Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective oil/water filter paper via a scalable one-pot hydrothermal growth of ZnO nanowires†
Yi Chenab,
Li Liub,
Hyun-Joong Chung*b and
John A. Nychka*b
aScion, Private Bag 3020, Rotorua 3046, New Zealand
bDepartment of Chemical and Material Engineering, University of Alberta, Edmonton, Alberta T6G 2V4, Canada. E-mail: chung3@ualberta.ca; jnychka@ualberta.ca
First published on 14th October 2015
Abstract
Uniform ZnO nanowires (NWs) were grown on cellulose filter paper via a modified one-step hydrothermal method. The ZnO coated filter paper exhibited superhydrophilicity and underwater superoleophobicity due to the surface roughness of the coated ZnO NWs. The coated cellulose filters demonstrated exceptional results at oil/water separation with high separation efficiency and experimentally proven recyclability for several oil/water mixtures. The outstanding separation performance and scalable growth method highlight potential for practical applications, such as wastewater treatment and polluted water purification.
In recent years, water cleanup by oil decontamination has been attracting extensive attention.1,2 Recent studies on modulating wettability by chemical modification and/or nanoscale texturizing have achieved a remarkable enhancement in separation efficiency of oil/water suspensions.3–7 However, the separated oil can cause frequent plugging of pores in the membrane, and subsequently results in low flux, low recyclability and secondary pollution.8,9 An underwater oleophobicity, which means that the oil can automatically be removed from the pores when saturated with water, may provide a fundamental solution to the plugging problem. This underwater oleophobicity feature can be utilized for gravity-driven separation membranes since water usually has higher density than oil.10–15
Various approaches have been developed to achieve underwater superoleophobic surfaces. For example, free standing CNTs/TiO2 network films,11 graphene oxide coated membrane,12,13 polydopamine/polyethylenimine modified polypropylene membranes,14 and Cu(OH)2 NW haired meshes15 have been suggested as examples of superoleophobic membranes. Nevertheless, critical drawbacks such as high cost, low flexibility and toxicity have hindered their application in practical applications. Hence, it remains as a challenge to develop cost-effective and scalable material processing pathways without environmentally harmful by-products so that they can be employed in large scale production and use in society.
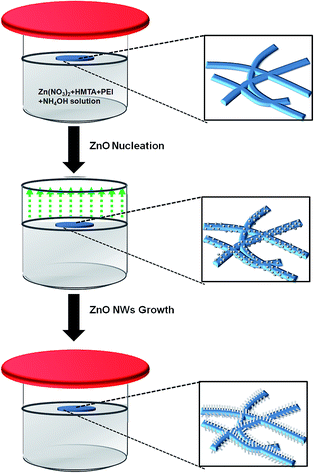
To address these issues, we report a novel one-pot hydrothermal growth method for ZnO nanowires (NWs) that can convert a commodity filter paper into a highly efficient and reusable oil/water separating membrane. Traditional growth of ZnO NWs requires a separate step to deposit a ZnO seed layer prior to NW growth, including metal-organic chemical vapour deposition (MOCVD), chemical vapor deposition (CVD) or pulsed laser deposition.16–18 In contrast to these methods, one pot methods as well as seedless ZnO growth have been receiving attention due to the potential to scale up.19,20 The advantage of our method lies in an exceptional simplicity of the one-pot synthesis, which provides unmatched compatibility for scalable production. In our method, seed nucleation and growth steps are simply switched by controlling the vapour pressure over the liquid precursor solution. By increasing the roughness of the filter surface, the ZnO NW coated filter paper shows superhydrophilic and underwater superoleophobic without post treatment. The superoleophobic filter paper is free from oil fouling and has high efficiency and recyclability for oil/water separation. The ZnO NWs growth method was developed for fabrication of large scale ZnO NWs coated cellulose filter paper as illustrated in Fig. 1. Particularly, the filter paper was placed at the interface of liquid-air. When the pristine filter paper is soaked with the solution at 90 °C (Fig. 1a), the lid is removed to drop the vapor pressure to allow water and NH3 gas to rapidly evaporate to form ZnO nuclei particles on the fiber surface, as described in eqn (1):21,22
| Zn(NH3)42+ + 2OH− → ZnO + 4NH3 + H2O. | (1) |
The cellulose fibers in the filter paper become uniformly decorated with the nuclei particles, as shown in Fig. S1.† Once the nuclei particle layer is continuous the beaker is closed with the lid again to increase the vapor pressure of ammonia and water. Now the formation of ZnO nuclei is suppressed, whereas NWs start to grow on the nuclei.15 More importantly, by selective absorption on the lateral facets and chelating Zn2+, polyethylenimine (PEI), a cationic polyelectrolyte, plays an essential role in controlling the NWs' length and diameter during growth by slowing down the lateral growth rate whilst maintaining the growth density and length,23,24 which directly affects the surface roughness and resulting controllable wettability.25,26
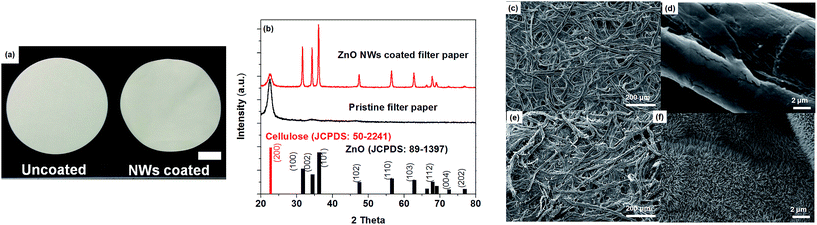
Fig. 2a shows photographs of pristine (left) and ZnO NWs coated filter paper (right), indicating that there is clearly no visible difference after ZnO NW coating. In the ZnO NW coated papers, however, XRD patterns confirm the wurtzite crystalline structure of ZnO (JCPDS no. 89-1397) (Fig. 2b). The strong and sharp diffraction peaks indicate that the ZnO NWs are highly crystalline. No other peaks besides the characteristic wurtzite ZnO pattern were detectable, except for a peak at around 22.58°. This extra peak corresponds to the (200) plane of cellulose.27 Fig. 2c and d are the secondary electron scanning electron microscopy (SEM) images of uncoated (pristine) cellulose fibers with a typical network structure at low (Fig. 2c) and higher magnification (Fig. 2d). It is revealed that a high density of ZnO NWs were grown on the fibers with uniform diameter of 30 nm (Fig. 2f). The characterization results indicate that ZnO NWs were successfully grown on a large scale filter paper (diameter of 90 mm) and indicate that the fabrication procedure appears to be scalable.
The wetting property of the ZnO NWs coated filter paper was tested by water and oil contact angle measurements for oil and water droplets in ambient atmosphere and for oil droplets in a water environment. As shown in Fig. 3a, when a water droplet was placed onto the as-coated filter paper in ambient air (with relative humidity of 40%), the drop spread very quickly and a 0° contact angle was recorded (Fig. 3a), and the spread took 0.66 s, as recorded in a high-speed video for dynamic water contact angle test (Fig. S2†). The sample also exhibited 0° contact angle for oil in air (Fig. 3b). For underwater oil contact angle measurement, 1,2-dichloroethane (DCE) was selected as a model oil to evaluate the underwater oil contact angle due to its higher density than water. The coated filter paper had a large underwater oil contact angle of 153° (Fig. 3c). The underwater superoleophobic property with low oil adhesion is attributed to the rough ZnO NWs and the superhydrophilic ZnO surface,25,28–30 thus the coating renders an ideal surface condition for gravity driven oil/water separating filter paper.
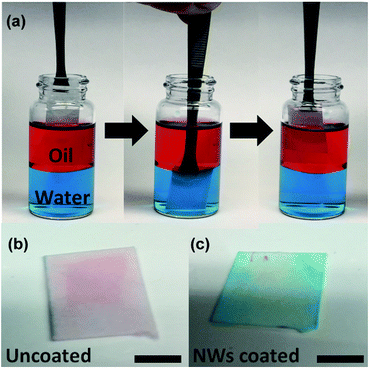
To further demonstrate the non-fouling characteristics of ZnO NWs coated filter paper, an oil/water selective absorption/desorption processes was demonstrated in Fig. 4. The filter paper was submerged into the oil/water mixture and taken out without any further treatment. After firstly being dipped into the top oil layer, the uncoated filter paper was wetted by oil (kerosene dyed with Oil Red O) due to the large pore size and oleophilic surface. The oil droplets were absorbed (red), which resulted in the formation of an oil fouling layer on the filter surface and blocked the absorption of water due to the difference in surface energy between oil and water (Fig. 4b). However, when the ZnO NWs coated filter paper was submerged into the oil initially, the superoleophobic nature of modified filter paper in water caused a desorption of oil absorption, followed by a full wetting by water, which was dyed with methylene blue, and no visible dyed oil was observed (Fig. 4c).
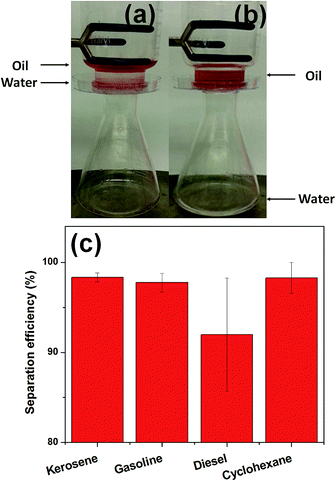
Fig. 5a and b demonstrate the oil/water separation procedure. The details for experimental setup are described in ESI.† When the surfactant-free oil/water mixture (prepared by mixing water and kerosene at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v)) was poured into the funnel, the superhydrophilic filter paper absorbed water quickly and become saturated, resulting in oil staying at the interface atop the water. Kerosene dyed with Oil Red O was mixed with water and poured into the funnel. Water quickly permeated through the filter (in a few minutes) and was collected by the flask below. Meanwhile, oil was retained above the filter paper. No external pressure was applied for the separation and no hints of oil droplets (i.e. red dye) were observed within the filtrate. To further determine the oil content in the filtrate after separation, UV-absorption spectra were collected and compared with dyed oil (Fig. S3†).31,32 There was no discernible dye absorption peak observed from the filtrate, suggesting high purity, selectivity, and effectiveness of the oil/water separation.
50 (v/v)) was poured into the funnel, the superhydrophilic filter paper absorbed water quickly and become saturated, resulting in oil staying at the interface atop the water. Kerosene dyed with Oil Red O was mixed with water and poured into the funnel. Water quickly permeated through the filter (in a few minutes) and was collected by the flask below. Meanwhile, oil was retained above the filter paper. No external pressure was applied for the separation and no hints of oil droplets (i.e. red dye) were observed within the filtrate. To further determine the oil content in the filtrate after separation, UV-absorption spectra were collected and compared with dyed oil (Fig. S3†).31,32 There was no discernible dye absorption peak observed from the filtrate, suggesting high purity, selectivity, and effectiveness of the oil/water separation.
To test the oil/water separation of modified filter paper, four types of light oil/water mixtures, including kerosene/water, gasoline/water, diesel/water and cyclohexane/water mixtures were selected. Oil/water separation efficiency was defined as mseparation/minitial, where mseparation is the mass of water after separation, and mintial is the mass of water before mixing.33–36 In all cases, high efficiency values between 91% and 99% were achieved. This result indicates that the modified filter paper was a useful oil/water separator. Moreover, the water flux dependence on different mixtures were also calculated by determining the completely permeated water volume within a certain time period (Fig. S4†). Solely driven by gravity, the filter paper allowed the water flux of 917, 871, 880 and 1060 L m−2 h−1 for kerosene/water, gasoline/water, diesel/water and cyclohexane/water mixtures, respectively. These flux values are comparable to polymeric or ceramic membranes for oil/water mixture separation.37–40
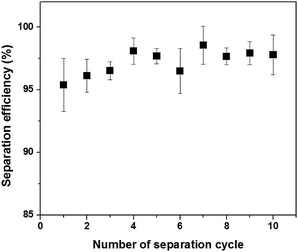
The recyclability is a key criterion for practical oil/water separation membranes or filters. The separation cycle performance is illustrated in Fig. 6. The filter paper was washed by water after every separation cycle, and dried before the next cycle.41 For the 10 cycles of sample reutilization, separation efficiency stayed relatively invariant, indicating a prolonged oil anti-fouling property and excellent recyclability.
In summary, superhydrophilic and underwater superoleophobic ZnO NWs coated filter paper was developed by a one pot synthesis method. The hierarchically rough ZnO NWs improved underwater oleophobicity of filter paper, and exhibited anti-fouling oil/water separation properties. The as-prepared filter paper, without further modification, showed high oil/water separation efficiency using gravity and could enable an energy-efficient and environmental-friendly separation process. More importantly, our one pot hydrothermal growth method for ZnO NWs also provides the possibility for scalable fabrication and large scale oil/water separation. Future studies may include the separation of emulsified oil/water mixture. We also note an international standard protocol for reproducible sessile-drop contact angle measurements.42
Acknowledgements
This work is supported by NSERC DG (HJC) & NSERC DG (JAN). Thank you to the NanoFAB at the UofA for imaging and characterization facility usage.Notes and references
- J. J. González, L. Viñas, M. A. Franco, J. Fumega, J. A. Soriano, G. Grueiro, S. Muniategui, P. López-Mahía, D. Prada, J. M. Bayona, R. Alzaga and J. Albaigés, Mar. Pollut. Bull., 2006, 53, 250–259 CrossRef PubMed
.
- E. Kintisch, Science, 2010, 329, 735–736 CrossRef CAS PubMed
.
- J. Zhang and S. Seeger, Adv. Funct. Mater., 2011, 21, 4699–4704 CrossRef CAS PubMed
.
- A. K. Kota, Y. Li, J. M. Mabry and A. Tuteja, Adv. Mater., 2012, 24, 5838–5848 CrossRef CAS PubMed
.
- L. Wen, Y. Tan and L. Jiang, Angew. Chem., Int. Ed., 2015, 54, 3387–3399 CrossRef CAS PubMed
.
- B. Wang, W. Liang, Z. Guo and W. Liu, Chem. Soc. Rev., 2015, 44, 336–361 RSC
.
- C. Gao, Z. Sun, K. Li, Y. Chen, Y. Cao, S. Zhang and L. Peng, Energy Environ. Sci., 2013, 6, 1147–1151 CAS
.
- J. Li, L. Shi, Y. Chen, Y. Zhang, Z. Guo, B. L. Su and W. Liu, J. Mater. Chem., 2012, 22, 9774–9781 RSC
.
- L. Feng, Z. Zhang, Z. Mai, Y. Ma, B. Liu, L. Jiang and D. Zhu, Angew. Chem., 2004, 116, 2046–2048 CrossRef PubMed
.
- A. K. Kota, J. M. Mabry and A. Tuteja, Surf. Innovations, 2013, 1, 71–83 CrossRef CAS
.
- S. J. Gao, Z. Shi, W. B. Zhang, F. Zhang and J. Jin, ACS Nano, 2014, 8, 6344–6352 CrossRef CAS PubMed
.
- Y. Huang, H. Li, L. Wang, Y. Qiao, C. Tang, C. Jung, Y. Yoon, S. Li and M. Yu, Adv. Mater. Interfaces, 2015, 2, 1400433 Search PubMed
.
- H. Li, Y. Huang, Y. Miao, W. L. Xu, H. J. Ploehn and M. Yu, Chem. Commun., 2014, 50, 9849–9851 RSC
.
- H. Yang, J. Pi, K. Liao, H. Huang, Q. Wu, X. Huang and Z. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 12566–12572 CAS
.
- F. Zhang, W. B. Zhang, Z. Shi, D. Wang, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 4192–4198 CrossRef CAS PubMed
.
- J. J. Wu and S. C. Liu, Adv. Mater., 2002, 14, 215–218 CrossRef CAS
.
- Y. Sun, G. M. Fuge and M. N. R. Ashfold, Chem. Phys. Lett., 2004, 396, 21–26 CrossRef CAS PubMed
.
- H. Z. Zhang, X. C. Sun, R. M. Wang and D. P. Yu, J. Cryst. Growth, 2004, 269, 464–471 CrossRef CAS PubMed
.
- J. Liu, R. Lu, G. Xu, J. Wu, P. Thapa and D. Moore, Adv. Funct. Mater., 2013, 23, 4941–4948 CrossRef CAS PubMed
.
- X. Wen, W. Wu, Y. Ding and Z. L. Wang, J. Mater. Chem., 2012, 22, 9469–9476 RSC
.
- J. Zhang, L. Sun, J. Yin, H. Su, C. Liao and C. Yan, Chem. Mater., 2002, 14, 4172–4177 CrossRef CAS
.
- K. Liu, W. Wu, B. Chen, X. Chen and N. Zhang, Nanoscale, 2013, 5, 5986–5993 RSC
.
- M. Law, L. E. Greene, J. C. Johnson, R. Saykally and P. Yang, Nat. Mater., 2005, 4, 455–459 CrossRef CAS PubMed
.
- S. H. Ko, D. Lee, H. W. Kang, K. H. Nam, J. Y. Yeo, S. J. Hong, C. P. Grigoropoulos and H. J. Sung, Nano Lett., 2011, 11, 666–671 CrossRef CAS PubMed
.
- T. Pauporté, G. Bataille, L. Joulaud and F. J. Vermersch, J. Phys. Chem. C, 2010, 114, 194–202 Search PubMed
.
- M. Gong, Z. Yang, X. Xu, D. Jasion, S. Mou, H. Zhang, Y. Long and S. Ren, J. Mater. Chem. A, 2014, 2, 6180–6184 CAS
.
- J. Kim, S. Yun and Z. Ounaies, Macromolecules, 2006, 39, 4202–4206 CrossRef CAS
.
- M. Lee, G. Kwak and K. Yong, ACS Appl. Mater. Interfaces, 2011, 3, 3350–3356 CAS
.
- Z. Cheng, H. Lai, Y. Du, K. Fu, R. Hou, N. Zhang and K. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 11363–11370 CAS
.
- X. Zheng, Z. Guo, D. Tian, X. Zhang, W. Li and L. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 4336–4343 CAS
.
- K. Rohrbach, Y. Li, H. Zhu, Z. Liu, J. Dai, J. Andreasen and L. Hu, Chem. Commun., 2014, 50, 13296–13299 RSC
.
- P. Chen and Z. Xu, Sci. Rep., 2013, 3, 2776 Search PubMed
.
- J. Li, L. Yan, Y. Zhao, F. Zha, Q. Wang and Z. Lei, Phys. Chem. Chem. Phys., 2015, 17, 6451–6457 RSC
.
- Q. Pan, M. Wang and H. Wang, Appl. Surf. Sci., 2008, 254, 6002–6006 CrossRef CAS PubMed
.
- X. Jin, B. Shi, L. Zheng, X. Pei, X. Zhang, Z. Sun, Y. Du, J. Kim, X. Wang, S. Dou, K. Liu and L. Jiang, Adv. Funct. Mater., 2014, 24, 2721–2726 CrossRef CAS PubMed
.
- Y. Dong, J. Li, L. Shi, X. Wang, Z. Guo and W. Liu, Chem. Commun., 2014, 50, 5586–5589 RSC
.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856–860 CrossRef CAS PubMed
.
- A. K. Kota, G. Kown, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef PubMed
.
- H. Yang, K. Liao, H. Huang, Q. Wu, L. Wan and Z. Xu, J. Mater. Chem. A, 2014, 2, 10225–10230 CAS
.
- A. Raza, B. Ding, G. Zainab, M. EI-Newhy, S. S. Al-Deyab and J. Yu, J. Mater. Chem. A, 2014, 2, 10137–10145 CAS
.
- M. Tao, L. Xue, F. Kiu and L. Jiang, Adv. Mater., 2014, 26, 2943–2948 CrossRef CAS PubMed
.
- J. Drelich, Surf. Innovations, 2013, 1, 248–254 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and more characterization are included. See DOI: 10.1039/c5ra15970a |
| This journal is © The Royal Society of Chemistry 2015 |