Electrocatalytic cycle of P450 cytochromes: the protective and stimulating roles of antioxidants
V. V. Shumyantseva*a,
A. A. Makhovab,
T. V. Bulkoa,
A. V. Kuzikova,
E. V. Shichb,
V. Kukesb and
A. I. Archakova
aInstitute of Biomedical Chemistry, Pogodinskaya Street, 10, Moscow 119121, Russia. E-mail: viktoria.shumyantseva@ibmc.msk.ru; Fax: +7 499 245 08 57; Tel: +7 499 246 58 20
bI. M. Sechenov First Moscow State Medical University, Russia
First published on 11th August 2015
Abstract
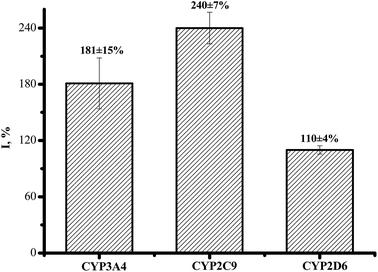
The electrochemical activity of cytochrome P450 (CYP) 3A4, 2C9 and 2D6 on a screen-printed graphite electrode nanostructured with gold nanoparticles and stabilized with didodecyldimethylammonium bromide was examined. The analysis of the CYP catalytic activity was carried out using a variety of electrochemical techniques, such as cyclic voltammetry, square wave voltammetry and amperometry. A sensitive electrochemical CYP-sensor system was proposed as a reliable candidate for investigating the influence of medicinal preparations – ethoxidol (2-ethyl-6-methyl-3-hydroxypyridine malate), mexidol (2-ethyl-6-methyl-3-hydroxypyridine succinate), cytochrome c and L-carnitine – on the CYP redox behavior and electrocatalytic activity. In the presence of antioxidants, the enhancement of the electrochemical signal of CYP was registered. It was shown that ethoxidol, in the concentration range of 90–600 μM, stimulates the electrochemical reduction of P450 cytochromes, with the maximum stimulating effects for CYP3A4, CYP2C9 and CYP2D6 being estimated as 181 ± 15%, 240 ± 7% and 110 ± 4% (at 540 μM ethoxidol), respectively.
Introduction
P450 cytochromes (CYPs) are typical external monooxygenases capable of catalyzing the oxygenation of a variety of organic substances using NAD(P)H and O2 as cosubstrates. CYPs can act as electron carriers in reducing compounds during the catalytic cycle and as an oxidase when generating superoxide anions and hydrogen peroxide as reactive oxygen species with organic substrate oxygenation. Water formation is characteristic of a CYP functioning as a monooxygenase, so the enzyme is also called the mixed-function oxidase. CYPs are able to act as four-electron oxidases and reduce oxygen to two water molecules.1–6 However, despite such multifunctionality, the main functions of P450 cytochromes are monooxygenation reactions, i.e. the hydroxylation of organic molecules, drugs and foreign chemicals in the body. From this viewpoint, the in vitro biocatalysis with CYPs is a promising approach for drug assays in practical clinical medicine as well as for the analysis of drug–drug interactions, and the substrate/inhibitor competence of these enzymes.Investigation of the catalytic activity of the isolated cytochromes from the P450 superfamily requires the presence of redox partners and electron donors (NADPH). However, the availability of redox partners is not obligatory upon the electrochemical reduction of the P450 family hemoproteins, so the catalytic system is essentially simplified.7–19 Electrochemical systems exhibit dual function: they substitute partner proteins and serve as a source of electrons for redox enzymes.
From a chemical viewpoint, this diversity of functions of CYPs is based on the oxidative–reductive properties of the heme iron of the P450 cytochromes. From an electrochemical viewpoint, the most characteristic feature of hemoproteins is their ability to perform direct electron transfer from the electrode surface to the heme iron and vice versa, as is reflected on the voltammogram (in the absence of oxygen) by the appearance of a pair of peaks corresponding to the oxidation and reduction processes. The electrode reaction of the heme group may be described with the equation:
| Fe(III) + e− (from the electrode) + H+ ↔ Fe(II) |
The relevance of the electrochemical approach is apparent as cytochrome P450-based electrodes may be used as biosensors in personalized medicine, high-throughput screening and drug interference studies, as well as in therapeutic drug monitoring. Studies aimed at the search of new drugs and estimation of their toxicity and drug–drug interactions have shown that P450 cytochromes are the most significant preparations for use in practical clinical medicine.
One of the most important methods in personalized medicine is the tracing of a dose of a clinical drug and correction of drug intake by taking into account specific features of the patient. Pharmacogenetic tests reveal the risk of side effects of a number of drugs (for example, the anticoagulant warfarin) and allow the doses of medicinal preparations to be changed and/or corrected. An individual reaction to warfarin is caused by single nucleotide polymorphisms of the genes coding for CYP2C9, and vitamin K-epoxy reductase (VKORC1). Thus, Roche developed genetic tests based on a microarray technique (Amplichip CYP450) for genomic predictions.5,17
Despite the importance and broad information content of pharmacogenetic tests, their results do not allow influence to be exerted on the activity of the drug metabolizing enzymes. Therefore, it is necessary to develop other approaches, based on the regulation of enzyme activity by means of biologically active compounds or metabolic drug preparations.
The functional significance of P450 cytochromes (CYPs) includes the metabolism of drugs, foreign chemicals and endogenous compounds. P450 cytochromes play an important role in the detoxification of bioactive compounds and hydrophobic xenobiotics, both coming from the outside (medicines, drugs, food supplements, environment pollutants) and being formed inside the cells (cholesterol, saturated and unsaturated fatty acids, steroids, prostaglandins and others) of living organisms. CYP3A4 recognizes and metabolizes a broad range of structurally diverse therapeutic agents.5,20 Consequently, many clinically relevant drug–drug interactions are associated with the inhibition and/or induction of this enzyme.21 Antioxidant therapy is used as an additional agent in cellular defense against reactive oxygen species (ROS) and in the treatment of different diseases.22
In the present study, we have investigated the influence of the metabolic drugs ethoxidol, mexidol and cytochrome c possessing antioxidant properties on the electrochemical activity of P450 enzymes. Medicinal preparations of ethoxidol and mexidol based on 2-ethyl-6-methyl-3-hydroxypyridine are widely used in clinical practice. They possess antioxidant, nootropic and cerebroprotective effects. We found that the drug-antioxidants mexidol, ethoxidol and cytochrome c stimulated the redox behavior of cytochromes P450 3A4, 2C9 and 2D6 and enhanced the electrochemical signal, corresponding to heme reduction. L-Carnitine had no effect on CYP electrochemical activity. The data obtained support the opportunity to regulate the pharmacokinetic parameters and the pronounced pharmacodynamic effects due to the impact of the antioxidant medicinal preparations on the activities of P450 3A4, 2C9 and 2D6.
Materials and methods
Apparatus
Electrochemical studies were performed using an AUTOLAB 12 potentiostat/galvanostat (Metrohm Autolab, the Netherlands) with GPES software (version 4.9.7). All measurements were taken at room temperature. Electrochemical studies on cytochrome P450 3A4, 2C9 and 2D6 were performed in 0.1 M potassium phosphate buffer containing 0.05 M NaCl, pH 7.4 (PBS). In this work, three-pronged screen-printed electrodes (SPE) were used (AvtoKOM, Russia; http://www.membrans.ru) with working and auxiliary graphite electrodes (graphite paste was from Acheson) and a Ag/AgCl reference electrode. The diameter of the working electrode was 2 mm. All potentials are referenced to the Ag/AgCl reference electrode.Cyclic voltammograms (CV) were recorded at the scan rate of 10–100 mV s−1. The parameters used in square wave voltammetry (SWV, reduction, aerobic conditions) were as follows: the initial potential, 100 mV; the final potential, −600 mV; the step potential, 5 mV; the amplitude, 20 mV and the frequency, 10 to 100 Hz. For the presentation of all electrochemical data, the average values of the maximum cathodic peak heights of SWV from three independent experiments were used. Electrolysis was conducted at the controlled potential of −0.5 V for 20 min (erythromycin) and 60 min (testosterone and diclofenac). The apparent Michaelis–Menten (Km) constant was determined using the electrochemical form of the Michaelis–Menten equation21 from the amperometry at the controlled reduction potential of −0.5 V in 1 mL of air-saturated buffer with the injection of erythromycin or testosterone. The apparent Michaelis–Menten (Km) constant for diclofenac was determined previously.7,23
Materials and reagents
The following reagents were used: didodecyldimethylammonium bromide (DDAB), HAuCl4·3H2O, sodium borohydride, itraconazole (Sigma-Aldrich, USA), diclofenac (2-(2,6-dichloraniline)phenylacetonic acid) (Novartis, Russia), ethoxidol (2-ethyl-6-methyl-3-hydroxypyridine malate), 50 mg mL−1 (Sintez, Russia), mexidol (2-ethyl-6-methyl-3-hydroxypyridine succinate), 50 mg mL−1 (Farmasoft, Russia), erythromycin (Sigma-Aldrich), acetic acid, ammonium acetate, acetylacetone (Spektr-Khim, Russia), and testosterone (Sigma-Aldrich).In electrochemical experiments, freshly prepared solutions of 10 mM diclofenac in water, 10 mM erythromycin in ethanol–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 10 mM testosterone in ethanol–water (7
3), 10 mM testosterone in ethanol–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and 10 mM itraconazole in dimethylsulfoxide were used. Recombinant cytochrome P450 3A4 (182 μM) and 2C9 (175 μM) were from the Institute of Bioorganic Chemistry (Minsk, Belarus) and 2D6 (30 μM) was from Sigma. The concentration of P450 enzymes was determined from the production of a reduced complex with carbon monoxide with the absorption coefficient24 ε450 = 91 mM−1 cm−1.
3) and 10 mM itraconazole in dimethylsulfoxide were used. Recombinant cytochrome P450 3A4 (182 μM) and 2C9 (175 μM) were from the Institute of Bioorganic Chemistry (Minsk, Belarus) and 2D6 (30 μM) was from Sigma. The concentration of P450 enzymes was determined from the production of a reduced complex with carbon monoxide with the absorption coefficient24 ε450 = 91 mM−1 cm−1.
P450-electrodes were prepared as earlier described.25,26 Briefly, the surface of the working graphite electrode was covered with 2 μL of a 5 mM colloidal gold solution in 0.1 M DDAB in chloroform and after the evaporation of chloroform (10 min), 1 μL of 18.2 μM P450 3A4, 1 μL of 17.5 μM P450 2C9 or 2 μL of 30 μM P450 2D6 was placed on the electrode. To prevent the complete drying of the electrodes, they were kept for 12 h at 4 °C in a humid chamber.
Determination of the N-demethylase activity of cytochrome P450 3A4
Formaldehyde is one of the products of the cytochrome P450 3A4-dependent electrocatalytic N-demethylation of erythromycin, and its accumulation was used to assess the activity of the enzyme immobilized on the electrode. The electrode with immobilized cytochrome P450 3A4 was placed into a 1 mL electrochemical cell that contained potassium-phosphate buffer (pH 7.4) and 100 μM of erythromycin. Electrolysis was performed at the controlled potential of E = −0.5 V for 20 min. After the electrolysis, the reaction mixture was mixed with the Nash reagent (4 M ammonium acetate, 0.1 M glacial acetic acid, 0.04 M acetyl acetone) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and incubated at 37 °C for 30 min to develop the color.27 The concentration of formaldehyde produced during the cytochrome P450-dependent electrocatalytic N-demethylation of erythromycin was determined spectrophotometrically using a Cary 100 Scan UV-Vis spectrophotometer (Agilent, the Netherlands), the absorption coefficient being ε412 = 4 mM−1 cm−1 as in ref. 28 and 29.
1 and incubated at 37 °C for 30 min to develop the color.27 The concentration of formaldehyde produced during the cytochrome P450-dependent electrocatalytic N-demethylation of erythromycin was determined spectrophotometrically using a Cary 100 Scan UV-Vis spectrophotometer (Agilent, the Netherlands), the absorption coefficient being ε412 = 4 mM−1 cm−1 as in ref. 28 and 29.
The HPLC/MS analysis of 6β-hydroxytestosterone and 4-hydroxydiclofenac was conducted as described previously.30
Results
The influence of antioxidants on the electrochemical reduction and electrochemical response of CYP3A4
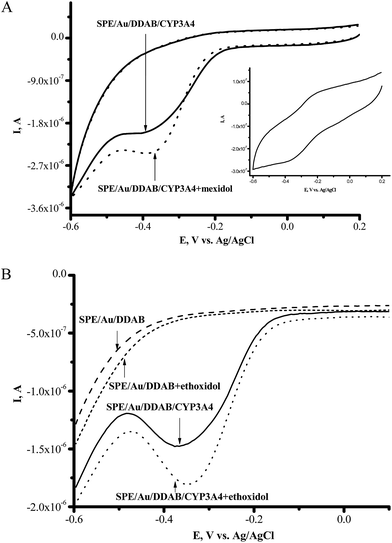
Screen-printed electrodes modified with a membrane-like synthetic surfactant (didodecyldimethylammonium bromide (DDAB)) and gold nanoparticles (SPE/Au/DDAB) were used for immobilization of CYP3A4 on the electrode surface (SPE/Au/DDAB/CYP3A4). To investigate the electroanalytical characteristics of electrochemical systems with cytochrome P450 immobilized on the nanostructured electrode surface, we have resorted to cyclic voltammetry, voltammetric analysis (square wave voltammetry) and amperometry at the controlled reduction potential of −0.5 V (vs. Ag/AgCl).A clear pair of peaks was observed using CV (in argon) with the formal redox potential E0 = (Ered + Eox)/2 = −0.302 V for CYP3A4 (Fig. 1A, inset). As we have shown earlier using SPE/Au/DDAB, with the increase of scan rate, the anodic and cathodic peak currents of anaerobic CV also increased and appeared to be proportional to the scan rate,25,26 showing typical evidence for the manifestation of a surface-controlled process.
The antioxidant/antihypoxant mexidol (2-ethyl-6-methyl-3-hydroxypyridine succinate) and ethoxidol (2-ethyl-6-methyl-3-hydroxypyridine malate) (both widely used in clinical practice) were tested as inducers/modulators of CYP3A4 activity. Ethoxidol and mexidol stimulated the electrochemical reduction of cytochrome P450 3A4 (Fig. 1A). Both of these compounds did not produce type I differential binding spectra as are typical for the substrates of CYP enzymes.1–3 In electrochemical experiments, drug-antioxidants serve as the modulating and/or stimulating additives with respect to the electrochemical activity of CYP due to their free-radical scavenging, antihypoxant, and/or electron mediator properties. The increase in the reductive cathodic current in the presence of ethoxidol was also studied by the more sensitive voltammetric analysis method, square wave voltammetry (SWV) (see Fig. 1B). Ethoxidol itself did not produce any pronounced peaks in the region of potentials of +0.1 to −0.7 V.
In our experiments, ethoxidol and mexidol enhanced the cathodic reduction current of CYP3A4 in a dose-dependent manner (Fig. 2). At an ethoxidol concentration of 540 μM, its stimulating effect on CYP3A4 reduction reached 180 ± 15%. Mexidol is not as active as ethoxidol in the range of 200–600 μM and only produces 155 ± 15% enhancement at 540 μM drug concentration. As we have shown earlier, the electrochemical response of CYP3A4 also increased in the presence of antioxidants such as vitamin C, vitamin A, vitamin E26 and taurine (2-aminoethanesulfonic acid).29
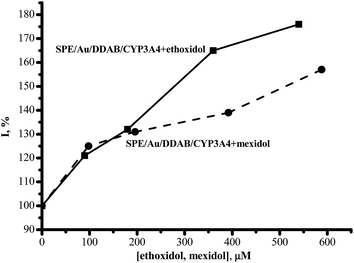 | ||
| Fig. 2 The dependence of the cathodic current intensity of the reductive peak of SWV (%) on the concentration of ethoxidol and mexidol. | ||
Cytochrome c is a metabolic preparation with antihypoxant properties. This drug is used in the therapy of processes dealing with pathological oxidative reactions. This cytochrome c stimulated the electrochemical reduction of CYP3A4 in a dose-dependent manner (Fig. 3). After the application of 20–100 μM cytochrome c, the maximum enhancement of the reductive peak current of CYP3A4 (140 ± 10%) was observed with 60 μM cytochrome c.
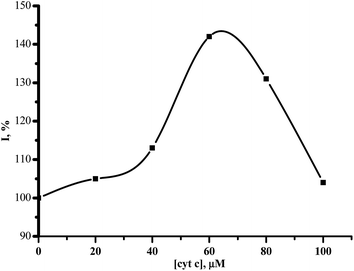 | ||
| Fig. 3 The dependence of the cathodic current intensity (%) on the concentration of cytochrome c (0–100 μM). | ||
The vitamin-like biologically active compound L-carnitine ((3R)-3-hydroxy-4-trimethylammonium butanoate) manifests itself as a strong antioxidant; it acts by reducing and scavenging free radicals and by enhancing the activity of antioxidant enzymes.31 To investigate the influence of L-carnitine on the electrochemical parameters of CYP3A4, we used SPE/Au//DDAB/CYP3A4. In the range of 100–600 μM L-carnitine, the voltammetric SWV peak for CYP3A4 in the presence and absence of this compound had the same position and intensity. L-Carnitine did not produce any reductive current enhancement or any inhibition of the cathodic current (Fig. 4).
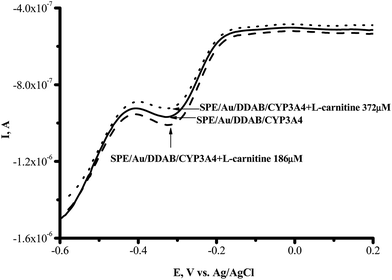 | ||
Fig. 4 Square-wave voltammograms (SWVs) (reductive curves) of SPE/Au/DDAB/CYP3A4 (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ); SPE/Au/DDAB/CYP3A4 + L-carnitine (186 μM) ( ); SPE/Au/DDAB/CYP3A4 + L-carnitine (186 μM) (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ); and SPE/Au/DDAB/CYP3A4 + L-carnitine (372 μM) (⋯). ); and SPE/Au/DDAB/CYP3A4 + L-carnitine (372 μM) (⋯). | ||
The absence of the stimulating effect of L-carnitine on the reductive peak and electrochemical response of CYP3A4 can possibly be explained by the polar structure of this substance and the lack of interaction with the heme or the active site of the CYP. It is known that the main function of a CYP enzyme is to catalyze oxidative transformations and to hydroxylate hydrophobic xenobiotics or endogenous compounds, thereby producing more polar products capable of being excreted.1–3
Electrocatalysis of the CYP enzymes in the presence of antioxidants
We have studied the influence of the antioxidant ethoxidol on the catalytic current generated by CYP3A4 enzymes in the presence of their typical substrates erythromycin, diclofenac and testosterone. We have conducted the cyclic voltammetry of CYP3A4 on the Au/DDAB modified electrode in the presence of 1 mM erythromycin as the substrate, in oxygenated 0.1 M potassium phosphate buffer (pH 7.4). In the case of erythromycin, the catalytic current was registered, indicating the occurrence of the CYP3A4-catalyzed drug metabolism reaction (Fig. 5).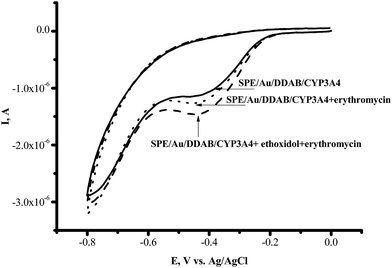 | ||
Fig. 5 Cyclic voltammograms of SPE/Au/DDAB/CYP3A4 (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ); SPE/Au/DDAB/CYP3A4 + erythromycin (1 mM) (⋯); SPE/Au/DDAB/CYP3A4 + erythromycin (1 mM) + ethoxidol (180 μM) ( ); SPE/Au/DDAB/CYP3A4 + erythromycin (1 mM) (⋯); SPE/Au/DDAB/CYP3A4 + erythromycin (1 mM) + ethoxidol (180 μM) (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). ). | ||
With erythromycin as the substrate, the ratio of the catalytic current in the presence of oxygen to the catalytic current in the presence of erythromycin corresponded to IO2/Ier = 1.30 ± 0.01 (100%), while with ethoxidol plus erythromycin the ratio corresponded to IO2/Ier+et = 1.70 ± 0.04 (131%). The catalytic current of CYP3A4 in the presence of diclofenac and testosterone as measured using SWV, was found to be stimulated by ethoxidol (Fig. 6).
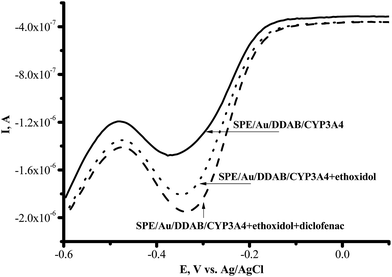 | ||
Fig. 6 Square-wave voltammograms (SWVs) (reductive curves) of SPE/Au/DDAB/CYP3A4 (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ); SPE/Au/DDAB/CYP3A4 + ethoxidol (90 μM) (⋯); SPE/Au/DDAB/CYP3A4 + ethoxidol (90 μM) + diclofenac (100 μM) ( ); SPE/Au/DDAB/CYP3A4 + ethoxidol (90 μM) (⋯); SPE/Au/DDAB/CYP3A4 + ethoxidol (90 μM) + diclofenac (100 μM) (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). ). | ||
The influence of ethoxidol on the N-demethylation of erythromycin was studied spectrophotometrically at 412 nm by means of formaldehyde formation using the Nash reagent.21,29,30 The catalytic constant kcat for the N-demethylation of erythromycin was calculated for CYP3A4 + erythromycin and for CYP3A4 + ethoxidol + erythromycin, via formaldehyde quantification using a Nash method.27 The values obtained in both cases were approximately the same: 3.1 ± 0.3 min−1 and 2.7 ± 0.3 min−1, respectively.
The product formation for the N-demethylation of erythromycin was measured using the Nash reagent as well as using HPLC/MS analysis of 6β-hydroxytestosterone and 4-hydroxydiclofenac. Analysis of the reaction products and the influence of antioxidants on the substrate conversion are summarized in Table 1. Only in the case of taurine was the increase of N-demethylation of erythromycin registered.29 The apparent Michaelis–Menten constant (Km) of CYP3A4 for erythromycin was 70 ± 8 μM and for testosterone was 67 ± 10 μM.
| Enzyme + substrate | Antioxidant | Km, μM | kcat, min−1 |
|---|---|---|---|
| a n.d. – not determined. *Product formation was determined using mass spectrometry. **Product formation was determined using the Nash reagent. | |||
| CYP3A4 + testosterone | — | 67 ± 10 | 0.003 ± 0.0005* |
| CYP3A4 + testosterone | Cytochrome c | — | 0.002 ± 0.0005*↓↑ |
| CYP3A4 + testosterone | Ethoxidol | — | n.d. |
| CYP3A4 + diclofenac | — | 40 ± 8 (ref. 7) | 0.01 ± 0.006* |
| CYP3A4 + diclofenac | Cytochrome c | — | 0.004 ± 0.0003*↓ |
| CYP3A4 + diclofenac | Ethoxidol | — | 0.003 ± 0.0006*↓ |
| CYP3A4 + erythromycin | — | 70 ± 8 | 3.1 ± 0.3** |
| CYP3A4 + erythromycin | Taurine | — | ↑3.6 ± 0.3**29 |
| CYP3A4 + erythromycin | Ethoxidol | — | 2.7 ± 0.3**↓ |
| CYP2C9 + diclofenac | — | 4.3 ± 2.4 (ref. 18) | 0.005 ± 0.0005* |
| CYP2C9 + diclofenac | Cytochrome c | — | 0.004 ± 0.0003*↓↑ |
| CYP2C9 + diclofenac | Ethoxidol | — | 0.002 ± 0.0001*↓ |
The clinical estimation of cytochrome P450 3A4 activity was carried out using a noninvasive approach by determining the concentration ratio of 6β-hydroxycortisol (produced from cortisol only under the catalysis of cytochrome P450 3A4) and cortisol (6β-hydroxycortisol/cortisol). Clinical experiments in ethoxidol-treated patients revealed a statistically significant increase in the concentration ratio of 6β-hydroxycortisol to cortisol before and after a course of ethoxidol intake, which suggests an increase in the cytochrome P450 3A4 activity towards cortisol as an endogenous substrate of this P450 isoenzyme. Concentrations of 6β-hydroxycortisol and cortisol were determined in urine using routine high-performance liquid chromatography (HPLC).32 The determination of the 6β-hydroxycortisol/cortisol ratio in urine showed that the ethoxidol intake enhances this ratio from 3.95 ± 0.88 to 4.92 ± 0.96 at a statistically relevant level.32 The results obtained proved the ability of antioxidants not only to stimulate the electrochemical reduction of CYP3A4, but also to influence the clinical substrate metabolism in patients.
Evaluation of ethoxidol’s influence on the redox parameters of CYP2C9 and CYP2D6
CYP2C9 is recognized as one of the most important drug-metabolizing enzymes in humans, responsible for the hepatic clearance of many non-steroidal and anti-inflammatory agents. CYP2C9 is involved in the metabolism of ∼16% of therapeutically important drugs such as the anticoagulant warfarin, hypoglycemic tolbutamide and glipzide. CYP2D6 metabolizes basic drugs and other xenobiotics containing a nitrogen atom, such as beta-blockers and tricyclic antidepressants.2 Both of these enzymes have a polymorphic human form with different catalytic constants towards widely used drugs.5,33–35 The influence of ethoxidol on the CYP2C9- and CYP2D6-produced electrochemical responses was studied using the SWV technique (Fig. 7 and 8). It is shown that ethoxidol stimulated the reductive processes of these CYP enzymes. Importantly, the same ethoxidol concentration had different stimulating effects on the CYP3A4, 2C9 and 2D6 enzymes (Fig. 9). The most effective stimulation by ethoxidol was registered for CYP2C9.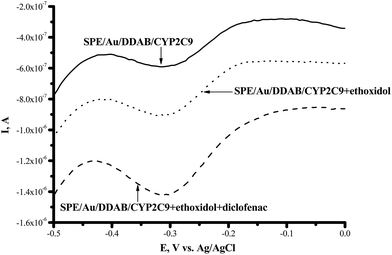 | ||
Fig. 7 Square-wave voltammograms (SWVs) (reductive curves) of SPE/Au/DDAB/CYP2C9 (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ); SPE/Au/DDAB/CYP2C9 + ethoxidol (90 μM) (⋯); SPE/Au/DDAB/CYP2C9 + ethoxidol (90 μM) + diclofenac (100 μM) ( ); SPE/Au/DDAB/CYP2C9 + ethoxidol (90 μM) (⋯); SPE/Au/DDAB/CYP2C9 + ethoxidol (90 μM) + diclofenac (100 μM) (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). ). | ||
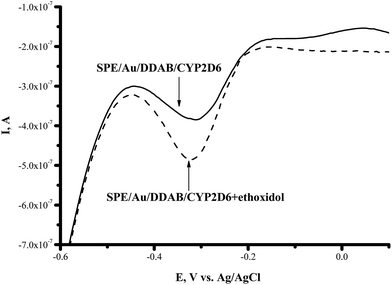 | ||
Fig. 8 Square-wave voltammograms (SWVs) (reductive curves) of SPE/Au/DDAB/CYP2D6 (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ); SPE/Au/DDAB/CYP2D6 + ethoxidol (90 μM) ( ); SPE/Au/DDAB/CYP2D6 + ethoxidol (90 μM) (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). ). | ||
We have found that antioxidants could influence the direct electron transfer of human CYP3A4, 2C9 and 2D6 enzymes immobilized on an electrode surface. In electrochemical experiments, drug-antioxidants served as modulating and/or stimulating additives with respect to the electrochemical activity of P450 cytochromes due to their free radical scavenging and antihypoxant properties.
Discussion
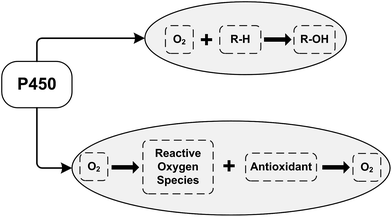
Our earlier electrochemical studies were performed to examine the influence of the B group vitamins on the catalytic activity of cytochrome P450 3A4 towards diclofenac.25 Diclofenac (voltaren) is a non-steroidal anti-inflammatory drug used for the treatment of arthritis, ankylosing spondylitis and acute muscle pain.25,31,36 We have shown that thiamine (vitamin B1) and riboflavin (vitamin B2) blocked the catalytic activity of cytochrome P450 3A4 towards diclofenac. Diclofenac produced a 137% increase of catalytic current without thiamine, but in the presence of thiamine, only 67% of the catalytic current can be measured. Thiamine acts as a noncompetitive inhibitor against organic substrates (diclofenac) with the electrochemical inhibition constant Ki = 0.45 ± 0.15 mM.25 Therefore, thiamine prevents the electrochemical reduction of P450 3A4 and reduces the catalytic activity of cytochrome P450 3A4 towards diclofenac. Riboflavin (vitamin B2) also exhibits inhibitory properties with respect to the electrocatalytic activity of P450 3A4 on the electrochemical SPE/DDAB/Au/CYP3A4 + diclofenac system. Our results demonstrate that based on the electrochemical behavior of the electrode/P450 3A4 system, it is possible to assess the influence of the B group vitamins on the catalytic activity of cytochrome P450 alone and to evaluate the substrate/inhibitor competence of this enzyme. As has been shown in pharmacokinetic experiments with volunteers, the application of B group vitamins allows the duration of diclofenac therapy to be shortened and the daytime dose of this drug to be reduced.25Vitamins with antioxidant properties, such as vitamin C, vitamin A, and vitamin E stimulate the electrochemical reduction of cytochrome P450 3A4.26 Vitamin C (in the range 0.03–1 mM) and vitamins A and E (in the range 10–100 μM) stimulated the dose-dependent growth of the cathodic peak current of cytochrome P450 3A4, corresponding to heme reduction according to the equation Fe(III) + 1e → Fe(II). Electrochemically-driven P450 catalysis is accompanied by ROS generation;31 therefore the influence of free radical-scavenging substances (ROS “traps” or antioxidants) on electrocatalysis may be reasonably expected (Scheme 1, R–H is the substrate for cytochrome P450; R–OH is the product of P450-catalyzed monooxygenase reaction).
Reactive oxygen species interact with cytochrome P450, causing inactivation of this protein.37–40 Antioxidants can diminish the level of ROS by the radical-scavenging effect, modulating the activities of CYPs, which are known to generate reactive intermediates. Scavenging substances are essential in the antioxidant defense against ROS, and can influence the catalytic functions of this hemoprotein. In CYP catalysis, the antioxidants may play the role of antihypoxant substances, increasing the local oxygen level (Scheme 1).
The modulation of the catalytic activity of CYP is a very important problem in the pharmacokinetic and therapeutic areas. CYP2C9 enzyme has two allelic variants (CYP2C9*2 and CYP2C9*3) due to a single nucleotide polymorphism. These allelic variants of drug-metabolizing enzymes possess different catalytic parameters (Km and kcat) towards the substrates S-warfarin and tolbutamide.33,34 This phenomenon must be taken into account during drug intake.
From this viewpoint, the search for substances capable of modulating the catalytic activity of CYP enzymes is a very promising task.
It was shown previously that vitamin C and cytochrome c can enhance the electron transfer in reactions mediating redox processes by serving as nonspecific redox activity facilitators for heme peroxidases such as chloroperoxidase and horseradish peroxidase.41 It was also shown that vitamin C, being a strong antioxidant, is capable of scavenging ROS in low concentration ranges, and possesses prooxidant capacity in high concentration.42 Our experimental data also revealed the active role of antioxidants in CYP electrocatalysis.
The electrochemically driven cytochrome P450 catalysis is an alternative model system for pharmacological research and drug–drug interaction studies. The increase in the reductive cathodic current might be attributed to the Fe3+ heme reduction, reflecting the stimulating effect of antioxidants. Based on the analysis of the electrochemical parameters of P450, an algorithm, allowing the elucidation of the influence of antioxidants on the electrochemical activity of CYP, was developed. Electrochemical investigations encompassed CYP3A4, CYP2C9 and CYP2D6 isoenzymes. The electrochemical experiments have elucidated the possible mechanism of the dose-dependent interactions of antioxidants with clinical drugs. These findings provide data for future clinical risk prediction studies – especially for those devoted to the interaction of drugs with antioxidants. Regulation and modulation of cytochrome P450 3A4 activity through the action of vitamin-antioxidants, upon their appointment in combination with clinical drugs metabolized by P450, will probably become an essential requirement in routine clinical practice. Antioxidant intake can lead to alteration of pharmacodynamic efficiency and demands special attention from the doctor since the prescribed medical product can bring about changes in the efficiency/safety profile.
It is necessary to keep in mind and to consider the interplay and balance between the antioxidant properties of drugs, dealing with (i) the ability of antioxidants to destroy hydrogen peroxide (a participant in CYP peroxide shunt reactions) on the one hand, and (ii) the stabilizing effect of antioxidants on hemeproteins (realizing itself through the lowering of the ROS level) on the other hand, and the antihypoxant properties of antioxidants (supplying CYPs by with additional oxygen) must be taken into account upon drug intake. Electrochemically driven CYP reactions may have practical relevance, providing a useful tool for drug assay studies.
Disclosure
The authors report no conflict of interest in this work.Acknowledgements
The work is done in the framework of the State Academies of Sciences fundamental research program for 2013–2020.References
- A. A. Archakov and G. I. Bachmanova, Cytochrome P450 and Active Oxygen, Taylor & Francis, London, 1990 Search PubMed.
- D. F. V. Lewis, Guide to Cytochromes P450: Structure and Function, Taylor & Francis, London, 2001 Search PubMed.
- P. R. Ortiz de Montellano, Cytochrome P450: Structure, Mechanism, and Biochemistry, Kluwer Academic/Plenum Publishers, New York, 2005 Search PubMed.
- E. G. Hrycay and S. M. Bandiera, Arch. Biochem. Biophys., 2012, 522, 71–89 CrossRef CAS PubMed.
- U. Zanger and M. Schwab, Pharmacol. Ther., 2013, 138, 103–141 CrossRef CAS PubMed.
- D. W. Nebert and D. W. Russel, Lancet, 2002, 360, 1155–1162 CrossRef CAS.
- V. Shumyantseva, T. Bulko, E. Suprun, Y. Chalenko, M. Vagin, Y. Rudakov, M. Shatskaya and A. Archakov, Biochim. Biophys. Acta, Proteins Proteomics, 2011, 1814, 94–101 CrossRef CAS PubMed.
- V. Shumyantseva, E. Suprun, T. Bulko, Y. Chalenko and A. Archakov, in Nanomedicine in Diagnostics, ed. N. Rozlosnik, CRC Press, Taylor & Francis Group, New York, 1st edn, 2012, ch. 4, pp. 68–95 Search PubMed.
- E. Schneider and D. Clark, Biosens. Bioelectron., 2013, 39, 1–13 CrossRef CAS PubMed.
- A. Yarman, U. Wollenberger and F. W. Scheller, Electrochim. Acta, 2013, 110, 63–72 CrossRef CAS PubMed.
- H. Colas, K. Ewen, F. Hannemann, N. Bistolas, U. Wollenberger, R. Bernhardt and P. de Oliveira, Bioelectrochemistry, 2012, 87, 71–77 CrossRef CAS PubMed.
- R. W. Estabrook, K. M. Faulkner, M. S. Shet and C. W. Fisher, Methods Enzymol., 1996, 272, 44–51 CAS.
- N. Bistolas, U. Wollenberger, C. Jung and F. W. Scheller, Biosens. Bioelectron., 2005, 20, 2408–2423 CrossRef CAS PubMed.
- V. Shumyantseva, T. Bulko and A. Archakov, J. Inorg. Biochem., 2005, 99, 1051–1063 CrossRef CAS PubMed.
- A. K. Udit and H. B. Gray, Biochem. Biophys. Res. Commun., 2005, 338, 470–476 CrossRef CAS PubMed.
- C. Baj-Rossi, J. T. Rezzonico, A. Cavallini, F. Grassi, G. de Michelli and S. Carrara, Biosens. Bioelectron., 2014, 53, 283–287 CrossRef CAS PubMed.
- C. Baj-Rossi, G. de Micheli and S. Carrara, in Biosensors for Health, Environment and Biosecurity, ed. A. Serra, InTech Publisher, Vienna, 2011, pp. 448–482 Search PubMed.
- A. Fantuzzi, L. H. Mak, E. Capria, V. Dodhia, P. Panicco, S. Collins and G. Gilardi, Anal. Chem., 2011, 83, 3831–3839 CrossRef CAS PubMed.
- P. Sun and Y. Wu, Sens. Actuators, B, 2013, 178, 113–118 CrossRef CAS PubMed.
- S. Zhou, C. Xue, X. Yu, C. Li and G. Wang, Ther. Drug Monit., 2007, 29, 687–708 CrossRef CAS PubMed.
- A. Sucheta, R. Cammack, J. Weiner and F. Armstrong, Biochemistry, 1993, 32, 5455–5465 CrossRef CAS.
- G. S. Celep and F. Marotta, Oxid. Antioxid. Med. Sci., 2014, 3, 5–8 CrossRef.
- S. Sadeghi, S. Ferrero, G. Di Nardo and G. Gilardi, Bioelectrochemistry, 2012, 86, 87–91 CrossRef CAS PubMed.
- T. Omura and R. Sato, J. Biol. Chem., 1964, 239, 2379–2385 CAS.
- A. A. Makhova, V. V. Shumyantseva, E. V. Shich, T. V. Bulko, V. G. Kukes, O. S. Sizova, G. V. Ramenskaya, S. A. Usanov and A. I. Archakov, BioNanoScience, 2011, 1, 46–52 CrossRef.
- V. V. Shumyantseva, A. A. Makhova, T. V. Bulko, A. V. Kuzikov, E. V. Shich, E. V. Suprun, V. Kukes, S. Usanov and A. Archakov, Oxid. Antioxid. Med. Sci., 2013, 2, 113–117 Search PubMed.
- T. Nash, Biochem. J., 1953, 55, 416–421 CrossRef CAS.
- V. V. Shumyantseva, T. V. Bulko, G. P. Kuznetsova, N. F. Samenkova and A. I. Archakov, Biochemistry, 2009, 74, 438–444 CAS.
- V. V. Shumyantseva, A. A. Makhova, T. V. Bulko, R. Bernhardt, A. V. Kuzikov, E. V. Shich, V. G. Kukes and A. I. Archakov, Biochemistry, 2015, 80, 366–373 CAS.
- O. V. Gnedenko, E. O. Yablokov, S. A. Usanov, D. V. Mukha, G. V. Sergeev, T. V. Bulko, A. V. Kuzikov, N. E. Moskaleva, V. V. Shumyantseva, A. S. Ivanov and A. I. Archakov, Chem. Phys. Lett., 2014, 593, 40–44 CrossRef CAS PubMed.
- G. Guerreiro, C. P. Mescka, A. Sitta, B. Donida, D. Marchetti, T. Hammerschmidt, J. Faverzani, D. Coelho, M. Wajner, C. S. Dutra-Filho and C. R. Vargas, Int. J. Dev. Neurosci., 2015, 42, 10–14 CrossRef CAS PubMed.
- V. A. Otdelenov, V. V. Smirnov, A. V. Dmitriev, V. V. Poroikov, V. V. Shumyntseva, L. M. Krasnykh, D. A. Sychev and V. G. Kukes, Lekarstv. Prep. Ratsional. Farmakoterap., 2013, 3, 30–36 Search PubMed.
- D. L. Johson, B. C. Lewis, D. J. Elliot, J. O. Miners and L. L. Martin, Biochem. Pharmacol., 2005, 69, 1533–1541 CrossRef PubMed.
- Y. Mie, E. Tateyama and Y. Komatsu, Electrochim. Acta, 2014, 115, 364–369 CrossRef CAS PubMed.
- A. Bogni, M. Monshouwer, A. Moscone, M. Hidestrand, M. Ingelman-Sundberg, T. Hartung and S. Coecke, Toxicol. In Vitro, 2005, 19, 621–629 CrossRef CAS PubMed.
- S. Shen, M. R. Marchick, M. R. Davis, G. A. Doss and L. R. Pohl, Chem. Res. Toxicol., 1999, 12, 214–222 CrossRef CAS PubMed.
- Y. O. Rudakov, V. V. Shumyantseva, T. V. Bulko, E. V. Suprun, G. P. Kuznetsova, N. F. Samenkova and A. I. Archakov, J. Inorg. Biochem., 2008, 102, 2020–2025 CrossRef CAS PubMed.
- H. Yasui, S. Hayashi and H. Sakurai, Drug Metab. Pharmacokinet., 2005, 20, 1–13 CrossRef CAS.
- F. P. Guengerich, Biochemistry, 1978, 17, 3633–3639 CrossRef CAS.
- S. Bondy and S. Naderi, Biochem. Pharmacol., 1994, 48, 155–159 CrossRef CAS.
- S. Gade, S. Bhattacharya and K. Manoj, Biochem. Biophys. Res. Commun., 2012, 419, 211–214 CrossRef CAS PubMed.
- C. Bian, H. Xiong, X. Zhang, Y. Ye, H. Gu and S. Wang, Sens. Actuators, B, 2012, 169, 368–373 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |