Real-time monitoring of self-assembling worm-like micelle formation by organic transistors†
V. Preziosi*a,
G. Tarabella*b,
P. D'Angelob,
A. Romeob,
M. Barraa,
S. Guidoc,
A. Cassinesea and
S. Iannottab
aCNR-SPIN and Physics Dep., University Federico II, Piaz. Tecchio 80, I-80125 Naples, Italy. E-mail: valentina.preziosi@unina.it
bIMEM-CNR, Parco Area delle Scienze 37/A, I-43124 Parma, Italy. E-mail: giuseppe.tarabella@imem.cnr.it
cDepartment of Chemical, Materials and Production Engineering, University Federico II, Piaz. Tecchio 80, I-80125 Naples, Italy
First published on 21st January 2015
Abstract
Thanks to their appealing rheological properties, nowadays micellar solutions play a fundamental role in many industrial sectors, including personal care products, cosmetics and pharmaceuticals. The use of these solutions and their effectiveness in the desired application is, however, strongly dependent on the specific micellar aggregation form and the related degree of entanglement. To this end, it should be mentioned that, still today, the type and size of the micellar aggregates in a solution can be only investigated by using sophisticated laboratory techniques, which are very difficult to be applied directly in real industrial production sites. In this study, we demonstrate that organic electrochemical transistors (OECTs) based on PEDOT:PSS active channels can be used to reveal the presence of worm-like micelles in surfactant–salt aqueous solutions. In the worm-like regime the response of these devices displays distinctive features in comparison with those exhibited when pure surfactant solutions, containing only spherical micelles, are analysed. Our results could pave the way to the development of a new class of portable and user-friendly devices, suitable for the in situ monitoring of morphological transitions, involving micellar aggregates.
Introduction
Nowadays, surfactants play an extraordinary role in the worldwide industry due to their almost ubiquitous applications in the finalization of products such as detergents, cosmetics, pharmaceuticals and foods, as witnessed by the current global production of surfactants largely exceeding 10 Mton per year.1 The importance of surfactants is related to the amphiphilic character of their structure, determined by the contemporary presence of hydrophilic (water-soluble) and hydrophobic (water-insoluble) groups. In this way, surfactants are able to promote the miscibility of otherwise immiscible liquids (e.g. water and oil) by decreasing the interfacial tension between them.2 This process favours the stabilization of emulsions and foams, and makes them suitable for use in consumer goods. Another distinctive feature of surfactants is their ability, when dispersed in an aqueous solution and above a certain critical concentration value (CMC), to aggregate forming micelles of various shape and size. This self-assembling process is basically driven by the surfactant requirement to reach a stable energy condition, obtained by the means of a structural rearrangement that minimizes the exposure of the hydrophobic tails to water.Over the last few years, the micellization phenomenon in surfactant systems has been widely studied, analysing the specific influence of several factors (e.g., the surfactant concentration, the solvent type, the solution temperature and the addition of other substances) on micelles formation and their final shape.3–7 In particular, it was demonstrated that the addition of organic or inorganic salts to surfactant aqueous solutions promotes the conversion of basic spherical micelles (i.e. the simplest micellar aggregation form) in more complex structures exhibiting worm-like shapes8 with a variable degree of entanglement.4,9 Nowadays, worm-like micellar solutions are widely studied for their peculiar rheological properties,7–13 which make them increasingly attractive in many industrial processes such as in enhanced oil recovery, where they are used as fracturing fluids, or in cosmetics and biomedical industries.14,15
As widely reported in previous studies, in worm-like systems the counterion nature of the added salt can induce strong structural changes, in terms of micellar flexibility, characteristic lengths and scission energy (that is the energy required to create two end-caps from a semi-infinite cylinder), according to whether or not it penetrates into the micelle creating a strong binding effect.7,16 However, small changes in the formulation of worm-like micelles can also drastically affect their structure and dynamics, resulting in a different macroscopic rheological response. As worm-like micelles show many analogies with polymers, they are also called “living polymers”17,18 and can be used as model fluids to investigate polymer behaviour.9 Nevertheless, unlike polymers, worm-like micelles can rather easily break and reversibly return to their spherical structure. Therefore, from a theoretical point of view, worm-like micelles can be described by a model based on the reptation theory,18 which describes the rheological properties of the entangled polymeric chains, but including the effects of reversible scission kinetics, derived by Cates9 and still widely used.19,20 The model basically states that micelles relax mono-exponentially with a single characteristic time τR, which takes into account both the reptation and breaking processes. In this situation their linear viscoelastic properties, represented by G′ and G′′ spectra, can be accurately described by the Maxwell-fluid model with a single relaxation time. In this model, it should be remembered that G′ is defined as the elastic (or storage) modulus, which takes into account the stored energy, while G′′ is defined as the viscous (or loss) modulus, which is, instead, a measure of the energy mechanically dissipated in the material. According to the Maxwell-fluid model, G′ and G′′ can be described by the following equations:
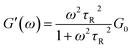 | (1) |
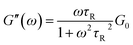 | (2) |
From previous reports it is also known that when a steady shear is applied to a worm-like structure, the solution undergoes a transition between a homogenous and a non-homogeneous shear flow, the latter being characterized by the formation of macroscopic regions in the fluid with different viscosity (shear banding).20–22 Shear banding is an abrupt transition that occurs at a critical shear rate, ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) *, in correspondence to which the flow curve exhibits a discontinuity of slope (τp), followed by a stress plateau that stretches over more than a decade in shear rates. At higher shear rates, a further increase in stress takes place. However, in some worm-like systems the plateau is not totally flat but exhibits a slight increase as a function of the shear rate.
*, in correspondence to which the flow curve exhibits a discontinuity of slope (τp), followed by a stress plateau that stretches over more than a decade in shear rates. At higher shear rates, a further increase in stress takes place. However, in some worm-like systems the plateau is not totally flat but exhibits a slight increase as a function of the shear rate.
Despite the enormous scientific interest in the rheological properties of worm-like systems and their related practical applications, it has to be pointed out that, still today, the presence of worm-like aggregates in a surfactant aqueous solution is checked by specific laboratory techniques displaying different levels of complexity and accuracy. Among these, Laser Light Scattering (LLS), rheometry, Cryogenic-Transmission-Electron Microscopy (Cryo-TEM) and Small Angle Neutron Scattering (SANS) are some of the most used experimental approaches.4,8,23–26
Recently, the possibility of using an organic electrochemical transistor (OECT) based on a PEDOT:PSS (poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonate)) channel as a portable device to monitor the formation of micellar aggregates in a surfactant (namely, cetyltrimethylammonium bromide – CTAB) solution27 and for liposome sensing28 have been reported. In an OECT, the PEDOT:PSS channel connects two electrodes (source and drain) and is in contact with an electrolytic medium, which acts as the gate dielectric. The device is completed by a third electrode (gate), which is directly immersed in the electrolyte solution. When a positive voltage (VGS) is applied between the gate and source, the positively charged species contained in the electrolyte are pushed away from the gate electrode and are injected into the PEDOT:PSS backbone, neutralizing the PSS− sites and de-doping the active channel.29,30 Hence, this process provides a reversible VGS – controlled decrease in the drain-source current (IDS), which can flow along the active channel for a given drain-source voltage (VDS). In detail, it was shown that if a CTAB solution is employed as the electrolyte for the OECT gating, the related current modulation rapidly increases when the surfactant concentration overcomes the CMC value.27 By means of a detailed UV/VIS optical analysis, this phenomenon was found to be directly related to the improved capability of CTA+ micelles with respect to metallic cations, for instance, to reversibly de-dope the PEDOT:PSS channel by electrically balancing the PSS− sites.
Inspired by this initial work, herein we have investigated the response of OECTs based on PEDOT:PSS in the presence of more complex micellar aggregates characterized by an elongated worm-like shape. To this purpose, two different CTAB systems, prepared through the addition of both organic (sodium salicylate – NaSal) and inorganic (potassium bromide – KBr) salts, have been employed. First, the rheological properties of the selected solutions have been studied to confirm the formation of the worm-like aggregation. Then, the OECT electrical responses in the presence of worm-like solutions have been evaluated by the means of both steady state and dynamic electrical measurements, which have been compared with those achievable in pure NaSal, KBr and CTAB solutions. Our results demonstrate that the OECT behaviour is strongly affected by the size of the micellar aggregates and the related level of entanglement. This last feature, in particular, seems to be a major factor in defining the way micelles can electrically interact with the PEDOT:PSS chains.
Materials and methods
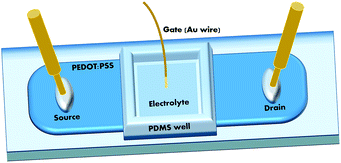
A PEDOT:PSS aqueous dispersion (Clevios PH500) was purchased from Heraeus Conductive Polymers Division. In order to achieve films with enhanced conductivity (up to 102 S cm−1), the commercial PEDOT:PSS dispersion was doped with ethylene glycol (Sigma Aldrich, 20% in vol.) and dodecyl-benzene sulfonic acid (DBSA) surfactant (Sigma Aldrich, 0.05% in vol.). The final solution was spun on glass slides, partially covered with Kapton tape in order to define the device active channel. Films were spin-coated with a first ramp of 6 s at 450 rpm with acceleration up to 1500 rpm, followed by a plateau of 30 s at 1500 rpm (final measured thickness of ∼80 nm), and then baked in air on a hotplate at 120 °C for 1 h. After the annealing process, the Kapton tape was removed resulting in a 2 cm long, 1 mm wide PEDOT:PSS channel. For all devices considered in this study, a poly(dimethylsiloxane) (PDMS) well was attached on the active channel to confine the electrolyte solution. In all cases, a Gold (Au) wire immersed in the solution was used as the gate electrode. Fig. 1 shows a schematic of the complete layout of the investigated devices. The OECT electrical responses were tested with two different measurement stations, both using 2-channel source-meters (Agilent B2902A and Keithley 2612A).As electrolytes, the aqueous solutions reported in Table 1 have been considered: CTAB (C19H42BrN, PM = 364.5, purity ≥98%) and NaSal (HOC6H4COONa, PM = 160.10, purity ≥98%) were purchased from Sigma Aldrich, whereas KBr (PM = 119.00) was purchased from VWR International Ltd.
| Electrolyte | C [M] | Conductivity [mS cm−1] | |
|---|---|---|---|
| 1 | CTAB | 0.01 | 0.29 |
| 2 | NaSal | 0.045 | 0.72 |
| 3 | CTAB | 0.05 | 0.37 |
| 4 | KBr | 0.25 | 28.75 |
| 5 | KBr | 0.5 | 52.00 |
| 6 | CTAB + KBr | 0.01–0.25 | 41.00 |
| 7 | CTAB + KBr | 0.01–0.5 | 55.00 |
| 8 | CTAB + NaSal | 0.05–0.045 | 4.74 |
The solutions were prepared under ambient conditions and their conductivity was estimated by a conductivity meter (PC 2700, Eutech Instruments) at ∼25 °C. Before their use as electrolytes, the worm-like micellar solutions were left to completely stabilize for an adequate amount of time (2 days). Prior to every OECT measurement, the micellar solutions were stirred for 30 min.
Rheological measurements were performed using a controlled-stress rheometer (Bohlin Instruments CVO 120) operating both in continuous shear and in dynamic oscillatory mode using a 60 mm, 1 deg smooth stainless steel cone-plate geometry. The sample was loaded on the plate center and possible air bubbles were removed. All measurements were performed at room temperature (∼23 °C).
Results and discussion
Rheological characterization
CTAB has been widely used in previous studies as a surfactant for its ability to form micellar structures with different shapes at concentrations higher than its critical micellar concentration (CMC ∼ 10−3 M at 298 K). It has also been reported that the addition of a salt to a CTAB solution can largely shift the CMC value, therefore favoring the formation of worm-like micelles.7Moreover, the structure and dynamics of worm-like micelles are highly modified by the specific nature of the salt counterion, which directly affects the macroscopic rheological behaviour of the final solutions.31–37 In the CTAB/salt systems, in particular, it has been widely demonstrated that the interaction mechanism of Sal− ions is different to that of Br− ions on the surfactant structure.38,39 Indeed, while the former can deeply penetrate the micellar structure (the aromatic part of the counter ion is solubilized in the micelle, increasing the degree of binding), the latter are non-penetrating counterions, providing a weak interaction with the surfactant and consequently a different rheological response.8
In order to experimentally assess the rheological features of the worm-like systems (CTAB + KBr and CTAB + NaSal) investigated in this study, their viscoelastic behaviour was evaluated by applying oscillatory deformations to the samples and measuring the corresponding G′ and G′′ spectra. In Fig. 2A, G′ and G′′ are plotted as a function of the angular frequency in the range 0.2–20 rad s−1 for the CTAB + KBr solution. For this system, the viscous modulus G′′ predominates over the elastic modulus G′ throughout almost the entire frequency range studied. Moreover, especially in the low frequency range, the low elasticity of this system results in G′ values close to the instrument sensitivity. Fig. S1A (see ESI†) represents the continuous shear test (viscosity vs. shear rate) performed on this sample, confirming the non-Newtonian character of this worm-like fluid and, in particular, the occurrence of a shear thinning behaviour with the viscosity decreasing as a function of the shear rate.40 In Fig. 2B, the linear viscoelastic response of the CTAB + NaSal system is shown. Herein, G′ and G′′ are measured in the frequency range 0.01–10 rad s−1, and both moduli values are at least two orders of magnitude higher than those of the CTAB + KBr system. The viscosity measurements reported in Fig. S1B† for this system reveals a Newtonian behaviour, featuring a constant viscosity up to about 0.3 s−1 and a decreasing trend occurring for higher shear rates. The calculated slope of the power law is about 0.85, which is in agreement with a semi-dilute polymer behaviour.41 In Fig. 2C, a Cole–Cole representation for the CTAB + NaSal system, where G′′ is plotted as a function of G′, is presented. As shown, the experimental data (symbols) can be very well fitted by a semi-circle equation (solid line) corresponding to an ideal Maxwell element with a single relaxation time (τR = 1.6 s).
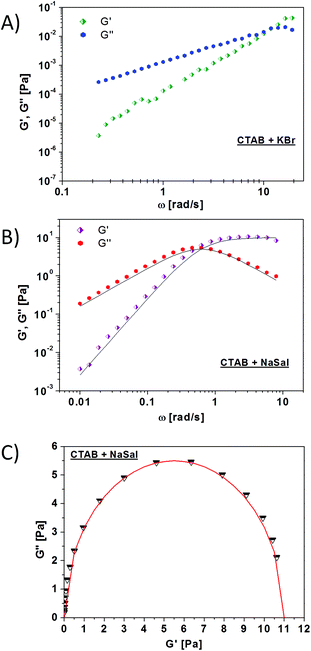 | ||
| Fig. 2 Elastic (G′) ad viscous (G′′) modulus as a function of the oscillation frequency measured for (A) CTAB + KBr and (B) CTAB + NaSal worm-like solutions. The solid lines in panel B are the fits calculated from eqn (1) and (2). (C) Cole–Cole plot for the CTAB + NaSal system (the dashed red line corresponds to the fit with the equation of a semi-circle). | ||
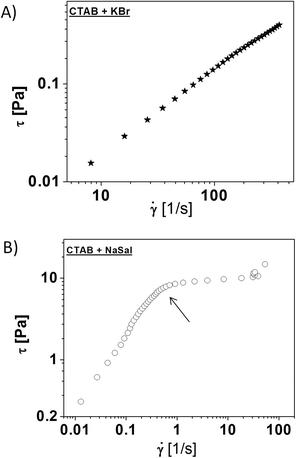
Fig. 3 shows the shear stress as a function of the shear rate for the two investigated systems. In Fig. 3A (CTAB + KBr), an increasing trend of the shear stress, τ, against the shear rate, ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) , is shown, whereas for CTAB + NaSal (Fig. 3B) three regions are visible. The first one corresponds to a Newtonian regime, where the stress varies linearly with the shear rate. In the second one, the shear stress increases up to a value of the shear rate,
, is shown, whereas for CTAB + NaSal (Fig. 3B) three regions are visible. The first one corresponds to a Newtonian regime, where the stress varies linearly with the shear rate. In the second one, the shear stress increases up to a value of the shear rate, ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) * = 0.5 s−1, where the flow curve exhibits a discontinuity of slope, followed by a stress plateau for two decades in
* = 0.5 s−1, where the flow curve exhibits a discontinuity of slope, followed by a stress plateau for two decades in ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . In the third one, an increase at higher shear rates (
. In the third one, an increase at higher shear rates (![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ∼ 20 s−1) occurs.
∼ 20 s−1) occurs.
All the rheological data discussed above make it clear that worm-like micellar structures are present in both the two CTAB-based systems analyzed. The micelles, however, are characterized by a different size and level of entanglement in close agreement with the data previously reported.9
Basically, the CTAB + KBr systems display a poor viscoelastic response with the related worm-like micelles being poorly entangled. Laser Light Scattering (LLS) measurements have revealed that, in the CTAB + KBr solutions, having a CTAB concentration equal to 0.01 M, the hydrodynamic correlation length (Rh) of the worm-like micelles increases from 20 nm up to about 40 nm when the KBr concentration, CKBr, goes from 0.2 to 0.6 M.38 Moreover, other experiments suggest that, in this system, the persistence length, lp of the micelles (defined as the maximum length of the uninterrupted polymer chain persisting in a particular direction) is about 15 nm.9,25,38
The situation is quite different for the previously observed CTAB + NaSal solution, which exhibits an enhanced viscoelastic behavior. According to the previous data, for a CTAB + NaSal solution with CTAB = 0.05 M and NaSal = 0.05 M, the hydrodynamic size Rh is found to be about 10 nm,36 whereas lp is instead in the order of 40 nm.18,20,39 Therefore, it can be concluded that in the presence of NaSal, which is able to penetrate the initial CTAB micellar structures, the final micelles assume a cylindrical shape having an increased aspect ratio (i.e. long and thin micelles). This feature strongly supports the formation of entangled structures, which make this fluid more similar to a gel-like system.
OECT electrical response
For the OECT characterization, the use of a gold wire as the gate electrode (see Fig. 1) for all the considered solutions allowed working in a purely capacitive mode, without the occurrence of any Faradaic reaction at the gate/electrolyte interface.42 Platinum was not taken into consideration as a gate material to avoid (as verified in some preliminary tests) the oxidation reaction, involving the –OH group in the salicylate anion (SaL−), similar to that observed for the OECT operation in an aqueous suspension of a synthetic eumelanin.43OECT modulation ratio
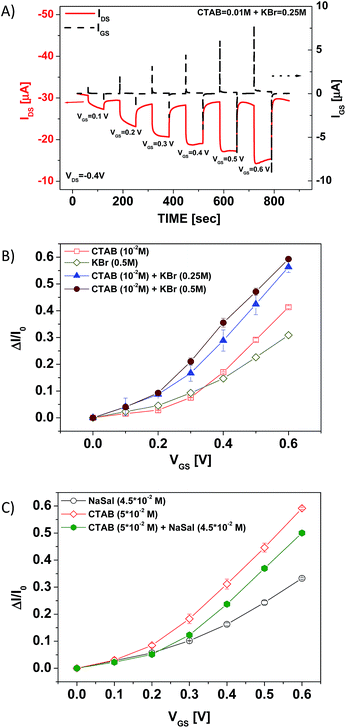
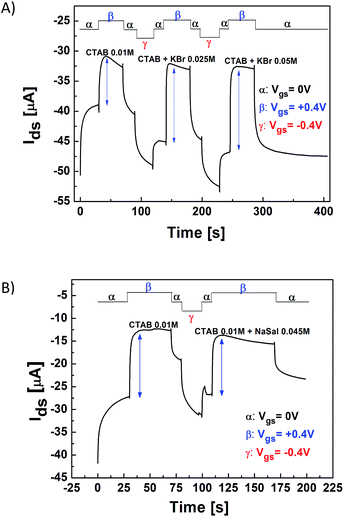
In the first set of experiments, the OECT response characterization was carried out by recording the drain-source current (IDS) and the corresponding gate-source current (IGS) as a function of time, whereas the drain-source (VDS) voltage was set constant at −0.4 V and the gate-source voltage (VGS) was pulsed from 0 to 0.6 V with a step (ΔVGS) of 0.1 V (transient current measurements). The duration of both VGS > 0 V and VGS = 0 V steps was set at 60 s, in order to allow the IDS current to reach a nearly steady state and to recover the initial ON current value (I0).27 According to our experimental results, for the complex electrolytic media considered in this study, the application of VGS voltages not exceeding 0.6 V remarkably improves the device testing reproducibility.Fig. 4A reports a typical IDS vs. time curve recorded for a CTAB + KBr worm-like solution covering the OECT active channel. In the same plot, the IGS measurement is also shown. These curves clearly indicate that the OECT can work properly also in the complex surfactant–salt mixed solutions investigated. Indeed, in agreement with the aforementioned OECT working principle, when a VGS > 0 pulse is applied, the IDS current decreases significantly, whereas the IGS remains orders of magnitude lower than IDS, neglecting the presence of the displacement current spikes, which correspond to the VGS changes and are related to the capacitive coupling between the gate electrode and the electrolyte.29
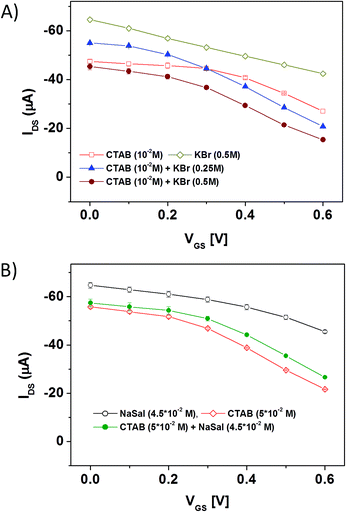
To compare the OECT performances in the different solutions, the current modulation ratio, defined as ΔI/I0 = |[I(VGS > 0) − I(VGS = 0)/I(VGS = 0)]|,44 was extracted from the transient current measurements as a function of VGS. Fig. 4B and C summarize the results achieved for the two different systems (CTAB + KBr and CTAB + NaSal) investigated in this study. In the case of CTAB + KBr, it is possible to observe that the OECT current modulation is significantly enhanced by the presence of worm-like micellar aggregates (CTAB + KBr solutions), in comparison with those recorded with pure KBr (0.5 M) salt or a solution with only spherical micelles (CTAB 0.01 M). In particular, these data demonstrate that the OECT sensitivity in the worm-like regime is the highest in the entire VGS range investigated. Fig. 4B shows also that, when the KBr concentration is increased (from 0.25 M to 0.5 M), the OECT current modulation undergoes a further enhancement. This feature cannot be simply ascribed to the increased presence of the salt, since (see the corresponding curves in Fig. S3A†) the OECT modulation response in pure KBr aqueous solutions is the same for these two KBr concentrations. Consequently, this finding is rather related to the shift of the average size of the worm-like aggregates, whose hydrodynamic correlation length (Rh) is almost doubled for CKBr going from 0.25 M to 0.5 M.38 The analysis of the OECT response for the CTAB + NaSal system (Fig. 4C) provides a very different scenario. In this case, indeed, it can be easily observed that the OECT current modulation (ΔI/I0) in the presence of the spherical micelles (CTAB 0.05 M) is the highest, in comparison, in particular, to what occurs with the worm-like aggregates obtained by mixing CTAB and NaSal.
Very significantly, this last result reveals that, for this system, the level of entanglement in the micellar structures rather than the simple presence of worm-like micelles was able to dominate the OECT current modulation behaviour.
FET-like transfers and real-time measurements
To get a further confirmation of the experimental results discussed above, a complementary OECT measurement was performed by directly recording the transfer-curves of the transistor (Fig. 5). Herein, VGS was varied between 0 and 0.6 V with steps of 0.1 V and differently from the transient IDS measurements, the time interval between the VGS application and the IDS value recording was set at 5 s, whereas VGS was continuously raised up from 0 V to 0.6 V without the restoring step at VGS = 0 V. The transfer curves gave a further indication about the selectivity of the OECT response according to the specific morphology of the investigated micellar aggregates. In particular, it was observed that those solutions, providing enhanced OECT current modulations (as reported in Fig. 4B and C), also tend to shift the transfer curves to lower gate voltages with an equivalent decrease of the ON current (IDS at VGS = 0 V).45 Interestingly, current modulation data extracted from the transfer-curves (see Fig. S2†) exhibit an almost perfect agreement with the results in Fig. 4. Therefore, while the CTAB + KBr worm-like solutions are shown to provide the OECT with an increased modulation capability when compared with the parent CTAB (0.01 M) solution containing only spherical micelles, the entangled worm-like micelles in the CTAB + NaSal system are confirmed to be less effective in de-doping the PEDOT:PSS active channel.Finally, in order to demonstrate with more evidence, the possibility to use OECTs for monitoring the real-time formation of micelles, we performed a set of flow measurements. For both the micellar solutions investigated here (CTAB + KBr and CTAB + NaSal), the IDS current was recorded upon progressive and real-time substitution of the electrolyte in the PDMS well in the following order: CTAB 0.01 M → CTAB 0.01 M + KBr 0.25 M → CTAB 0.01 M + KBr 0.5 M and CTAB 0.05 M → CTAB 0.05 M + NaSal 0.045 M, respectively. These measurements provide a direct and intuitive dynamic picture of the changes taking place in complex systems, witnessing the general ability of the electrochemical devices in displaying real-time variations that can be induced in the rheological systems of interest. The two surfactant–salt systems were analysed separately as shown in Fig. 6. VGS was swept during the different phases of the experiment between the values +0.4 V and −0.4 V, as reported explicitly in Fig. 6. VDS, instead, was set again at −0.4 V. It should be mentioned that, in this case, the application of negative gate-steps allowed inducing a fast recovery of the original value of the IDS current, corresponding to the ON state (VGS = 0 V) of the device. In this respect, the recovery of the channel current induced by the depletion of micelles in the polymer channel is field-assisted (application of negative VGS), unlike the case of current modulation (depletion assisted by a diffusive mechanism) and FET-like transfer characteristic measurements (where the step-like increase of VGS corresponds to a progressive injection of micelles into the polymer).
In any case, in full agreement with the previously discussed data, the real-time flow measurements confirm once again the different behavior of the two worm-like micellar aggregates with respect to their corresponding spherical micelles.
The main aspect emerging from these real-time measurements regards the reliability of all the measurements carried out so far. In fact, the self-consistent trends, showed by both the current modulation curves and transfer characteristics recorded in the presence of different micellar aggregates are the result of reversible interactions between the conducting channel and the analysed electrolytes. Therefore, irreversible changes, involving the polymer conducting state, such as those induced by repeated measurements in presence of other complex electrolytes, can be ruled out in these experiments.
Discussion and conclusions
In this study, we have tested the electrical response of OECTs based on PEDOT:PSS active channels when surfactant–salt aqueous solutions, containing worm-like micelles, are employed as electrolytic media. First of all, our results demonstrate that OECTs are able to work properly and reliably also in the presence of these complex liquids.More specifically, it was found that, in comparison with a CTAB solution having the same surfactant concentration, CTAB + KBr worm-like micelles provide an enhanced current modulation and the related transfer-curves tend to shift towards the lower VGS voltages. The opposite trends were observed for the CTAB + NaSal gel-like system, whose entangled worm-like micelles are less effective in de-doping the PEDOT:PSS channel.
It is quite important to highlight, once again, that these differences in the OECT behaviour are not trivially related to the different types or concentrations of the salts contained in the solutions. Despite this, the salt concentration affects basically the final solution conductivity σ, (see Table 1, for the worm-like solutions σ is always higher than the corresponding one in the spherical micellar regime). Fig. S3A† demonstrates unambiguously that the OECT modulation ratio is practically the same for all the types and concentrations of salts considered in this study. In particular, in all pure salt aqueous solutions investigated, ΔI remains about 0.3 even for VGS = 0.6 V.
Even more importantly, the changes in the OECT response detected for the previously analyzed CTAB + KBr and CTAB + NaSal solutions cannot be ascribed to the difference in the CTAB concentration (0.01 M vs. 0.05 M). In this regard, we performed a further experiment, investigating the OECT response in a worm-like solution containing CTAB 0.05 M + KBr 0.5 M. In Fig. S3B,† the corresponding current modulation ratio was compared with that achieved for the pure CTAB 0.05 M solution (with spherical micelles) and with the gel-like CTAB 0.05 M + NaSal 0.045 M fluid. This comparison confirms that, although keeping the surfactant concentration (CTAB 0.05 M) fixed, the OECT response is remarkably dependant on the size of the micellar structures and, notably, on their level of entanglement.
As a whole, our experimental observations seem to depict a scenario, where the ability of the cationic species contained in the solutions to penetrate into the porous PEDOT:PSS film plays the fundamental role in determining the final OECT modulation response. Consequently, in this framework, the level of entanglement in the micellar structures can strongly limit their effectiveness to interact and electrically balance the PSS− sites, therefore providing a reduced de-doping effect in the transistor active channel (i.e. lower ΔI).
In conclusion, the results discussed in this study confirm the relevance of PEDOT:PSS OECTs when applied as portable and miniaturized electronic devices, operating in complex surfactant solutions for monitoring the presence and the in situ formation of micellar aggregates with different shapes and sizes. Future work will be devoted to analyze the OECT behaviour when in contact with micellar solutions based on various surfactants, exhibiting different CMC values and progressively increasing size of the starting spherical micelles.
Acknowledgements
The authors would like to thank Prof. Luigi Paduano for the stimulating discussion on worm-like solutions. Eng. Antonio Perazzo is also acknowledged for the stimulating discussion on rheological measurements. This study has been financially supported by the Provincia Autonoma di Trento, call “Grandi progetti 2012”, project “Madelena”, the N-Chem project within the CNR-NANOMAX Flagship program and the Mastri project (CUP B25B09000010007).Notes and references
- D. Myers, Surfactant science and technology, John Wiley & Sons, 2005 Search PubMed
.
- V. B. Fainerman, N. Mucic, V. Pradines, E. V. Aksenenko and R. Miller, Langmuir, 2013, 29, 13783–13789 CrossRef CAS PubMed
.
- Z. Chu, C. A. Dreiss and Y. Feng, Chem. Soc. Rev., 2013, 42, 7174–7203 RSC
.
- R. D. Koehler, S. R. Raghavan and E. W. Kaler, J. Phys. Chem. B, 2000, 104, 11035–11044 CrossRef CAS
.
- Z. Lin, J. Cai, L. Scriven and H. Davis, J. Phys. Chem., 1994, 98, 5984–5993 CrossRef CAS
.
- Z. Lin, Langmuir, 1996, 12, 1729–1737 CrossRef CAS
.
- C. Oelschlaeger, P. Suwita and N. Willenbacher, Langmuir, 2010, 26, 7045–7053 CrossRef CAS PubMed
.
- C. A. Dreiss, Soft Matter, 2007, 3, 956–970 RSC
.
- M. Cates and S. Candau, J. Phys.: Condens. Matter, 1990, 2, 6869–6892 CrossRef CAS
.
- B. Lin, S. Mohanty, A. V. McCormick and H. T. Davis, Mesoscale Phenomena in Fluid Systems, American Chemical Society, 2003, pp. 313–326 Search PubMed
.
- M. Vasudevan, A. Shen, B. Khomami and R. Sureshkumar, J. Rheol., 2008, 52, 527–550 CrossRef CAS
.
- N. Dubash, J. Cardiel, P. Cheung and A. Q. Shen, Soft Matter, 2011, 7, 876–879 RSC
.
- J. Yang, Curr. Opin. Colloid Interface Sci., 2002, 7, 276–281 CrossRef CAS
.
- R. Cressely and V. Hartmann, Eur. Phys. J. B, 1998, 6, 57–62 CrossRef CAS
.
- V. P. Torchilin, Pharmaceut. Res., 2007, 24, 1–16 CrossRef CAS PubMed
.
- T. H. Ito, P. C. Miranda, N. H. Morgon, G. Heerdt, C. A. Dreiss and E. Sabadini, Langmuir, 2014, 30, 11535–11542 CrossRef CAS PubMed
.
- M. A. Fardin and S. Lerouge, Soft Matter, 2014, 10, 8789–8799 RSC
.
- M. Cates, Macromolecules, 1987, 20, 2289–2296 CrossRef CAS
.
- M. E. Cates and S. M. Fielding, Adv. Phys., 2006, 55, 799–879 CrossRef CAS
.
- J. F. Berret, Molecular gels, Springer, 2006, pp. 667–720 Search PubMed
.
- R. L. Moorcroft and S. M. Fielding, Phys. Rev. Lett., 2013, 110, 086001–086005 CrossRef
.
- S. Caserta, M. Simeone and S. Guido, Phys. Rev. Lett., 2008, 100, 137801–137804 CrossRef
.
- T. Clausen, P. Vinson, J. Minter, H. Davis, Y. Talmon and W. Miller, J. Phys. Chem., 1992, 96, 474–484 CrossRef CAS
.
- E. Cappelaere, J. Berret, J. Decruppe, R. Cressely and P. Lindner, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1997, 56, 1869–1878 CrossRef CAS
.
- U. Olsson, O. Soederman and P. Guering, J. Phys. Chem., 1986, 90, 5223–5232 CrossRef CAS
.
- N. C. Das, H. Cao, H. Kaiser, G. T. Warren, J. R. Gladden and P. E. Sokol, Langmuir, 2012, 28, 11962–11968 CrossRef CAS PubMed
.
- G. Tarabella, G. Nanda, M. Villani, N. Coppedè, R. Mosca, G. G. Malliaras, C. Santato, S. Iannotta and F. Cicoira, Chem. Sci., 2012, 3, 3432–3435 RSC
.
- G. Tarabella, A. G. Balducci, N. Coppedè, S. Marasso, P. D'Angelo, S. Barbieri, M. Cocuzza, P. Colombo, F. Sonvico and R. Mosca, Biochim. Biophys. Acta, 2013, 4374 CrossRef CAS PubMed
.
- D. A. Bernards and G. G. Malliaras, Adv. Funct. Mater., 2007, 17, 3538–3544 CrossRef CAS
.
- D. Nilsson, M. Chen, T. Kugler, T. Remonen, M. Armgarth and M. Berggren, Adv. Mater., 2002, 14, 51–54 CrossRef CAS
.
- H. Rehage and H. Hoffmann, Mol. Phys., 1991, 74, 933–973 CrossRef CAS
.
- F. Kern, R. Zana and S. Candau, Langmuir, 1991, 7, 1344–1351 CrossRef CAS
.
- T. Shikata, H. Hirata and T. Kotaka, Langmuir, 1988, 4, 354–359 CrossRef CAS
.
- F. Kern, P. Lemarechal, S. Candau and M. Cates, Langmuir, 1992, 8, 437–440 CrossRef CAS
.
- G. Porte, J. Appell and Y. Poggi, J. Phys. Chem., 1980, 84, 3105–3110 CrossRef CAS
.
- S. Amin, T. W. Kermis, R. M. van Zanten, S. J. Dees and J. H. van Zanten, Langmuir, 2001, 17, 8055–8061 CrossRef CAS
.
- A. Koike, T. Yamamura and N. Nemoto, Colloid Polym. Sci., 1994, 272, 955–961 CAS
.
- W. Zhang, G. Li, J. Mu, Q. Shen, L. Zheng, H. Liang and C. Wu, Chin. Sci. Bull., 2000, 45, 1854–1857 CrossRef CAS
.
- V. Aswal, P. Goyal and P. Thiyagarajan, J. Phys. Chem. B, 1998, 102, 2469–2473 CrossRef CAS
.
- C. W. Macosko and J. Mewis, Suspension Rheology, VCH, 1994 Search PubMed
.
- J. D. Ferry, Viscoelastic properties of polymers, John Wiley & Sons, 1980 Search PubMed
.
- G. Tarabella, C. Santato, S. Y. Yang, S. Iannotta, G. G. Malliaras and F. Cicoira, Appl. Phys. Lett., 2010, 97, 1233041–1233043 CrossRef PubMed
.
- G. Tarabella, A. Pezzella, A. Romeo, P. D'Angelo, N. Coppedè, M. Calicchio, M. d'Ischia, R. Mosca and S. Iannotta, J. Phys. Chem. B, 2013, 1, 3843–3849 CAS
.
- Z. T. Zhu, J. T. Mabeck, C. Zhu, N. C. Cady, C. A. Batt and G. G. Malliaras, Chem. Commun., 2004, 1556–1557 RSC
.
- P. Lin, F. Yan and H. L. Chan, ACS Appl. Mater. Interfaces, 2010, 2, 1637–1641 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14118k |
| This journal is © The Royal Society of Chemistry 2015 |