Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Large, heterometallic coordination cages based on ditopic metallo-ligands with 3-pyridyl donor groups†
Matthew D.
Wise
a,
Julian J.
Holstein
bf,
Philip
Pattison
cd,
Celine
Besnard
e,
Euro
Solari
a,
Rosario
Scopelliti
a,
Gerard
Bricogne
f and
Kay
Severin
*a
aInstitut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland. E-mail: kay.severin@epfl.ch; Fax: +41 21-693-9305
bGZG, Abteilung Kristallographie, Georg-August-Universität Göttingen, Goldschmidtstr. 1, 37077 Göttingen, Germany
cSwiss-Norwegian Beamline, ESRF, F-38043 Grenoble, Switzerland
dLaboratory of Crystallography, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
eLaboratory of X-ray Crystallography, University of Geneva, CH-1211-Geneva 4, Switzerland
fGlobal Phasing Ltd., Sheraton House, Castle Park Cambridge CB3 0AX, England
First published on 11th November 2014
Abstract
Ditopic N-donor ligands with terminal 4-pyridyl groups are omnipresent in coordination-based self-assembly. The utilization of ligands with 3-pyridyl donor groups is significantly less common, because the intrinsic conformational flexibility of these ligands tends to favor the formation of small aggregates. Here, we show that large Pd6L1212+ cages can be obtained by reaction of Pd(II) salts with metallo-ligands L bearing terminal 3-pyridyl groups. The easy-to-access metallo-ligands contain an Fe(II) clathrochelate core. These sterically demanding clathrochelate complexes prevent the formation of smaller aggregates, which is observed for less bulky analogous building blocks. The cages were shown to bind BF4− and BPh4− anions in aqueous solvent mixtures, whilst the lateral size of the clathrochelate significantly affects their guest encapsulation behavior.
Introduction
Coordination-based self-assembly is underpinned by a set of well-established design principles. These geometrical tenets have enabled chemists to rationally target molecular architectures possessing an enormous range of structural and functional characteristics.1,2 The directional bonding approach is perhaps the most intuitive design strategy.1g,3 This approach entails the combination of donor (ligand) and acceptor (metal complex) units, the geometries of which determine the structure of the assembly formed. A fundamental requirement of this strategy is that these building blocks are shape-persistent; possessing conformational rigidity and well-defined coordinate vectors. The self-assembly behavior of building blocks without these characteristics is less predictable and, consequently, their strategic incorporation into supramolecular structures inherently difficult.Ditopic N-donor ligands with terminal 4-pyridyl groups are amongst the most extensively exploited building blocks in the preparation of coordination-based supramolecular assemblies. The simplest member of this family of tectons is 4,4′-bipyridine, which has been incorporated into countless discrete1 and polymeric self-assembled structures.3 The insertion of a linker group between the 4-pyridyl moieties has led to ever more elaborate assemblies.4,5 In contrast to the ubiquity of 4-pyridyl terminated N-donor ligands, closely related 3-pyridyl terminated ligands are far less common.1–3Scheme 1 alludes to why this is the case. The coordinate vectors of a generic ditopic N-donor ligand L1 with terminal 4-pyridyl groups are fixed, regardless of the linking group R between the heterocycles, provided that R is rigid. Free rotation about the R–pyridine bond does not affect the orientation of the non-bonding sp2 orbitals of the N atoms (Scheme 1a). However, in the case of a generic 3-pyridyl terminated ligand L2, rotation about this bond changes the coordinate vectors of the pyridine N atoms (Scheme 1b).
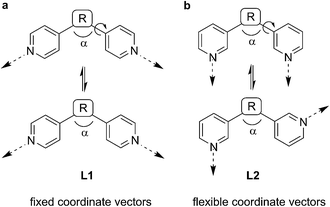 | ||
| Scheme 1 For ditopic ligands L2 with 3-pyridyl donor groups, the relative orientation of the coordinate vectors is more flexible than for ligands L1 with 4-pyridyl donor groups. | ||
As a consequence of the conformational flexibility of 3-pyridyl terminated N-donor ligands, entropically favored small6 aggregates are obtained upon coordination to metal ions. Several research groups have shown that the combination of Pd2+ with ‘banana-shaped’ ligands of type L2 (α < 180°) typically results in the formation of dinuclear aggregates of the formula [Pd2(L2)4]4+ (Scheme 2).7,8 Fujita and co-workers have examined the reaction of Pd2+ with linear ligands of type L2 (α = 180°) and observed the formation of [Pd3(L2)6]6+ and [Pd4(L2)8]8+ complexes.9 These results are in contrast to reactions of Pd2+ with ligands of type L1, which were found to give polynuclear coordination cages of type [Pd12(L1)24]24+ and [Pd24(L1)48]48+.4 Inspection of the assemblies obtained with L2-type ligands reveals that they all feature Pd(L2)2Pd macrocycles as part of their structure.10 We hypothesized that increasing the steric bulk of L2-type ligands would overcome their entropic propensity to form small aggregates. Bulky lateral R group substituents should disfavor Pd(L2)2Pd macrocycles and thus result in more expanded assemblies. Below we show that this strategy can indeed be used to access unprecedented, large coordination cages based on ditopic 3-pyridyl ligands. In particular, we demonstrate that clathrochelate-based metalloligands enable the synthesis of octahedral [Pd6(L2)12]12+ cages with a diameter of more than 20 Å. Despite the lateral size of the metalloligands, the encapsulation of reasonably large guest molecules such as tetraphenylborate (BPh4−) is possible.
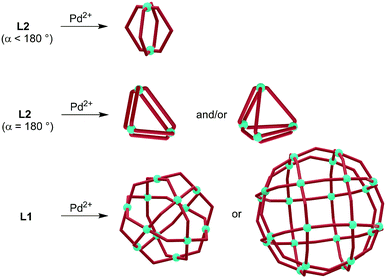 | ||
| Scheme 2 Reactions of Pd2+ with ligands of type L2 give di-, tri- and tetranuclear aggregates, whereas large coordination cages are obtained upon reaction with ligands of type L1. | ||
Results and discussion
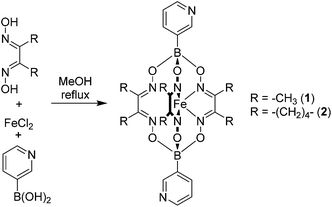
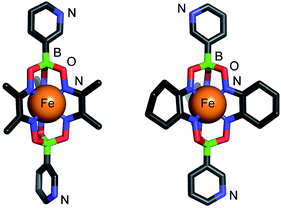
Bipyridyl ligands 1 and 2 were isolated in 96% and 79% yield respectively, following a one-pot synthesis similar to that previously described for 4-pyridyl functionalized clathrochelates (Scheme 3).11 All the starting materials used in this procedure—1,2-cyclohexanedione dixoime (nioxime) or dimethylglyoxime, anhydrous iron(II) chloride and pyridin-3-ylboronic acid—are commercially available.Single crystal X-ray diffraction unambiguously confirmed the formation of 1 and 2 (Fig. 1). The crystal structures reveal the conformational flexibility of the coordination vectors of these 3-pyridyl appended ligands. In 1, the two planes of the pyridine rings are close to orthogonal (85.8°). Conversely, in 2, they are almost coplanar (7.8°), yet the nonbonding sp2 orbitals of the pyridine N atoms are essentially parallel and divergent. The bond lengths and angles found for the trigonal prismatic Fe core of 1 and 2 are within the range observed for other Fe-based clathrochelate complexes.12
The inherent conformational flexibility of 1 and 2 makes the prediction of the structure of an assembly comprising these building blocks difficult, even when combined with a metal acceptor of well-defined geometry, such as a naked Pd2+ ion (square planar geometry). As outlined above, we envisaged that the considerable lateral steric bulk of 1 and 2 would play a structure-directing role and prevent the formation of macrocyclic Pd(L2)2Pd motifs. Consequently, we anticipated that larger cage structures could be formed by combination of 1 or 2 and a naked Pd2+ ion, rather than small aggregates.
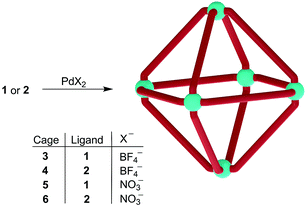
NMR-scale experiments were subsequently undertaken to investigate this hypothesis. A solution of [Pd(CH3CN)4](BF4)2 in acetonitrile-d3 was added to two equivalents of solid 1 or 2, both of which are poorly soluble in acetonitrile-d3 at room temperature, giving a pale yellow suspension. These samples were then heated at 70 °C and 1H NMR spectra were recorded periodically. Over time, the reaction mixtures progressively became less turbid and turned red. After 3 hours, neither solution contained any precipitate and a single set of sharp peaks was observed in the 1H NMR spectrum in both cases, suggesting the formation of a single highly symmetric, discrete species (ESI, Fig. S19 and S20†). The syntheses were subsequently performed on a preparative scale, without deuterated solvent (Scheme 4). The ESI-MS spectra of the products show major peaks which can be attributed to complexes of the formula (Pd6(L2)12) (BF4)n12−n+ (n = 4–6, L2 = 1 or 2, ESI Fig. S1 and S2†). This result confirmed that higher order assemblies (3, 4) had indeed been formed from 1 and 2 respectively, rather than small aggregates. The complexes 5 and 6 were prepared in an analogous fashion by heating a suspension of 1 or 2 and Pd(NO3)2(H2O)n in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetonitrile
1 mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water for 2 hours at 70 °C. The 1H NMR spectra once more showed a single set of signals for each product and ESI-MS confirmed the molecular formulas of the cations to be [(Pd6(1)12)]12+ and [(Pd6(2)12)]12+ (ESI, Fig. S3 and S4†). Isolation of all complexes was achieved by precipitation with diethyl ether (isolated yields: 82–94%).
water for 2 hours at 70 °C. The 1H NMR spectra once more showed a single set of signals for each product and ESI-MS confirmed the molecular formulas of the cations to be [(Pd6(1)12)]12+ and [(Pd6(2)12)]12+ (ESI, Fig. S3 and S4†). Isolation of all complexes was achieved by precipitation with diethyl ether (isolated yields: 82–94%).
Single crystals of 3 suitable for X-ray diffraction were grown by diffusion of diethylether into a solution of the assembly in acetonitrile, whilst those of 5 and 6 were grown by layering an acetonitrile solution onto toluene. Synchrotron radiation was required to obtain diffraction data of sufficient quality, which were collected at the Swiss Norwegian Beamline at ESRF Grenoble. Assemblies of this size pose a number of difficulties due in part to their extensive inherent disorder and high solvent content within the crystal. Synchrotron radiation mitigates some of these difficulties, but we have also employed a series of carefully and rigorously adapted macromolecular refinement techniques13 in order to build a molecular model (see ESI† for details).
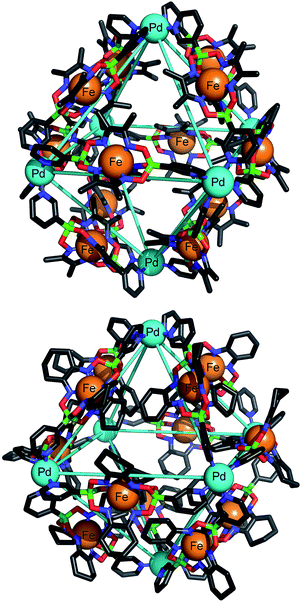
Fig. 2 shows the molecular structures of 5 and 6 in the crystal (the structure of 3 is similar to that of 5 and thus not shown). In all structures, the six Pd2+ ions of the assembly form an octahedron of close to regular geometry, with Pd–Pd distances of 2.19–2.32 nm across the body of the framework, and 1.52–1.63 nm within each triangular face. The internal angles of the facial triangles vary from 57.3° to 61.4°. The structural regularity of the Pd framework is not, however, reflected in the conformation of the bridging ligands themselves. In 5, the planes of the pyridine rings of the six ligands along the edges of two opposing faces of the octahedral framework deviate significantly from parallel (plane dihedral angles 90.0°, 89.2° and 66.8°). In contrast, the planes of the pyridine rings of the remaining six ligands are much closer to parallel (20.6°, 9.3° and 13.7°). The situation is yet more disordered in the cases of 3 and 6, where a range of dihedral angles between pyridine rings within each clathrochelate is observed (10.8–89.9°). This illustrates the intrinsic conformational flexibility of ligands of the type L2. Their variable coordination vectors determine the most energetically favorable conformation of the assembly as a whole, and enable this arrangement to be attained.
However, the different conformations adopted by 1 and 2 in the self-assembled architectures 3–6 are not manifested in the solution phase NMR spectra at room temperature. Only one set of peaks is visible, implying that the pyridyl rings of the ligands 1 and 2, as well as the tris(dioxime) clathrochelate core, undergo rapid conformational changes (on the NMR timescale). The 1H NMR spectra of 3 and 4 were also recorded at 233 K (CD3CN). Significant line broadening was observed in all cases (ESI, Fig. S23 and S24†). The extent of line broadening was far greater in the case of 4 than 3. This result is unsurprising, given the bulky cyclohexyl substituents of ligand 2 impose a higher steric barrier to rotation than the relatively small methyl substituents of ligand 1.
The octahedral cages possess internal void space, which is largely occupied by solvent molecules and anions (both ordered and disordered) in the crystal. The X-ray structures of 3, 5 and 6 show NO3− or BF4− anions in interior binding pockets adjacent to the Pd2+ ions at the vertices of the octahedral assemblies. The presence of close anion–Pd contacts is in line with what has been observed for many cages of type [Pd2(L2)4]4+.7 Simple geometric approximations reveal the volume of the octahedra described by the six Pd2+ ions in 3, 5 and 6 to be ∼5.2 nm3. However, much of the interior volume of the assembly is occupied by clathrochelate oxime substituents.14 The influence of the lateral size of the clathrochelate complexes over the void volume within each assembly was quantified by VOIDOO calculations (see ESI† for details).15 In the solid state, assembly 3 possesses a cavity volume of 1.4 nm3 or 1.8 nm3 (two independent cages found in the asymmetric unit of the crystal structure), cage 5 a cavity volume of 1.3 nm3 and cage 6 a much smaller cavity volume of 1.1 nm3. From these values it is clear that it is not only the oxime substituents that significantly affect the internal space within each assembly, but also the conformation of the clathrochelate metalloligands. However, given the dynamic and fluxional nature of these cages evidenced by 1H NMR, this conformational influence was not expected to contribute to the solution phase void volume, and hence guest binding behavior, of assemblies 3–6. Any differences in solution phase behavior can be attributed to the oxime substituents alone. To investigate whether oxime steric bulk would influence the kinetics and thermodynamics of guest binding, 19F NMR spectra of 3 and 4 were recorded at 298 K in acetonitrile-d3. A single peak was observed at −150.55 ppm for the BF4− anion in 3, whereas peaks at −146.37 ppm and −151.98 ppm were observed in the case of 4 (referenced to hexafluorobenzene at −164.90 ppm). These data suggest that the rate of exchange between internal and external BF4− is fast on the NMR timescale at 298 K in the case of 3 (one signal), but slow in the case of 4 (two signals). 19F NMR spectra of 3 and 4 were subsequently recorded over a range of temperatures (see ESI, Fig. S25 and S26†). These experiments revealed a coalescence temperature of approximately 255 K for the BF4− anion signals of 3, and 345 K for those of 4. The difference of 90 K corresponds to a difference of ∼16 kJ mol−1 for the activation free energy16 of the anion exchange process and neatly illustrates one key characteristic of clathrochelate-based building blocks in general—the ease with which we can modulate their steric bulk.
In addition to the anion binding sites situated adjacent to the Pd2+ metal centers, cages 3–6 possess a central cavity. The internal environment of this cavity is hydrophobic due to the methyl or cyclohexyl substituents of the clathrochelate ligands, yet the overall charge of the cages is 12+. Consequently, we expected coulombic interactions and the hydrophobic effect to dominate the guest binding behavior of assemblies 3–6. Therefore, the lipophilic tetraphenylborate (BPh4−) anion appeared to be a potentially suitable guest molecule. The BPh4− anion was of particular interest not only given its perceived size and electrostatic match, but also the limited number of host complexes that have been found to bind BPh4− as a guest. To the best of our knowledge, only certain cyclodextrins,17 cavitands,18 liposomes19 and the external face of a metallosupramolecular cube20 have been observed to associate with BPh4− in a host—guest manner.
Complexation studies with cage 4 were hampered by the formation of precipitates upon addition of NaBPh4, and ultimately abandoned. However, addition of one equivalent of NaBPh4 to 3 (0.62 mM) in CD3CN did not lead to precipitation, and significant changes in the 1H NMR signals of both species were apparent.21 Two sets of peaks were immediately visible for the protons of the phenyl groups of the anionic guest, and a new set of signals was also observed for aromatic protons and methyl groups of the clathrochelate ligands. These observations were consistent with the formation of a host—guest complex between the cage and the BPh4− anion, the rate of exchange of bound and unbound BPh4− being slow on the NMR timescale. To confirm the encapsulation of the BPh4− anion within the interior cavity of 3, DOSY and NOESY NMR experiments were performed. DOSY NMR revealed a common diffusion coefficient (4.94 × 10−6 cm2 s−1) for the assembly and one of the two sets of BPh4− protons (ESI, Fig. S40–S43†), whilst NOESY cross peaks were observed between the CH3 groups of the clathrochelate oxime ligand and the same set of BPh4− peaks (ESI, Fig. S46–S49†).
The apparent association constant for the complexation of BPh4− by 3 was calculated through integration of the NMR signals corresponding to bound and unbound guest across a range of concentrations, using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (see ESI† for details). The resulting value is Ka = 2.4(±0.3) × 103 M−1. We subsequently recorded the 1H NMR spectra of 3 in the presence of one equivalent of NaBPh4 in a 2
1 binding model (see ESI† for details). The resulting value is Ka = 2.4(±0.3) × 103 M−1. We subsequently recorded the 1H NMR spectra of 3 in the presence of one equivalent of NaBPh4 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CD3CN and D2O, and a 1
1 mixture of CD3CN and D2O, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CD3CN and CDCl3 in order to elucidate the effect of solvent polarity upon BPh4− binding. In 2
1 mixture of CD3CN and CDCl3 in order to elucidate the effect of solvent polarity upon BPh4− binding. In 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD3CN
1 CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O, the binding constant was calculated to be of the order of 105 M−1, whereas in 1
D2O, the binding constant was calculated to be of the order of 105 M−1, whereas in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD3CN
1 CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCl3, the intensities of the peaks corresponding to bound BPh4− were too low to integrate reliably. The large increase in binding affinity observed upon changing the solvent from neat CD3CN to the more polar 2
CDCl3, the intensities of the peaks corresponding to bound BPh4− were too low to integrate reliably. The large increase in binding affinity observed upon changing the solvent from neat CD3CN to the more polar 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD3CN
1 CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O mixture, as well as the dramatic drop observed in the much less polar 1
D2O mixture, as well as the dramatic drop observed in the much less polar 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD3CN
1 CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCl3 mixture, enforces the hypothesis that the hydrophobic effect plays a significant role in guest binding. These apparent association constants take into account not only the encapsulation of BPh4− by 3, but also the thermodynamically unfavorable concomitant ejection of internal BF4−. Consequently, the affinity of 3 for BPh4− is, in isolation, presumably greater than the composite association constant recorded as discussed above.
CDCl3 mixture, enforces the hypothesis that the hydrophobic effect plays a significant role in guest binding. These apparent association constants take into account not only the encapsulation of BPh4− by 3, but also the thermodynamically unfavorable concomitant ejection of internal BF4−. Consequently, the affinity of 3 for BPh4− is, in isolation, presumably greater than the composite association constant recorded as discussed above.
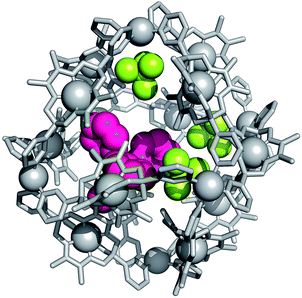
Encapsulation of BPh4− by 3 was unambiguously confirmed by single crystal X-ray crystallography. A ten-fold excess of NaBPh4 was added to a solution of 3 in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CD3CN
1 CD3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O, and from the resulting suspension single crystals of a mixed salt (7) containing both BPh4− and BF4− anions were isolated. Despite the relatively poor quality of the diffraction data, due to the size and complexity of the structure, a sufficient solution was obtained, which unquestionably established the connectivity within the [Pd6112] assembly, as well as the presence of encapsulated BPh4−. Unsurprisingly, it was impossible to locate all BF4− and BPh4− anions due to the extent to which they are disordered, hence precise formulation of this salt cannot be undertaken using the X-ray diffraction data. Rather, the general formula [BPh4@Pd6112][BF4]m[BPh4]n, where m + n = 11, must be used to describe 7. Two independent cages were found in the asymmetric unit, each of which contained a single encapsulated BPh4− (see ESI, Fig. S31†). One of the two independent cages additionally contained three ordered BF4− (Fig. 3), whilst a single ordered BF4− was located in the second. The positions of the different anions in the former illustrate the differences in size of the two interior cavities of 3—the smaller BF4− anions occupy binding pockets adjacent to the Pd2+ vertices of one face of the octahedral framework, whilst the non-coordinating BPh4− resides within the larger central cavity of the assembly. Two of the phenyl groups of BPh4− sit snugly between the methyl substituents of the clathrochelate oxime ligands of two neighboring faces of the [Pd6112]12+ assembly. This conformation alludes to the contribution of hydrophobic interactions to BPh4− encapsulation, and is consistent with the upfield change in chemical shift observed for the protons of the CH3 groups of the oxime substituents as they are exposed to the diamagnetic ring current of the phenyl rings (see ESI, Fig. S37†).
D2O, and from the resulting suspension single crystals of a mixed salt (7) containing both BPh4− and BF4− anions were isolated. Despite the relatively poor quality of the diffraction data, due to the size and complexity of the structure, a sufficient solution was obtained, which unquestionably established the connectivity within the [Pd6112] assembly, as well as the presence of encapsulated BPh4−. Unsurprisingly, it was impossible to locate all BF4− and BPh4− anions due to the extent to which they are disordered, hence precise formulation of this salt cannot be undertaken using the X-ray diffraction data. Rather, the general formula [BPh4@Pd6112][BF4]m[BPh4]n, where m + n = 11, must be used to describe 7. Two independent cages were found in the asymmetric unit, each of which contained a single encapsulated BPh4− (see ESI, Fig. S31†). One of the two independent cages additionally contained three ordered BF4− (Fig. 3), whilst a single ordered BF4− was located in the second. The positions of the different anions in the former illustrate the differences in size of the two interior cavities of 3—the smaller BF4− anions occupy binding pockets adjacent to the Pd2+ vertices of one face of the octahedral framework, whilst the non-coordinating BPh4− resides within the larger central cavity of the assembly. Two of the phenyl groups of BPh4− sit snugly between the methyl substituents of the clathrochelate oxime ligands of two neighboring faces of the [Pd6112]12+ assembly. This conformation alludes to the contribution of hydrophobic interactions to BPh4− encapsulation, and is consistent with the upfield change in chemical shift observed for the protons of the CH3 groups of the oxime substituents as they are exposed to the diamagnetic ring current of the phenyl rings (see ESI, Fig. S37†).
Conclusions
Herein, we have reported the syntheses and the structures of two clathrochelate-based metalloligands with terminal 3-pyridyl groups (1 and 2). Reactions of these ligands with Pd2+ afford large octahedral coordination cages (3–6), which were comprehensively characterized (including crystallographic analysis for three of the four cages). The formation of octahedral Pd cages from ditopic ligands containing 3-pyridyl groups is unprecedented. Typically, conformationally flexible ligands with terminal 3-pyridyl groups give rise to small aggregates (Scheme 2). In our case, the lateral size of the metalloligands prevents the formation of macrocyclic Pd(L2)2Pd structures. This enthalpic effect (steric hindrance) is sufficient to overcome the entropically driven propensity of a coordination-based supramolecular system to form small aggregates. Consequently, larger octahedral structures are obtained, the assembly of which is favored to such an extent that no side products are observed. Despite the fact that we have used steric effects to enlarge the assembly, we were still able to obtain cages with rather large cavities. Notably, cage 3 was found to bind the ‘non-coordinating’22 anion BPh4− with a solvent-dependent association constant of the order of 105 M−1.Our work also highlights the advantageous characteristics of clathrochelate complexes as building blocks for supramolecular chemistry.11,23 These complexes are straightforward to synthesize and their lateral size and functionality24 can be modulated by the boronic acid capping groups and the oxime substituents. The ability to modify the structure of the metalloligands without substantial synthetic efforts represents an important advantage for implementing or optimizing a certain function of the final assembly. As a proof-of-principle study, we have shown that the kinetics of BF4− exchange are strongly affected by the nature of the oxime-derived side chain (methyl vs. cyclohexyl). We believe that judicious design of clathrochelate building blocks will enable many more structurally and functionally novel self-assembled architectures to be obtained, and we are continuing to pursue the applications of these ligands in coordination-based self-assembly.
Acknowledgements
The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007–2013/under REA grant agreement no 264645 (MDW and JJH). We thank Mathieu Marmier, EPFL, for help with the graphical material.Notes and references
- For selected recent review articles about molecularly defined assemblies see: (a) S. Mukherjee and P. S. Mukherjee, Chem. Commun., 2014, 50, 2239 RSC; (b) M. D. Ward and P. R. Raithby, Chem. Soc. Rev., 2013, 42, 1619 RSC; (c) J. G. Hardy, Chem. Soc. Rev., 2013, 42, 7881 RSC; (d) N. J. Young and B. P. Hay, Chem. Commun., 2013, 49, 1354 RSC; (e) T. K. Ronson, S. Zarra, S. P. Black and J. R. Nitschke, Chem. Commun., 2013, 49, 2476 RSC; (f) H. Amouri, C. Desmarets and J. Moussa, Chem. Rev., 2012, 112, 2015 CrossRef CAS PubMed; (g) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef CAS PubMed; (h) M. J. Wiester, P. A. Ulmann and C. A. Mirkin, Angew. Chem., Int. Ed., 2011, 50, 114 CrossRef CAS PubMed.
- For selected recent review articles about polymeric assemblies see: (a) W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tina, M. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC; (b) V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudi, Chem. Soc. Rev., 2014, 43, 6141 RSC; (c) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974 CrossRef CAS PubMed; (d) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef CAS PubMed; (e) W. Xuan, C. Zhu, Y. Liu and Y. Cui, Chem. Soc. Rev., 2012, 41, 1677 RSC; (f) N. N. Adarsh and P. Dastidar, Chem. Soc. Rev., 2012, 41, 3039 RSC; (g) F. A. Almeida, J. Klinowski, S. M. F. Vilela, J. P. C. Tomé, J. A. S. Cavaleiro and J. Rocha, Chem. Soc. Rev., 2012, 41, 1088 RSC; (h) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933 CrossRef CAS PubMed.
- B. H. Northrop, D. Chercka and P. J. Stang, Tetrahedron, 2008, 64, 11495 CrossRef CAS PubMed.
- Review: K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703 RSC.
- Selected examples: (a) S.-L. Huang, Y.-J. Lin, T. S. A. Hor and G.-X. Jin, J. Am. Chem. Soc., 2013, 135, 8125 CrossRef CAS PubMed; (b) D. Fujita, K. Suzuki, S. Sato, M. Yagi-Utsumi, Y. Yamaguchi, N. Mizuno, T. Kumasaka, M. Takata, M. Noda, S. Uchiyama, K. Kato and M. Fujita, Nat. Commun., 2012, 3, 1093 CrossRef PubMed; (c) J. Bunsen, J. Iwasa, P. Bonakdarzadeh, E. Numata, K. Rissanen, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2012, 51, 3161 CrossRef PubMed; (d) Q.-F. Sun, T. Murase, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2011, 50, 10318 CrossRef CAS PubMed; (e) Q.-F. Sun, J. Iwasa, D. Ogawa, Y. Ishido, S. Sato, T. Ozeki, Y. Sei, K. Yamaguchi and M. Fujita, Science, 2010, 328, 1144 CrossRef CAS PubMed.
- The notion ‘small’ refers here to the formation of aggregates containing a small number of building blocks. They can be large in terms of size if large ligands are employed.
- Review: M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848 RSC.
- Selected examples: (a) J. E. M. Lewis, A. B. S. Elliott, C. J. McAdam, K. C. Gordonab and J. D. Crowley, Chem. Sci., 2014, 5, 1833 RSC; (b) C. Desmarets, G. Gontard, A. L. Cooksy, M. N. Rager and H. Amouri, Inorg. Chem., 2014, 53, 4287 CrossRef CAS PubMed; (c) M. Han, R. Michel, B. He, Y.-S. Chen, D. Stalke, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2013, 52, 1319 CrossRef CAS PubMed; (d) G. H. Clever, W. Kawamura, S. Tashiro, M. Shiro and M. Shionoya, Angew. Chem., Int. Ed., 2012, 51, 2606 CrossRef CAS PubMed; (e) J. E. M. Lewis, E. L. Gavey, S. A. Cameron and J. D. Crowley, Chem. Sci., 2012, 3, 778 RSC; (f) M. Han, J. Hey, W. Kawamura, D. Stalke, M. Shionoya and G. H. Clever, Inorg. Chem., 2012, 51, 9574 CrossRef CAS PubMed; (g) N. Kishi, Z. Li, K. Yoza, M. Akita and M. Yoshizawa, J. Am. Chem. Soc., 2011, 133, 11438 CrossRef CAS PubMed; (h) P. Liao, B. W. Langloss, A. M. Johnson, E. R. Knudsen, F. S. Tham, R. R. Julian and R. J. Hooley, Chem. Commun., 2010, 46, 4932 RSC; (i) G. H. Clever, S. Tashiro and M. Shionoya, Angew. Chem., Int. Ed., 2009, 48, 7010 CrossRef CAS PubMed.
- D. K. Chand, K. Biradha, M. Kawano, S. Sakamoto, K. Yamaguchi and M. Fujita, Chem.–Asian J., 2006, 1, 82 CrossRef CAS PubMed.
- The reaction of linear ligands of type L2 with cis-blocked (L-L)Pd2+ complexes gives also (L-L)Pd(L2)2Pd(L-L) macrocycles. See: M.-E. Moon, H.-J. Lee, B.-S. Ko and K.-W. Chi, Bull. Korean Chem. Soc., 2007, 28, 311 CrossRef CAS.
- M. D. Wise, A. Ruggi, M. Pascu, R. Scopelliti and K. Severin, Chem. Sci., 2013, 4, 1658 RSC.
- Recent examples include: (a) O. A. Varzatskii, S. V. Shul'ga, A. S. Belov, V. V. Novikov, A. V. Dolganov, A. V. Vologzhanina and Y. Z. Voloshin, Dalton Trans., 2014 10.1039/c4dt01557f; (b) O. A. Varzatskii, I. N. Denisenko, A. S. Belov, A. V. Vologzhanina, Y. N. Bubnov, S. V. Volkov and Y. Z. Voloshin, Inorg. Chem. Commun., 2014, 44, 134 CrossRef CAS PubMed; (c) V. V. Novikov, I. V. Ananyev, A. A. Pavlov, M. V. Fedin, K. A. Lyssenko and Y. Z. Voloshin, J. Phys. Chem. Lett., 2014, 5, 496 CrossRef CAS; (d) O. A. Varzatskii, I. N. Denisenko, S. V. Volkov, A. V. Dolganov, A. V. Vologzhanina, Y. N. Bubnov and Y. Z. Voloshin, Inorg. Chem. Commun., 2013, 33, 147 CrossRef CAS PubMed; (e) O. A. Varzatskii, I. N. Denisenko, S. V. Volkov, A. S. Belov, A. V. Dolganov, A. V. Vologzhanina, V. V. Novikov, Y. N. Bubnov and Y. Z. Voloshin, Eur. J. Inorg. Chem., 2013, 3178 CrossRef CAS.
- (a) A. Thorn, B. Dittrich and G. M. Sheldrick, Acta Cryst. A, 2012, 68, 448 CrossRef CAS; (b) O. S. Smart, T. O. Womack, C. Flensburg, P. Keller, W. Paciorek, A. Sharff, C. Vonrhein and G. Bricogne, Acta Cryst. D, 2012, 68, 368 CAS.
- For examples of octahedral Pd cages see: (a) C. Gütz, R. Hovorka, C. Klein, Q.-Q. Jiang, C. Bannwarth, M. Engeser, C. Schmuck, W. Assenmacher, W. Mader, F. Topić, K. Rissanen, S. Grimme and A. Lützen, Angew. Chem., Int. Ed., 2014, 53, 1693 CrossRef PubMed; (b) K. Li, L.-Y. Zhang, C. Yan, S.-C. Wei, M. Pan, L. Zhang and C.-Y. Su, J. Am. Chem. Soc., 2014, 136, 4456 CrossRef CAS PubMed; (c) K. Suzuki, M. Tominaga, M. Kawano and M. Fujita, Chem. Commun., 2009, 1638 RSC.
- G. J. Kleywegt and T. A. Jones, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1994, 50, 178–185 CrossRef CAS PubMed.
- J. Sandström, Dynamic Nmr Spectroscopy, Academic Press, 1982 Search PubMed.
- (a) M. D. Johnson and V. C. Reinsborough, Aust. J. Chem., 1992, 45, 1961 CrossRef CAS; (b) T. Nhujak and D. M. Goodall, Electrophoresis, 2001, 22, 117 CrossRef CAS.
- S. S. Zhu, H. Staats, K. Brandhorst, J. Grunenberg, F. Gruppi, E. Dalcanale, A. Lützen, K. Rissanen and C. A. Schalley, Angew. Chem., Int. Ed., 2008, 47, 788 CrossRef CAS PubMed.
- H. Osanai, T. Ikehara, S. Miyauchi, K. Shimono, J. Tamogami, N. Nara and N. Kamo, J. Biophys. Chem., 2013, 4, 11 CrossRef CAS.
- W. J. Ramsay and J. R. Nitschke, J. Am. Chem. Soc., 2014, 136, 7038 CrossRef CAS PubMed.
- Binding studies were not performed with cage 5 and 6 because they display a lower solubility compared to 3 and 4.
- Receptors for weakly-coordinating anions are described in: (a) S. Lee, C.-H. Chen and A. H. Flood, Nat. Chem., 2013, 5, 704 CrossRef CAS PubMed; (b) Y. R. Hristova, M. M. J. Smulders, J. K. Clegg, B. Breiner and J. R. Nitschke, Chem. Sci., 2011, 2, 638 RSC.
- (a) Y.-Y. Zhang, Y.-J. Lin and G.-X. Jin, Chem. Commun., 2014, 50, 2327 RSC; (b) M. Pascu, M. Marmier, C. Schouwey, R. Scopelliti, J. J. Holstein, G. Bricogne and K. Severin, Chem.–Eur. J., 2014, 20, 5592 CrossRef CAS PubMed.
- For clathrochelate complexes with different functional groups see: E. G. Lebed, A. S. Belov, A. V. Dolganov, A. V. Vologzhanina, A. Szebesczyk, E. Gumienna-Kontecka, H. Kozlowski, Y. N. Bubnov, I. Y. Dubey and Y. Z. Voloshin, Inorg. Chem. Commun., 2013, 30, 53 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1024735–1024740. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc03046j |
| This journal is © The Royal Society of Chemistry 2015 |