting that the different ratio of POSS-MA–BMAB in the prepolymerization solution obviously affects the morphological structures of hybrid monoliths. For example, a continuous reticular skeleton was observed in column B and C, whereas the nanoglobular aggregated morphology was obtained in column D and E. This finding is similar to the results reported previously by Zou's group.27 The significant difference in morphology may be attributed to the phase separation process. The increase of BMAB crosslinker content may result in high local degree of polymerization and form crosslinked nanoglobular polymers separating from solvents.39Fig. 3 shows the separation of ABs on POSS–BMAB hybrid monoliths with different molar ratio of POSS-MA–BMAB. It was observed that increasing the molar ratio of POSS-MA–BMAB in the polymerization mixture improved retention and separation efficiency. Considering peak efficiency and retention of analytes, the molar ratio of POSS-MA–BMAB with 1
3 were examined (column A–E, Table 1). It was observed that the back pressure and BET surface area of the hybrid monoliths gradually decreased with the decrease of the ratio of POSS-MA–BMAB, and the permeability of corresponding monoliths increased from 0.47 × 10−14 to 30.80 × 10−14 m2. The SEM images of column B–E were also obtained and are in Fig. 2. It could be seen that these hybrid monoliths have the morphology of a continuous skeleton and macro through-pores and good attachment to the inner wall of the capillary. It is interesting that the different ratio of POSS-MA–BMAB in the prepolymerization solution obviously affects the morphological structures of hybrid monoliths. For example, a continuous reticular skeleton was observed in column B and C, whereas the nanoglobular aggregated morphology was obtained in column D and E. This finding is similar to the results reported previously by Zou's group.27 The significant difference in morphology may be attributed to the phase separation process. The increase of BMAB crosslinker content may result in high local degree of polymerization and form crosslinked nanoglobular polymers separating from solvents.39Fig. 3 shows the separation of ABs on POSS–BMAB hybrid monoliths with different molar ratio of POSS-MA–BMAB. It was observed that increasing the molar ratio of POSS-MA–BMAB in the polymerization mixture improved retention and separation efficiency. Considering peak efficiency and retention of analytes, the molar ratio of POSS-MA–BMAB with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (column B in Table 1) was chosen for further experiments.
1.5 (column B in Table 1) was chosen for further experiments.
 | ||
| Fig. 2 SEM images of hybrid monoliths of column B (a and b), column C (c and d), column D (e and f) and column E (g and h). | ||
Similar to the other radical polymerization synthesis of polymer monoliths, the thiol-ene click reaction temperature is an important factor in the formation of hybrid monoliths. In this experiment, the effect of thiol-ene click reaction temperature on the permeability, back pressure and retention value of benzene are shown in Table 1. High permeability but low column efficiency and resolution were obtained as the click reaction temperature was set at 50 and 60 °C (Fig. 4a and c). In contrast, a good chromatographic performance was observed on column B with a dense reticular monolithic network when a reaction temperature of 55 °C was applied (Fig. 4b).
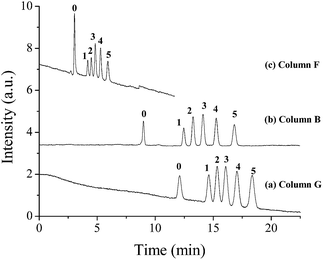 | ||
| Fig. 4 Effect of thiol-ene click reaction temperature on separation of ABs on POSS–BMAB hybrid monolith. Conditions: supplement pressure, 1000 psi (a and b), 75 psi (c). Other conditions are the same as in Fig. 3. | ||
A binary porogenic system of toluene–dodecanol was employed, where toluene and dodecanol acted as a good solvent for POSS-MA and BMAB, respectively. As shown in Table 1, the ratio of toluene–dodecanol was investigated from 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 to 130
240 to 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 (w/w). The results demonstrate that the ratio of toluene–dodecanol higher than 130
180 (w/w). The results demonstrate that the ratio of toluene–dodecanol higher than 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 results in high back pressure of more than 25.0 MPa. Contrarily, the morphology of hybrid monolith was too slack and detached from the capillary inner wall when using the ratio of toluene–dodecanol lower than 70
180 results in high back pressure of more than 25.0 MPa. Contrarily, the morphology of hybrid monolith was too slack and detached from the capillary inner wall when using the ratio of toluene–dodecanol lower than 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240. Fig. 5 showed the effect of the ratio of toluene–dodecanol on the separation of ABs on POSS–BMAB hybrid monoliths. By comparing the separation efficiency for ABs on columns B and I, ABs were co-eluted with column I whereas baseline resolution was observed for column B. Based on above experimental results, column B was considered to be the optimal, and chosen for further experiments.
240. Fig. 5 showed the effect of the ratio of toluene–dodecanol on the separation of ABs on POSS–BMAB hybrid monoliths. By comparing the separation efficiency for ABs on columns B and I, ABs were co-eluted with column I whereas baseline resolution was observed for column B. Based on above experimental results, column B was considered to be the optimal, and chosen for further experiments.
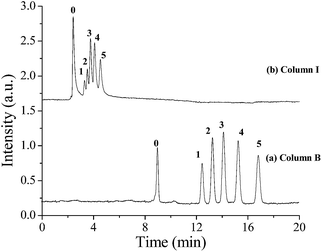 | ||
| Fig. 5 Effect of the ratio of toluene–dodecanol on separation of ABs on POSS–BMAB monoliths. Conditions: supplement pressure, 1000 psi (a), 100 psi (b). Other conditions are the same as in Fig. 3. | ||
3.2 Characterization of POSS–BMAB hybrid monolith
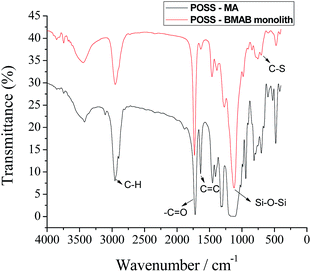
FT-IR spectroscopic analysis was used to characterize thiol-ene click reaction. As can be seen from Fig. 6, the signals of characteristic peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretches (1630 cm−1), which are associated with the presence of vinyl groups of the POSS-MA monomer, are almost completely absent. In addition, there is appearance of a new C–S adsorption (700 cm−1) associated with the covalent linkage between POSS-MA and BMAB. These results confirmed the thiol-ene click reaction of POSS-MA monomer with the BMAB crosslinker.
C stretches (1630 cm−1), which are associated with the presence of vinyl groups of the POSS-MA monomer, are almost completely absent. In addition, there is appearance of a new C–S adsorption (700 cm−1) associated with the covalent linkage between POSS-MA and BMAB. These results confirmed the thiol-ene click reaction of POSS-MA monomer with the BMAB crosslinker.
The thermal stability of POSS–BMAB hybrid monolith was investigated by TGA. As can be seen from Fig. 7a, an endothermic mass loss begins at 360 °C and continues up to 600 °C as a result of the pyrolysis of organic moieties, indicating good thermostability of the POSS–BMAB hybrid monolith. Column pressure drops were measured using MeOH to evaluate the mechanical stabilities of the synthesized POSS–BMAB hybrid monolith. As can be seen from Fig. 7b, a good linear dependence of flow rate on column back pressure even at high pressure was observed, indicating these monoliths have good mechanical stability.
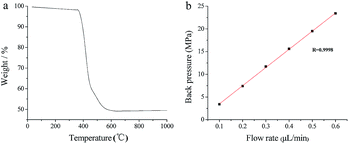 | ||
| Fig. 7 TGA of POSS–BMAB hybrid monolith at a heating rate of 10 °C min−1 in air (a) and the relationships between flow rate and the back pressure drop of POSS–BMAB hybrid monolith (b). | ||
To evaluate POSS–BMAB hybrid monolith column efficiency, the dependence of column efficiency on the linear velocity of the mobile phase was investigated. Fig. 8 shows the Van Deemter plots of thiourea and benzene. The minimum plate heights of 12.6 and 13.7 μm were achieved for thiourea and benzene, which corresponds to the column efficiencies of 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and 73
400 and 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plates per m, respectively. Compared with the previous report34 on poly(POSS-co-bisphenol A dimethacrylate) monolith with 23
000 plates per m, respectively. Compared with the previous report34 on poly(POSS-co-bisphenol A dimethacrylate) monolith with 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600–27
600–27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 plates per m for five ABs, the proposed POSS–BMAB hybrid monolith exhibited higher column performance. In addition, it can be seen that the column efficiency was satisfactory over a range of linear velocity from 0.3 to 0.9 mm s−1. Moreover, the good reproducibility for column B was demonstrated with the intra-day RSDS (≤0.5%, n = 5) and day-by-day RSDS (≤1.5%, n = 5) for the retention factors of ABs. The column-to-column reproducibility was similarly evaluated through the RSD for retention factor of ABs at the same conditions. It was calculated that the reproducibility based on column-to-column preparation from the same batch of polymerization solution was less than 5.3% (n = 3). No significant decline of column efficiency (<4.5%) and retention factors (<3.6%) of five ABs were observed during the course of the stability study when comparing cLC runs performed at the start of the study and after performing over 200 injections within two months. These results indicate that the prepared POSS–BMAB hybrid monolith has good stability and reproducibility.
200 plates per m for five ABs, the proposed POSS–BMAB hybrid monolith exhibited higher column performance. In addition, it can be seen that the column efficiency was satisfactory over a range of linear velocity from 0.3 to 0.9 mm s−1. Moreover, the good reproducibility for column B was demonstrated with the intra-day RSDS (≤0.5%, n = 5) and day-by-day RSDS (≤1.5%, n = 5) for the retention factors of ABs. The column-to-column reproducibility was similarly evaluated through the RSD for retention factor of ABs at the same conditions. It was calculated that the reproducibility based on column-to-column preparation from the same batch of polymerization solution was less than 5.3% (n = 3). No significant decline of column efficiency (<4.5%) and retention factors (<3.6%) of five ABs were observed during the course of the stability study when comparing cLC runs performed at the start of the study and after performing over 200 injections within two months. These results indicate that the prepared POSS–BMAB hybrid monolith has good stability and reproducibility.
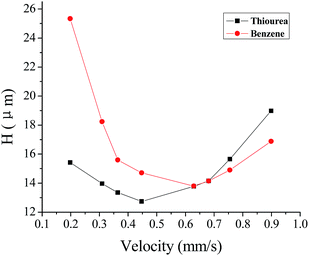 | ||
| Fig. 8 Dependence of the plate height of analytes on the linear velocity of mobile phase by the column B. Conditions: mobile phase, ACN–H2O (75/25, v/v). Other conditions are the same as in Fig. 3. | ||
Owing to the hydrophobicity of the POSS-MA, the resulting hybrid monoliths were applicable to chromatographic separation in reversed-phase (RP) mode. The retention properties of POSS–BMAB hybrid monolith were investigated using ABs as model compounds. Typical baseline separation of a mixture of ABs was showed in Fig. 5a. The retention and the elution order of ABs are benzene < toluene < ethylbenzene < propylbenzene < butylbenzene, which is well in agreement with the hydrophobicity of the ABs. Effect of varying ACN content in the mobile phases in the range of 60 to 85% (v/v) on the retention factors of ABs was investigated. It appears that lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k almost linearly decreases with increasing organic modifier composition, where all linear correlation coefficients (R) were higher than 0.9941 (Fig. 9). All these effects can be deduced that the separation of ABs on the POSS–BMAB hybrid monolith was based on typical RP chromatographic retention mechanism.
k almost linearly decreases with increasing organic modifier composition, where all linear correlation coefficients (R) were higher than 0.9941 (Fig. 9). All these effects can be deduced that the separation of ABs on the POSS–BMAB hybrid monolith was based on typical RP chromatographic retention mechanism.
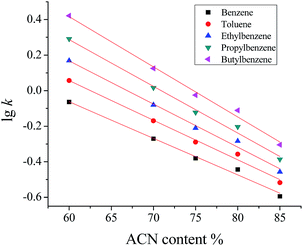 | ||
Fig. 9 The effect of ACN content in the mobile phase on the retention factor lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k of ABs on column B. Conditions are the same as in Fig. 3. k of ABs on column B. Conditions are the same as in Fig. 3. | ||
3.3 Chromatographic performance
The chromatographic performance of POSS–BMAB hybrid monolith was investigated by separating PAHs, phenols and anilines in cLC. Fig. 10 represents the separation of five PAHs in cLC using ACN–H2O (90/10, v/v) as mobile phase. The five PAHs were well separated with column efficiency up to 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–69
800–69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 plates per m according to their hydrophobicity, in order from low to high, which indicates the retention mechanism is based on hydrophobic interactions.
900 plates per m according to their hydrophobicity, in order from low to high, which indicates the retention mechanism is based on hydrophobic interactions.
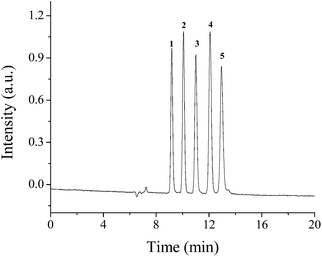 | ||
| Fig. 10 Separations of PAHs on the column B in RP-cLC. Conditions: mobile phase, ACN–H2O (90/10, v/v). Other conditions are the same as in Fig. 3. Solutes 1, naphthalene; 2, fluorene; 3, phenanthrene; 4, fluoranthene; 5, pyrene. | ||
The prepared hybrid monoliths were also applied for separation of weakly acidic polar compounds. A mixture of six phenols was used to survey the chromatographic performance of the POSS–BMAB hybrid monolith. As can be seen from Fig. 11, good separation of all these weakly acidic polar compounds was achieved with the mobile phase containing ACN–H2O (55/45, v/v). The high column efficiency for all phenols ranged from 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 to 69
600 to 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 plates per m.
200 plates per m.
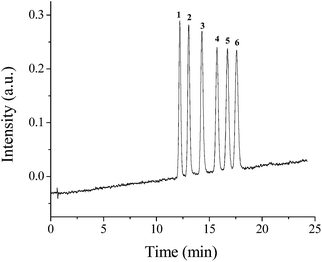 | ||
| Fig. 11 Separations of phenols on the column B. Conditions: mobile phase, ACN–H2O (55/45, v/v). Other conditions are the same as in Fig. 3. Solutes 1, phloroglucinol; 2, hydroquinone; 3, 2-aminophenol; 4, 4-methoxyphenol; 5, phenol; 6, 4-nitrophenol. | ||
It is well known that the separation of basic compounds is a troublesome task on traditional silica-based stationary phase in HPLC due to the secondary interactions between basic analytes and residual silanol groups of the stationary phases, which often lead to peak tailing and increased retention. However, this problem should be eliminated due to the neutral surface character of the POSS–BMAB hybrid without silanol groups. The separation of weak basic compounds on the POSS–BMAB hybrid monolith was also investigated. Fig. 12 presents the separation of six basic anilines on POSS–BMAB hybrid monolith. Good peak shape and high separation efficiency were observed for the separation of six basic analytes on POSS–BMAB hybrid monolith without adding any competing amines in the mobile phase. For instance, the tailing factors and column efficiency of six anilines were in the range of 1.05–1.18 and 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–64
800–64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 plates per m, respectively. These results demonstrated the higher separation ability of the POSS–BMAB hybrid monolith in RP-cLC.
500 plates per m, respectively. These results demonstrated the higher separation ability of the POSS–BMAB hybrid monolith in RP-cLC.
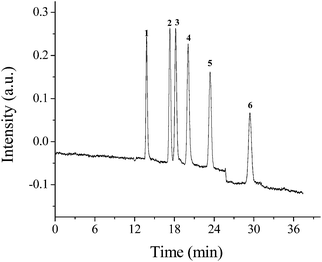 | ||
| Fig. 12 Separations of anilines on the column B in cLC. Conditions: mobile phase, ACN–H2O (65/35, v/v). Other conditions are the same as in Fig. 5. Solutes 1, o-phenylenediamine; 2, aniline; 3, p-toluidine; 4, benzidine; 5, 2,4-dinitroaniline; 6, 1-aminonaphthalene. | ||
4. Concluding remarks
In this work, a novel POSS-based hybrid monolith was facilely synthesized by thiol-ene click reaction of POSS-MA with BMAB. The synthetic method was very simple and convenient. The obtained POSS–BMAB hybrid monolith exhibited good permeability and thermostability and high mechanical stability. A typical RP chromatographic retention mechanism was observed for separation of the neutral analytes such as ABs. The POSS–BMAB hybrid monolith also demonstrated its excellent chromatographic performance for separation of ABs, PAHs, phenols and anilines in RP-cLC mode. These successful separations suggest that the proposed hybrid monolith is a good separation media for complex sample analysis.Acknowledgements
The financial support from the National Natural Science Foundation of China (nos 21065002, 21365005), Guangxi Natural Science Foundation of China (2012GXNSFAA053024, 2014GXNSFGA118002) and Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (Guangxi Normal University), and Ministry of Education of China (CMEMR2014-A09) are gratefully acknowledged.Notes and references
- R. D. Arrua, T. J. Causon and E. F. Hilder, Analyst, 2012, 137, 5179 RSC.
- T. L. Chester, Anal. Chem., 2013, 85, 579 CrossRef CAS PubMed.
- P. Aggarwal, H. D. Tolley and M. L. Lee, J. Chromatogr., A, 2012, 1219, 1 CrossRef CAS PubMed.
- M. Wu, R. Wu, Z. Zhang and H. Zou, Electrophoresis, 2011, 32, 105 CrossRef CAS PubMed.
- J. Ou, H. Lin, Z. Zhang, G. Huang, J. Dong and H. Zou, Electrophoresis, 2013, 34, 126 CrossRef CAS PubMed.
- A. Marechal, R. El-Debs, V. Dugas and C. Demesmay, J. Sep. Sci., 2013, 36, 2049 CrossRef CAS PubMed.
- C. Chu and R. Liu, Chem. Soc. Rev., 2011, 40, 2177 RSC.
- Z. M. Guo, A. W. Lei, Y. P. Zhang, Q. Xu, X. Y. Xue, F. F. Zhang and X. M. Liang, Chem. Commun., 2007, 2491 RSC.
- S. Bräse, C. Gil, K. Knepper and V. Zimmermann, Angew. Chem., Int. Ed., 2005, 44, 5188 CrossRef PubMed.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540 CrossRef CAS PubMed.
- A. Shen, Z. Guo, L. Yu, L. Cao and X. Liang, Chem. Commun., 2011, 47, 4550–4552 RSC.
- Y. Chen, M. Wu, K. Wang, B. Chen, S. Yao, H. Zou and L. Nie, J. Chromatogr., A, 2011, 1218, 7982 CrossRef CAS PubMed.
- Y. Chen, K. Wang, H. Yang, Y. Liu, S. Yao, B. Chen, L. Nie and G. Xu, J. Chromatogr., A, 2012, 1233, 91 CrossRef CAS PubMed.
- K. Wang, Y. Chen, H. Yang, Y. Li, L. Nie and S. Yao, Talanta, 2012, 91, 52 CrossRef CAS PubMed.
- Y. Lv, Z. Lin and F. Svec, Analyst, 2012, 137, 4114 RSC.
- X. Peng, T. Liu, S. Ji and Y. Feng, J. Sep. Sci., 2013, 36, 2571 CrossRef CAS PubMed.
- A. Shen, X. Li, X. Dong, J. Wei, Z. Guo and X. Liang, J. Chromatogr., A, 2013, 1314, 63 CrossRef CAS PubMed.
- X. Cheng, X. Peng, Q. Yu, B. Yuan and Y. Feng, J. Chromatogr., A, 2013, 1302, 81 CrossRef CAS PubMed.
- I. Tijunelyte, J. Babinot, M. Guerrouache, G. Valincius and B. Carbonnier, Polymer, 2012, 53, 29 CrossRef CAS PubMed.
- A. Laaniste, A. Marechal, R. El-Debs, J. Randon, V. Dugas and C. Demesmay, J. Chromatogr., A, 2014, 1355, 296 CrossRef CAS PubMed.
- X. Yao, T. Thatt, Y. Tan and Y. Wang, J. Chromatogr., A, 2014, 1326, 80 CrossRef CAS PubMed.
- Z. Liu, J. Ou, H. Lin, H. Wang, J. Dong and H. Zou, J. Chromatogr., A, 2014, 1342, 70 CrossRef CAS PubMed.
- Y. Liu, Y. Chen, H. Yang, L. Nie and S. Yao, J. Chromatogr., A, 2013, 1283, 132 CrossRef CAS PubMed.
- M. Chen, J. Zhang, Z. Zhang, B. Yuan, Q. Yu and Y. Feng, J. Chromatogr., A, 2013, 1284, 118 CrossRef CAS PubMed.
- Z. Lin, X. Tan, R. Yu, J. Lin, X. Yin, L. Zhang and H. Yang, J. Chromatogr., A, 2014, 1355, 228 CrossRef CAS PubMed.
- H. Yang, Y. Chen, Y. Liu, L. Nie and S. Yao, Electrophoresis, 2013, 34, 510 CrossRef CAS PubMed.
- Z. Liu, J. Ou, H. Lin, Z. Liu, H. Wang, J. Dong and H. Zou, Chem. Commun., 2014, 50, 9288 RSC.
- M. Wu, R. Wu, R. Li, H. Qin, J. Dong, Z. Zhang and H. Zou, Anal. Chem., 2010, 82, 5447 CrossRef CAS PubMed.
- X. Lin, N. Zheng, J. Wang, X. Wang, Y. Zheng and Z. Xie, Analyst, 2013, 138, 5555 RSC.
- H. Lin, J. Ou, Z. Zhang, J. Dong and H. Zou, Chem. Commun., 2013, 49, 23 Search PubMed.
- H. Lin, Z. Zhang, J. Dong, Z. Liu, J. Ou and H. Zou, J. Sep. Sci., 2013, 36, 2819 CrossRef CAS PubMed.
- X. Lin, X. Wang, T. Zhao, Y. Zheng, S. Liu and Z. Xie, J. Chromatogr., A, 2012, 1260, 174 CrossRef CAS PubMed.
- X. Xiong, Z. Yang, Y. Li, L. Xiao, L. Jiang, Y. Chen, M. Ma and B. Chen, J. Chromatogr., A, 2013, 1304, 85 CrossRef CAS PubMed.
- J. Ou, Z. Zhanga, H. Lin, J. Dong and H. Zou, Anal. Chim. Acta, 2013, 761, 209 CrossRef CAS PubMed.
- F. Alves and I. Nischang, Chem. - Eur. J., 2013, 19, 17310 CrossRef CAS PubMed.
- J. Ou, G. T. T. Gibson and R. D. Oleschuk, J. Chromatogr., A, 2010, 1217, 3628 CrossRef CAS PubMed.
- P. A. Bristow and J. H. Knox, Chromatographia, 1977, 10, 279 CAS.
- P. S. Nikam, L. N. Shirsat and M. Hasan, J. Chem. Eng. Data, 1998, 43, 732 CrossRef CAS.
- C. E. Hoyle, T. Y. Lee and T. Roper, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5301 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |