Sample-pretreatment-free based high sensitive determination of aflatoxin M1 in raw milk using a time-resolved fluorescent competitive immunochromatographic assay
Abstract
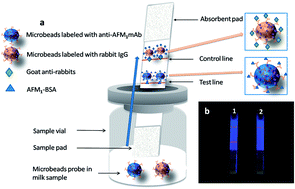
A highly-sensitive time-resolved fluorescent immunochromatographic assay (TRFICA) was developed to detect aflatoxin M1 (AFM1) in raw milk samples within 6 minutes without any sample pretreatments. This method could meet the requirement for rapid and sensitive milk monitoring in dairy farms and milk industries. Based on a competitive format and the home-made monoclonal antibody 2C9 against AFM1, this assay enhanced the sensitivity from 0.3 ng mL−1 (by using nanogold-strip method previously reported) to 0.03 ng mL−1 (by using this TRFICA method). The improved sensitivity could probably result from the increases in both the higher affinity of monoclonal antibody 2C9 against AFM1 and detection signals of immunoassay probes (with each europium microbead coupled with numbers of 2C9 antibodies). The TRFICA method showed a considerable dynamic range of 0.1–2.0 ng mL−1 and spiked recoveries of 80–110% for AFM1 quantification in raw milk. The results via TRFICA method was found to be high consistency (R2 = 0.988) with results via standard high-performance liquid chromatography (HPLC) method, when detecting AFM1 in 17 blind milk samples.

 Please wait while we load your content...
Please wait while we load your content...