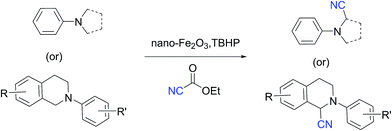
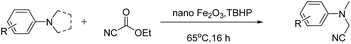
Magnetically recyclable iron oxide nanoparticles for the α-cyanation of amines under acid-free conditions and the formal synthesis of praziquantel†
Mahendra
Patil
,
Anant R.
Kapdi
and
A. Vijay
Kumar
*
Department of Chemistry, Institute of Chemical Technology, Matunga, Mumbai, Maharashtra, India–400019. E-mail: vijayakki@gmail.com; Fax: +91 2233611020; Tel: +91 2233612614
First published on 15th June 2015
Abstract
A sustainable protocol for the α-cyanation of amines has been developed using cheap and affordable iron oxide nanoparticles under acid free conditions with an easy-to-handle, user-friendly cyanide source, ethyl cyanoformate. The magnetic property of the iron oxide nanoparticles facilitated their easy recovery and the recovered particles were found to be reusable up to five cycles with no loss of activity. Further, this methodology was applied for the formal synthesis of the anti-schistosome drug praziquantel. The use of non-toxic, affordable and recyclable iron oxide nanoparticles as a catalyst makes this a green protocol.
The Cross-Dehydrogenative-Coupling (CDC) reaction is a versatile tool for the synthesis of various molecules and precursors which are medicinally and biologically important.1 The CDC strategy for the synthesis of α-cyanated amines is advantageous since the imines are generated in situ via C–H bond activation thus precluding their separate synthesis from their respective amines. The α-cyanation of amines is reported with various catalytic systems viz. Ru,2 Cu,3 V,4 Au,5 Mo,6 Co,7 Fe,8 I2,9 photo redox conditions,10 acetic acid,11 TEMPO/acetic acid12 along with non-user friendly cyanide sources. The protocols not only generate metal wastes but also require specialised metal scavengers to make the final products free of metal salt contaminations. These requisites thus escalate the cost and render them from being adopted for making pharmaceuticals and drug molecules. Due to the shortcomings of the existing protocols, a robust protocol more suitable to be adopted for industry and academia with features such as easy recoverability of catalyst, less harsh conditions and user friendly reagents is highly desirable. Over the recent years magnetically retrievable nanoparticles based catalysts have received much attention owing to the advantages of easy recoverability and non-toxicity.13 Iron being cheap, abundant and non-toxic makes it an affordable catalyst of choice to be employed in organic synthesis and subsequently qualify them to be adopted for large scale syntheses. Considering the above facts, herein we present a protocol based on easily recyclable iron oxide nanoparticles for the α-cyanation of amines under milder acid free conditions with a user friendly cyanide source – ethyl cyanoformate (Scheme 1). Taking into consideration of these advantages we have screened different iron based catalysts like nano-Fe3O4, Fe-BTC MOF (Basolite F-300™),14 bulk Fe2O3, FeIII(OH)(OAc)2, FeCl3, nano-Fe2O3, CuFe2O4, etc. for the alpha-cyanation of N,N-dimethylaniline with various cyanide sources such as TMSCN,15 mandelonitrile, ethyl cyanoformate,16D/L-lactonitrile, malononitrile,17 benzyl nitrile18 and KSCN19 in combination with oxidants such as H2O2, tert-butyl hydrogen peroxide (TBHP), O2 under solvent and solvent-free conditions.
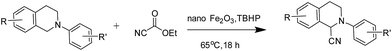
Of all the catalysts, oxidants and cyanide sources screened, Fe2O3 nanoparticles (Fe2O3 nps) and ethyl cyanoformate combination in methanol solvent were found to be best conditions which afforded the cyanated product in 80% isolable yield (Table, ESI†). With these optimized conditions we have further explored the scope of the method by varying different amine precursors. We were able to achieve the smooth conversions of different amines like tetrahydroisoquinolines, pyrrolidines, piperidines, N,N-dimethylanilines, and morpholines to their respective cyanated products. Almost all of them gave good to moderate yields of mono cyanated products (Tables 1 and 2).
| Substrate | Product | Yielda (%) |
|---|---|---|
| a Isolated yield, isolated yield, Fe2O3 nps (10 mol%), ethyl cyanoformate (2 equiv.), TBHP (2 equiv.), MeOH (2 mL), 65 °C and 18 h. | ||
| R = H, R′ = H | R = H, R′ = H | 80 |
| R = H, R′ = p-Cl | R = H, R′ = p-Cl | 78 |
| R = H, R′ = p-OMe | R = H, R′ = p-OMe | 85 |
| R = 6,7-OMe, R′ = H | R = 6,7-OMe, R′ = H | 72 |
| R = 6,7-OMe, R′ = p-Cl | R = 6,7-OMe, R′ = p-Cl | 70 |
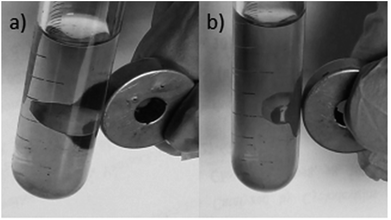
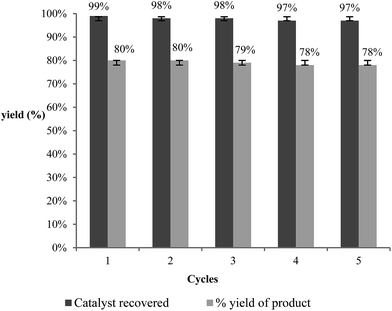
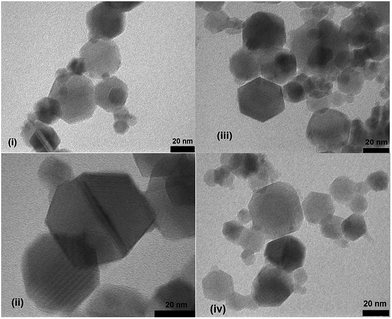
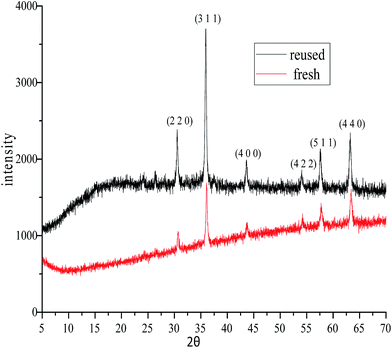
Our primary goal in choosing iron oxide nps as catalyst for this transformation was to bring them in utility to be useful as recyclable catalysts. So initially the recovered particles were checked for their recyclability. The particles were easily recovered with the aid of a simple external laboratory magnet after the completion of the reactions without the need of any specialised equipment like ultracentrifuge (or) nanofilters (Fig. 1). The collected particles were used for the next cycle after methanol wash followed by simple oven drying at 70 °C. The particles were recyclable for five cycles with no loss of activity and gave the comparable product yields for each cycle (Fig. 2). The surface degradation of nanoparticles and subsequent leaching is possible in nanocatalysis. Hence it is mandatory to investigate for surface modifications and crystallinity of the particles. This was investigated with the help of techniques like TEM imaging, XRD and ICP studies. Firstly, the native and the used nanoparticles (fifth cycle) were analysed by Transmission Electron Microscopy (TEM) for any possible degradations of surface. Both the TEM images were almost comparable with no signs of surface degradation, thus confirming the intactness of particles shape and size (Fig. 3). Similarly the powder X-ray patterns of native and reused catalysts showed no extra peaks strongly suggesting no modification in crystallinity despite their usage for several cycles (Fig. 4).
Thus these two spectroscopic techniques endorsed that the particles are tolerant and non-degradative to the reagents and conditions employed. Even though the catalyst is found to be unchanged as seen by XRD,20 FTIR21 and TEM, it is always advisory to probe for the chances of leached metal species in the reaction mixture.
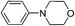
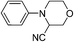
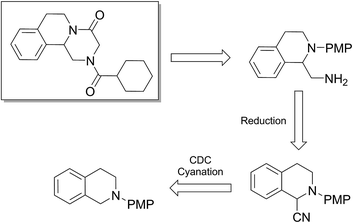
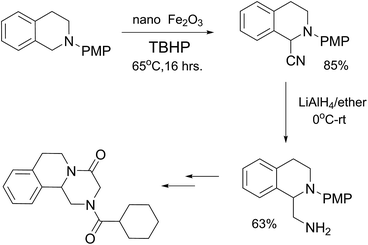
To find out this we have carefully filtered the particles from reaction mixtures after the complete conversion of the reactants and subjected them to ICP-AES analysis for Fe species coming off the particles. The amount of Fe leached into the solution was found to be only negligible (4 ppm) as revealed by ICP-AES analysis. In another control experiment, the catalyst was removed from the reaction after 50% conversion of the starting materials and the reaction was continued without catalyst. No progress of the starting material conversion was observed even after prolonged heating. All these results strongly suggests that the reaction is catalyzed by the Fe nps heterogeneously and the true catalyst being the iron nps and not the leached species. It is noteworthy to mention that the reaction did not proceed when only Fe and peroxides are added separately. The combination of both Fe and peroxides was essential for the smooth conversion of the substrates. When TEMPO was added to the reaction mixture the reaction was found to be non-progressive, which suggests that the reaction proceeds through a radical pathway. Thus, based on all these findings, we envisage that there might be a synergistic effect of iron nps and peroxides which dually activates the cyanide reagent and amine (imine generation through the electrophilic radical intermediate followed by proton abstraction) and subsequent cyanide addition leading to the product formation (refer ESI† for possible mechanism). This can be the only possible explanation we can offer at this moment and foresee to further investigate in detail to understand the mechanism. Having studied the substrate scope and recyclability, we were interested in the synthesis of a bioactive compound (or) drug using this protocol. Surprisingly, amongst the substrates studied in this methodology, the tetrahydroisoquinoline derivatives afforded the maximum yields, so our attention was drawn into the synthesis of a tetrahydroisoquinoline ring containing drug molecule praziquantel. It is also noteworthy to mention that praziquantel is the sole drug for the treatment of schistosomiasis, a parasitic infectious disease which is very prevalent amongst the regions of Africa and South-East Asia and for these reasons the drug is enlisted in the World Health Organization's List of Essential Medicines.22 We also found that the CDC cyanation strategy was seldom put to utility for this drug's synthesis except a few.23 The retrosynthetic analysis for the drug synthesis as elucidated could be fragmented to the respective synthetic equivalents i.e. the N-PMP protected diamine precursor which can be synthesised by the reduction of cyanated product, which in turn could be synthesised by using the developed CDC cyanation of the N-PMP protected tetrahydroisoquinoline (Scheme 2).
The synthesis was carried out as planned according to our retrosynthetic analysis. The cyanated derivative was synthesized using our Fe nps protocol without any difficulty.
Further reduction using Lithium Aluminium Hydride (LAH) of the cyanated derivative in dry diethyl ether gave the N-PMP protected diamine derivative in 63% yield and the final overall yield for the two steps is 74% (Scheme 3).24 Finally, we have succeeded in applying the cyanation methodology developed by us for the formal synthesis of praziquantel. Further studies to elucidate the mechanism of the reaction and to utilise the methodology for the synthesis of various natural products, drug molecules is being pursued in our group.
In conclusion we have developed a recyclable protocol for the α-cyanation of amines by a CDC reaction under acid free conditions using benign and affordable iron oxide nanoparticles in combination with a user friendly cyanide source – ethyl cyanoformate. The mild reaction conditions and recyclability of the catalyst makes this a sustainable protocol for the synthesis of α-cyanated amines. Also the formal total synthesis of the anti-schistosomiasis drug praziquantel was achieved with the developed cyanation method as the key step.
Acknowledgements
The authors are grateful to SAIF-IITB for the providing the TEM, ICP-AES and XRD analysis. MP is grateful to CSIR, Govt. of India for the research fellowship. AVK is thankful to DST, Govt. of India for the INSPIRE Faculty Award [IFA12-CH-40] and research funding. Institute of Chemical Technology (ICT) is acknowledged for providing the research facilities.Notes and references
- (a) C. J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) From C–H to C–C Bonds Cross-Dehydrogenative-Coupling, ed. C. J. Li, Royal Society of Chemistry, Cambridge, England, 2014, pp. 331 Search PubMed; (c) R. Yang, Q. Ruan, B. Y. Zhang, Z. L. Zheng, F. Miao, L. Zhou and H. L. Geng, Molecules, 2014, 19, 8051 CrossRef PubMed.
- (a) S.-I. Murahashi, N. Komiya and H. Terai, Angew. Chem., 2005, 117, 7091 ( Angew. Chem., Int. Ed. , 2005 , 44 , 6931 ) CrossRef; (b) S. I. Murahashi, T. Nakae, H. Terai and N. Komiya, J. Am. Chem. Soc., 2008, 130, 11005 CrossRef CAS PubMed; (c) S. Verma, S. L. Jain and B. Sain, ChemCatChem, 2011, 3, 1329 CrossRef CAS.
- (a) Z. Li and C.-J. Li, Eur. J. Org. Chem., 2005, 3173 CrossRef CAS; (b) R. Hudson, S. Ishikawa, C. J. Li and A. Moores, Synlett, 2013, 1637 CAS.
- S. Singhal, S. L. Jain and B. Sain, Chem. Commun., 2009, 237 Search PubMed.
- Y. Zhang, H. Peng, M. Zhang, Y. Cheng and C. Zhu, Chem. Commun., 2011, 47, 2354 RSC.
- K. Alagiri and K. R. Prabhu, Org. Biomol. Chem., 2012, 10, 835 CAS.
- N. Sakai, A. Mutsuro, R. Ikeda and T. Konakahara, Synlett, 2013, 1283 CrossRef CAS.
- (a) A. Wagner, W. Han, P. Mayer and A. R. Ofial, Adv. Synth. Catal., 2013, 355, 3058 CrossRef CAS; (b) D. Verma, S. Verma, A. K. Sinha and S. L. Jain, ChemPlusChem, 2013, 78, 860 CrossRef CAS.
- (a) T. Nobuta, N. Tada, A. Fujiya, A. Kariya, T. Miura and A. Itoh, Org. Lett., 2013, 15, 574 CrossRef CAS PubMed; (b) J. D. Kumar, M. Lamani, K. Alagiri and K. R. Prabhu, Org. Lett., 2013, 15, 1092 CrossRef PubMed.
- (a) P. Kumar, S. Varma and S. L. Jain, J. Mater. Chem. A, 2014, 2, 4514 RSC; (b) M. Rueping, S. Zhu and R. M. Koenigs, Chem. Commun., 2011, 47, 12709 RSC.
- H. Ueda, K. Yoshida and H. Tokuyama, Org. Lett., 2014, 16, 4194 CrossRef CAS PubMed.
- C. Yan, Y. Liu and Q. Wang, RSC Adv., 2014, 4, 60075 RSC.
- For recent reviews, books and articles on magnetically recoverable catalysts and nanocatalysis please refer these and the references cited therein (a) B. Karimi, F. Mansouri and H. M. Mirzaei, ChemCatChem, 2015, 7, 1736 CrossRef CAS; (b) R. B. N. Baig and R. S. Varma, Chem. Commun., 2013, 49, 752 RSC; (c) M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC; (d) V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed; (e) C. W. Lim and I. S. Lee, Nano Today, 2010, 5, 412 CrossRef CAS; (f) Nanocatalysis: Synthesis and Applications, ed. V. Polishettiwar and T. Asefa, John Wiley & Sons Inc., Hoboken, New Jersey, 2013 Search PubMed; (g) Nanomaterials in Catalysis, ed. P. Serp and K. Philippot, Wiley-VCH Verlag & Co. KGaA, Weinheim, Germany, 2013 Search PubMed.
- The MOF structure disintegrated during the reaction.
- L. Liu, Z. Wang, X. Fu and C.-H. Yan, Org. Lett., 2012, 14, 5692 CrossRef CAS PubMed.
- (a) K. T. V. Rao, B. Haribabu, P. S. S. Prasad and N. Lingaiah, ChemCatChem, 2012, 4, 1173 CrossRef CAS; (b) K. H. V. Reddy, G. Satish, V. P. Reddy, B. S. P. A. Kumar and Y. V. D. Nageswar, RSC Adv., 2012, 2, 11084 RSC.
- D. P. Hari and B. König, Org. Lett., 2011, 13, 3852 CrossRef CAS PubMed.
- C. Zhang, C. Liu, Y. Shao, X. Bao and X. Wan, Chem.–Eur. J., 2013, 19, 17917 CrossRef CAS PubMed.
- A. Wagner and A. R. Ofial, J. Org. Chem., 2015, 80, 2848 CrossRef CAS PubMed.
- The X-ray diffraction pattern of nanocrystalline γ-Fe2O3 was comparable with the XRD pattern reported in literature: T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. B. Na, J. Am. Chem. Soc., 2001, 123, 12798 CrossRef CAS PubMed.
- Refer ESI† for IR of native and used catalysts.
- (a) P. Steinmann, J. Keiser, R. Bos, M. Tanner and J. Utzinger, Lancet Infect. Dis., 2006, 6, 411 CrossRef PubMed; (b) C. R. Caffrey, Curr. Opin. Chem. Biol., 2007, 11, 433 CrossRef CAS PubMed; (c) A. L. Chenine, E. Shai-Kobiler, L. N. Steele, H. Ong, P. Augostini, R. Song, S. J. Lee, P. Autissier, R. M. Ruprecht and W. E. Secor, PLoS Neglected Trop. Dis., 2008, 2, 265 Search PubMed; (d) A. Alalade, S. Leeson and U. Andrady, Gynecol. Surg., 2009, 6, 177 CrossRef; (e) H. Feldmeier, I. Krantz and G. Poggensee, Int. J. STD AIDS, 1994, 5, 368 CAS; (f) E. F. Kjetland, P. D. Ndhlovu, E. Gomo, T. Mduluza, N. Midzi, L. Gwanzura, P. R. Mason, L. Sandvik, H. Friis and S. G. Gundersen, AIDS, 2006, 20, 593 CrossRef PubMed; (g) P. J. Hotez, A. Fenwick and E. F. Kjetland, PLoS Neglected Trop. Dis., 2009, 3, e430 Search PubMed; (h) J. Seubert, R. Pohlke and F. Loebich, Experientia, 1977, 33, 1036 CrossRef CAS PubMed; (i) T. M. Bilharz, Z. Wiss. Zool., Abt. A, 1853, 4, 53 Search PubMed; (j) T. M. Bilharz, Wien. Med. Wochenschr., 1856, 6(49), 65 Search PubMed; (k) T. M. Bilharz, Z. kais.-kgl. Ges. Ärzte Wien., 1858, 14, 447 Search PubMed; (l) C. H. King and A. A. Mahmoud, Ann. Intern. Med., 1989, 110, 290 CrossRef CAS PubMed; (m) World Health Organization, WHO Model List of Essential Medicines, World Health Organization, Geneva, 18th edn, 2013, pp. 1–43 Search PubMed.
- A. S. K. Tsang, K. Ingram, J. Keiser, D. B. Hibbert and M. H. Todd, Org. Biomol. Chem., 2013, 11, 4921 CAS.
- All our efforts to synthesize the amine by hydrogenation using RANEY®Ni afforded the decyanated N-PMP protected tetrahydroisoquinoline.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H-NMR, 13C-NMR for all compounds are available. See DOI: 10.1039/c5ra10552h |
| This journal is © The Royal Society of Chemistry 2015 |