Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The consequences for human health of stratospheric ozone depletion in association with other environmental factors
R. M.
Lucas
*ab,
M.
Norval
c,
R. E.
Neale
d,
A. R.
Young
e,
F. R.
de Gruijl
f,
Y.
Takizawa
gh and
J. C.
van der Leun
i
aNational Centre for Epidemiology and Population Health, The Australian National University, Canberra 2601, Australia. E-mail: robyn.lucas@anu.edu.au
bTelethon Kids Institute, University of Western Australia, Perth 6008, Australia
cBiomedical Sciences, University of Edinburgh Medical School, Edinburgh EH8 9AG, Scotland, UK
dQIMR Berghofer Medical Research Institute, Brisbane 4029, Australia
eKing's College London (KCL), St John's Institute of Dermatology, London SE1 9RT, UK
fDepartment of Dermatology, Leiden University Medical Centre, P.O. Box 9600, NL-2300 RC Leiden, The Netherlands
gAkita University Graduate School of Medicine, Akita-shi, Akita Prefecture, Japan
hNational Institute for Minamata Diseases, Minamata-sh, Kumamoto Prefecture, Japan
iEcofys, Kanaalweg 16G, NL-3526 KL Utrecht, The Netherlands
First published on 10th November 2014
Abstract
Due to the implementation of the Montreal Protocol, which has limited, and is now probably reversing, the depletion of the stratospheric ozone layer, only modest increases in solar UV-B radiation at the surface of the Earth have occurred. For many fair-skinned populations, changing behaviour with regard to exposure to the sun over the past half century – more time in the sun, less clothing cover (more skin exposed), and preference for a tan – has probably contributed more to greater levels of exposure to UV-B radiation than ozone depletion. Exposure to UV-B radiation has both adverse and beneficial effects on human health. This report focuses on an assessment of the evidence regarding these outcomes that has been published since our previous report in 2010. The skin and eyes are the organs exposed to solar UV radiation. Excessive solar irradiation causes skin cancer, including cutaneous malignant melanoma and the non-melanoma skin cancers, basal cell carcinoma and squamous cell carcinoma, and contributes to the development of other rare skin cancers such as Merkel cell carcinoma. Although the incidence of melanoma continues to increase in many countries, in some locations, primarily those with strong sun protection programmes, incidence has stabilised or decreased over the past 5 years, particularly in younger age-groups. However, the incidence of non-melanoma skin cancers is still increasing in most locations. Exposure of the skin to the sun also induces systemic immune suppression that may have adverse effects on health, such as through the reactivation of latent viral infections, but also beneficial effects through suppression of autoimmune reactivity. Solar UV-B radiation damages the eyes, causing cataracts and pterygium. UV-B irradiation of the skin is the main source of vitamin D in many geographic locations. Vitamin D plays a critical role in the maintenance of calcium homeostasis in the body; severe deficiency causes the bone diseases, rickets in children and osteomalacia in adults. Although many studies have implicated vitamin D deficiency in a wide range of diseases, such as cancer and cardiovascular disease, more recent evidence is less compelling, with meta-analyses of supplementation trials failing to show a beneficial effect on the health outcomes that have been tested. It continues to be difficult to provide public health messages to guide safe exposure to the sun that are accurate, simple, and can be used by people with different skin types, in different locations, and for different times of the year or day. There is increasing interest in relating sun protection messages to the UV Index. Current sun protection strategies are outlined and assessed. Climatic factors affect the amount of UV radiation received by the skin and eyes, separately from the effect of ozone depletion. For example, cloud cover can decrease or increase the intensity of UV radiation at Earth's surface and warmer temperatures and changes in precipitation patterns may alter the amount of time people spend outdoors and their choice of clothing. The combination of changes in climate and UV radiation may affect the number of pathogenic microorganisms in surface waters, and could have an impact on food security through effects on plant and aquatic systems. It remains difficult to quantify these effects and their possible importance for human health.
Introduction
Stratospheric ozone limits the amount of biologically damaging UV radiation in the UV-B waveband (280–315 nm) that reaches the Earth's surface – a 1000-fold reduction in mutagenic UV radiation.1 Depletion of the ozone layer results in greater potential exposure to UV-B radiation, while, for humans, actual exposure also depends on their behaviour, such as time spent outdoors, use of shade, and wearing of sun protective clothing. Global climate change is altering the recovery of the stratospheric ozone layer and, through effects on cloud cover, will modify the levels of UV radiation at Earth's surface (for detail, see Bais et al.2). Loss of snow cover will decrease albedo in mountain regions, possibly reducing UV radiation incident on body surfaces. Changing climate may also alter human behaviour with regard to exposure to the sun. Warmer temperatures may accelerate the genesis of skin cancer and vitamin D production. In the following sections, we assess the health risks associated with ozone depletion, focussing on effects related to UV radiation in the UV-B wavelengths. In addition, the risks and benefits of changing personal exposure to UV radiation under the combined effects of ozone depletion and global climate change are considered.Effects of solar UV radiation on the skin
Human skin comprises an outer thin epidermis of about 10 cell layers and typically <100 μm deep and an inner layer, the dermis, that consists mainly of connective tissue and gives skin its mechanical properties. Epidermal cells are mostly keratinocytes, with melanocytes in the basal layer producing melanin that determines pigmentation of the skin. UV radiation is absorbed in the skin by specific molecules called chromophores, with the ensuing chemical changes initiating multiple biological processes. UV-A radiation (315–400 nm) penetrates the skin to a greater depth (into the dermis) than UV-B radiation. Exposure to solar UV radiation may cause skin cancer and photo-ageing, but also initiates the synthesis of vitamin D, which is critical for human health.Skin cancer
Solar UV radiation is the major environmental risk factor for both melanoma and non-melanoma skin cancers (NMSCs).3 Cutaneous malignant melanoma (CMM) is the least common of the skin cancers, but accounts for most deaths due to skin cancer. NMSCs include basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and other rarer skin cancers. BCC occurs approximately 3–4 times more frequently than SCC. It has the lowest mortality rate but can cause significant ill-health due to extensive local invasion, particularly when it arises on the face.It is difficult to assess whether, or to what level, alterations in ozone or climate have contributed to the rising incidence of skin cancer globally. Over the 20th century, increasing travel to sunny locations and changed styles of clothing, at least in some populations, have led to higher personal exposure to the sun. This has probably been a more important driver of the increasing incidence of skin cancer than changes in ozone or climate.4 Changes in the diagnostic criteria for skin cancers, including the use of dermoscopy or epiluminescent microscopy and molecular classification,5 a lack of accurate recording of lesions, and a more general growing awareness of skin cancer and conservatism (erring on the safe side) in diagnoses,6 may also have introduced temporal biases in some instances.
Cutaneous malignant melanoma
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases of CMM and 55
000 new cases of CMM and 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in 2012.7 The incidence varies widely from country to country (Table 1), with the highest age-standardised annual incidence rates (∼35 per 100
000 deaths in 2012.7 The incidence varies widely from country to country (Table 1), with the highest age-standardised annual incidence rates (∼35 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population in 2012) in Australia and New Zealand.7 CMM is rare in darker skinned populations, such as in South Asia.8
000 population in 2012) in Australia and New Zealand.7 CMM is rare in darker skinned populations, such as in South Asia.8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population)
000 population)
| Country | Year | Incidence | Year | Incidence | Year | Incidence (projected) | Year | Incidence (projected) |
|---|---|---|---|---|---|---|---|---|
| a Data from ref. 10. b Data from http://www.aihw.gov.au. c Data from ref. 7. d Data from ref. 11. | ||||||||
| Men | ||||||||
| Denmarka | 1990 | 10.0 | 2007 | 16.0 | 2010 | 16.0 | 2015 | 18.0 |
| Englanda | 1990 | 4.6 | 2006 | 10.7 | 2010 | 12.2 | 2015 | 14.5 |
| Spaina | 1990 | 3.1 | 2004 | 8.1 | 2010 | 8.1 | 2015 | 9.3 |
| Netherlandsa | 1989 | 7.4 | 2007 | 13.6 | 2010 | 15.1 | 2015 | 17.5 |
| Australiab | 1990 | 31.1 | 2000 | 38.3 | 2012 | 40.5 | — | — |
| New Zealandc,d | 1998–2002 | 34.8 | 2012 | 39.2 | — | — | ||
| Canadac,d | 1988–1992 | 7.7 | 1998–2002 | 10.9 | 2012 | 10.4 | — | — |
| USAc,d | 1998–2002 | 15.1 | 2012 | 16.8 | — | — | ||
| Women | ||||||||
| Denmarka | 1990 | 12.8 | 2007 | 19.5 | 2010 | 21.1 | 2015 | 24.4 |
| Englanda | 1990 | 6.0 | 2006 | 12.3 | 2010 | 14.2 | 2015 | 16.9 |
| Spaina | 1990 | 3.6 | 2004 | 9.5 | 2010 | 9.5 | 2015 | 10.7 |
| Netherlandsa | 1989 | 10.7 | 2007 | 17.3 | 2010 | 19.0 | 2015 | 21.9 |
| Australiab | 1990 | 25.0 | 2000 | 28.8 | 2012 | 30.0 | — | — |
| New Zealandc,d | 1998–2002 | 31.4 | 2012 | 33.1 | — | — | ||
| Canadac,d | 1988–1992 | 6.9 | 1998–2002 | 9.3 | 2012 | 9.1 | — | — |
| USAc,d | 1998–2002 | 11.4 | 2012 | 12.6 | — | — | ||
The incidence of CMM in fair-skinned populations has approximately doubled every 10–20 years since the 1960s and this trend is projected to continue for at least 20 more years9 (Table 1).
The rise in incidence of CMM has been attributed to changes in recreational behaviour leading to increased exposure to the sun.12 The incidence rate has stabilised or is decreasing in younger age groups (<44 years) in some countries, such as Australia,9 but continues to increase elsewhere.13,14 Public health campaigns to encourage protection from the sun, beginning in the 1980s, have probably contributed to the decrease in incidence in younger age groups in Australia.15
Although CMM accounts for only 4% of all skin cancer cases, it is responsible for about 80% of deaths from skin cancer.9 Mortality due to CMM is increasing in Southern and Eastern Europe,16,17 particularly in elderly men who tend to have thicker lesions that are more invasive and less likely than the thinner lesions to respond to treatment.18 Here, the increase in deaths is probably due to the rising incidence of CMM. Mortality due to CMM is stable or decreasing in Australia,19 some parts of the USA,13 and Western Europe,20 with this decrease likely due to earlier detection of the tumours, possibly as a result of more self-awareness of risks of skin cancer in subgroups of the population.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was 35.8 in New Zealand and 34.9 in Australia, compared to 14.6 in the UK and 9.6 in Canada.7 Epidemiological studies have shown an increased risk of CMM in people who self-report higher levels of sunburn, and in those with phenotypic characteristics associated with greater sensitivity to UV radiation, including fair skin, light hair and eye colour, poor ability to tan, freckling, and having multiple naevi (moles). Childhood exposure to the sun may be particularly important; for example, migration from a high to a low latitude location before age 10 years34 or 20 years35 confers a greater risk of melanoma than migration at an older age.
000) was 35.8 in New Zealand and 34.9 in Australia, compared to 14.6 in the UK and 9.6 in Canada.7 Epidemiological studies have shown an increased risk of CMM in people who self-report higher levels of sunburn, and in those with phenotypic characteristics associated with greater sensitivity to UV radiation, including fair skin, light hair and eye colour, poor ability to tan, freckling, and having multiple naevi (moles). Childhood exposure to the sun may be particularly important; for example, migration from a high to a low latitude location before age 10 years34 or 20 years35 confers a greater risk of melanoma than migration at an older age.
Recent experimental studies indicate that both UV-A and UV-B radiation are involved in the development of CMM.36 Initiation of melanoma following UV-A irradiation involves oxidative damage to DNA and requires the presence of melanin, whereas UV-B-induced melanoma is independent of melanin and involves direct UV-B-induced damage to DNA.36 A two-hit model proposes that initiation of the tumour follows DNA damage induced by UV radiation, and then progression to melanoma depends on the host's genetic make-up (particularly for melanoma of the trunk) and/or tumour promotion by ongoing exposure to both UV-A and UV-B radiation.37
There is growing interest in the effects of exposures in early life on the risks of diseases that develop later in life. One indication that exposure early in life is important for melanoma comes from observations that, in predominantly fair-skinned populations, people with the disease are more likely to have been born in particular months of the year, compared with the general population. In two studies, young women (aged 15–24 years) with CMM from northern England38 and Sweden39 were significantly more likely to have been born in March (early Spring). The data are consistent with the two-hit model suggested in animal studies37 – early exposure to UV radiation during the months soon after birth initiates the first event, then later exposure causes progression to CMM. It is interesting to note that, in the past, exposure of infants to sunlight was encouraged in some countries, a practice that has not continued in more recent years.40–43
The higher incidence of small (<2 mm) melanomas during summer compared to winter in Northern Ireland (1984–2006) is consistent with exposure to UV radiation having a short-term promotional effect on melanocytes.44
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 point mutations per cell, and hundreds of mutations in protein-coding genes. Tumours from sun-exposed skin have the greatest number of mutations, most of which are characteristic of those induced by exposure to UV radiation.49,50 It is difficult to distinguish causative from incidental mutations, but special intron–exon comparative analyses provide evidence of likely causative mutations that are related to UV radiation.47
000 point mutations per cell, and hundreds of mutations in protein-coding genes. Tumours from sun-exposed skin have the greatest number of mutations, most of which are characteristic of those induced by exposure to UV radiation.49,50 It is difficult to distinguish causative from incidental mutations, but special intron–exon comparative analyses provide evidence of likely causative mutations that are related to UV radiation.47
Non-melanoma skin cancer
Despite these difficulties, there is a strong association between intensity of ambient UV radiation and incidence of both BCC and SCC.52 In a recent review, the highest annual incidence rates were in Australia (>1000 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population for BCC) and the lowest in Africa (<1 per 100
000 population for BCC) and the lowest in Africa (<1 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population).53 Data for the latter are sparse and the low incidence masks relatively high incidence in some sub-populations, for example among people with oculocutaneous albinism (OCA) [see section below], and in Caucasians in South Africa, where BCC and SCC are typically among the top 5 or 10 cancers reported (depending on year).54
000 population).53 Data for the latter are sparse and the low incidence masks relatively high incidence in some sub-populations, for example among people with oculocutaneous albinism (OCA) [see section below], and in Caucasians in South Africa, where BCC and SCC are typically among the top 5 or 10 cancers reported (depending on year).54
There have been substantial increases in the incidence of NMSC over the past several decades. Across Europe, the annual incidence of BCC was estimated to be around 50 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons in 1980.53 It has more than doubled since then in many parts of this region, and has quadrupled in the Netherlands.55 The incidence of SCC was approximately 10 per 100
000 persons in 1980.53 It has more than doubled since then in many parts of this region, and has quadrupled in the Netherlands.55 The incidence of SCC was approximately 10 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons in Europe in 198053 with an increase to about 25 per 100
000 persons in Europe in 198053 with an increase to about 25 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by 2000.53 There are no estimates of SCC and BCC separately for the United States, but a study of a study of data from national Medicare claims suggests that the age-adjusted rate of procedures for skin cancer increased by 77% between 1992 and 2006.56
000 by 2000.53 There are no estimates of SCC and BCC separately for the United States, but a study of a study of data from national Medicare claims suggests that the age-adjusted rate of procedures for skin cancer increased by 77% between 1992 and 2006.56
A recent quantitative review of data published between 1979 and 2012 showed that, in fair-skinned populations worldwide, after adjustment for age, sex, and the levels of ambient UV radiation, the average annual increases in SCC and BCC incidence were 4% and 1%, respectively.52 The incidence of SCC increased over time in both the older (≥60 years) and younger (<60 years) age groups, but only in the older age group for BCC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) of reported SCC was 4.6, 7.0, 41.5 and 101.3, and of reported BCC was 4.7, 13.0, 85.7 and 311.1 in the Black, Asian, Coloured and White population groups respectively.30
000) of reported SCC was 4.6, 7.0, 41.5 and 101.3, and of reported BCC was 4.7, 13.0, 85.7 and 311.1 in the Black, Asian, Coloured and White population groups respectively.30
The epidemiology, clinical presentation, and prognosis of NMSC differ between people with fair skin and those with darker skin. For example, in several studies, SCC was more common than BCC in those with deeply pigmented skin29,67 and these tumours typically arose in sites of chronic inflammation or scarring, so that solar UV radiation may not be the major risk factor.31 In contrast, the site-distribution of BCCs is similar in fair- and dark-skinned populations, occurring predominantly on the head or neck, suggesting that exposure to UV radiation is an important risk factor.
Over time, there has been little increase in the incidence of NMSC in Asian populations and almost none in dark-skinned populations (reviewed in Agbai et al.31 and Gloster & Neal,68). However, mortality and morbidity from NMSC is disproportionately high in dark-skinned populations in comparison with incidence, due to diagnosis occurring at a more advanced stage, atypical presentation, lack of screening, and socio-economic factors.31 Public health education regarding protection against the sun and self-awareness, tailored appropriately for each population group, should be expanded to include people of all skin types.69
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold in patients with xeroderma pigmentosum, a disorder where the repair of UV-induced DNA damage is severely impaired.72
000-fold in patients with xeroderma pigmentosum, a disorder where the repair of UV-induced DNA damage is severely impaired.72
SCCs and BCCs are the cancers with the highest mutational loads (33 and 76 mutations per million DNA-bases, respectively), especially those from skin regularly exposed to the sun.73,74 The majority of these mutations bear the “UV signature” (cytosine to tyrosine transitions at cyclobutane pyrimidine dimers, CPD, Fig. 1). Both BCC and SCC show UV-signature mutations in tumour suppressor genes (PTCH1 and p53 respectively),74–76 suggesting these are key abnormalities driving the development of these tumours.77 These genetic studies clearly indicate the causal role of exposure to UV radiation in the development of NMSC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people have OCA (with about 1 in 70 people carrying the OCA gene),79,80 but this figure can be considerably higher, such as 1 in 3900 in South Africa.81
000 people have OCA (with about 1 in 70 people carrying the OCA gene),79,80 but this figure can be considerably higher, such as 1 in 3900 in South Africa.81
People with OCA experience visual impairment including photophobia (discomfort from exposure to light, leading to avoidance).82 They are also highly susceptible to skin damage induced by solar UV radiation and it has been estimated that the risk of NMSC in people with OCA in Africa is one thousand times greater than that of the general population.83 In most instances, the skin cancer occurs at 20–30 years of age, which is considerably younger than in those without OCA.84 Cutaneous tumours in Africans with OCA are predominantly SCCs, with BCCs less frequent, and CMMs only occasionally seen,85,86 although the last may be under-reported as they are normally amelanotic (non-pigmented).87 Most commonly, the skin cancers in Africans with OCA occur on the head and neck and tend to progress rapidly with metastasis to the cartilage, bone, and muscle, resulting in high mortality.84,88 The key role of exposure to the sun in the oncogenic process is emphasised by finding an increased frequency of skin tumours and lower life expectancy in people with OCA living in equatorial regions of sub-Saharan Africa than in parts further from the Equator.84
Viruses and skin cancer
There are two instances (see following sections) where an association between certain viruses and skin cancer has been demonstrated. In both cases, the tumours occur predominantly on body sites most exposed to the sun, suggesting that exposure to solar UV radiation is likely to play a crucial role in the carcinogenic process.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 between 1986 and 2001 in the USA.92 This could be explained by increasing life expectancy, greater exposure to the sun and the rising number of immunosuppressed people in the general population as a result of an increasing number and range of organ transplants93 and infection with HIV.89
000 between 1986 and 2001 in the USA.92 This could be explained by increasing life expectancy, greater exposure to the sun and the rising number of immunosuppressed people in the general population as a result of an increasing number and range of organ transplants93 and infection with HIV.89
Merkel cell polyomavirus is present in around 80% of MCCs. The viral DNA is integrated into the host DNA and is thought to cause cancer after genomic mutations that eliminate its ability to replicate but maintain its oncogenic function (reviewed in Arora et al.94). Exposure to UV radiation may play a role in the integration or mutagenic processes (for example, Demetriou et al.95 and Mogha et al.95,96) or in suppression of the immune response to the virus.97
Photoageing
Chronic exposure to solar UV radiation results in photoaged skin, which is wrinkled, leathery, shows loss of elasticity, and is often associated with the development of SCC. Photoageing results from the UV-induced degradation of proteins such as collagen and elastin in the extracellular matrix of the dermis. UV-A radiation may be primarily responsible for chronic photoageing, given its greater depth of penetration.104 In addition, a role for UV-B radiation is indicated. First, UV-B radiation can directly degrade some proteins, such as fibrillin and fibronectin, involved in maintaining the structure of the dermis.105 Second, the action spectrum for the induction of matrix metalloproteinase (an enzyme that degrades collagen) in human skin is similar to that for erythema (reddening of the skin, inflammation), suggesting that this is primarily an effect of exposure to UV-B radiation.106 While not a risk to health per se, photoageing of the skin incurs considerable costs through the use of cosmetic and hydrating agents to improve the appearance and feel of the skin.Melasma
Melasma appears as dark, macular, pigmented patches on the brow, cheek, upper lip and jaw, and is due to a localised increase in melanin production.107 It can result in profound emotional and psychological stress, significantly reducing quality of life.108 Melasma is particularly common in adult women living in tropical areas of the world and occurs more frequently in individuals with skin types of intermediate pigmentation (i.e., types III–V) than in those with fair (skin types I and II) or very dark skin (skin type VI).107,109 Its prevalence has been estimated as 3.4% in the general population in Beirut, Lebanon, 10.1% in Cuzco, Peru,107 34% in adult women in Botucatu, Brazil,110 and 40% in adult women and 20% in adult men in South-East Asia.111The precise pathophysiology of melasma is unclear, but is known to be complex. There is a genetic predisposition and several environmental triggers, one of which is exposure to sunlight. For example, a report from Tunisia indicated that 51% of patients recognised exposure to the sun as a triggering factor and 84% as an aggravating factor, with high lifetime exposure to the sun increasing the risk of severe melasma three-fold.112 A case-control study in Brazil found that patients with melasma had a greater number of years of seaside or rural residence and greater exposure to the sun at work or during leisure than the controls; there was a lack of association with sunburn, implying that cumulative exposure to the sun may be more important than acute exposure.109 Solar UV radiation can induce proliferation and migration of melanocytes113 and the production of several cytokines that increase the production of melanin. In addition, inflammatory cells, especially mast cells, which produce a variety of potent pro-inflammatory substances, are likely to play key roles.114
Effects of solar UV radiation on the eye
The eye is partly protected from direct UV radiation by the brow ridges. This means that reflected radiation is likely to contribute more to the total UV radiation received than occurs for the skin. Higher intensity UV radiation induces the most damage, although less intense exposure over a long period also increases the risk of disease. Transmission of UV radiation through the eye (Fig. 2) generally decreases with increasing age, but there is wide inter-individual variability.115 The cornea filters out wavelengths less than about 280 nm, although this is relevant for artificial sources only, as sunlight at the Earth's surface is confined to wavelengths >290 nm. There is further absorption in the aqueous humour (Fig. 2). The lens of a young child (e.g., <5 years) transmits close to 100% of the visible light spectrum (wavelength >400 nm), but absorbs UV radiation, except for a small window at 320 nm.116 The lens of an older person (e.g., 60+ years) commonly filters out even some of the short blue visible light (400–500 nm) so that this, and UV radiation of shorter wavelengths, do not reach the retina.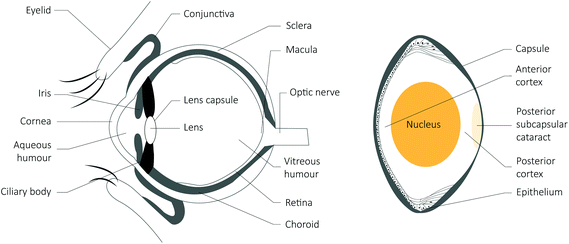 | ||
| Fig. 2 A schematic drawing of a section through the human eye, with an enlarged schematic of the lens to the right. | ||
Exposure to UV radiation increases the risk of a number of ocular conditions, with the strongest evidence of a specific effect of UV-B radiation for photokeratitis, pterygium, and cataract.
Photokeratitis and photoconjunctivitis
Exposure of the eye to high-dose UV radiation from the sun can result in inflammation of the cornea (photokeratitis) and/or conjunctiva (photoconjunctivitis) (Fig. 2). The maximum sensitivity is to the UV-B wavelengths.117 The damage is probably caused by oxidative stress,118–120 with the squamous cells of the epithelium of the cornea, the keratocytes of the stromal layer of the cornea, and the endothelial cells lining the back of the cornea, being affected.Transmission of UV radiation decreases across the cornea from the centre to the periphery, due to scattering and absorption.121 The centrally located endothelial cells receive a higher dose of UV radiation, and show evidence of higher oxidative stress than cells in the periphery. It is the damage to these central corneal endothelial cells that particularly causes corneal swelling and temporary loss of vision in UV-induced photokeratitis.121
Pterygium
Pterygium, a wing-shaped invasive growth of the conjunctiva (Fig. 3), is common in adults living in environments with high UV radiation. For example, it affects at least one eye in approximately 10% of: adults (≥15 years) on Norfolk Island, Australia;122 south Indians (≥40 years) in Chennai, India;123 and indigenous people (≥40 years) in Central Australia.124 Some recent studies show that the prevalence of pterygium increases with increasing age and is more common in men than women,123 but the increase with age is not consistently found.125 Key risk factors for pterygium are greater time outdoors (including sports with high ocular exposure to UV radiation, such as surfing126), rural residence, having a skin type that tans122 and non-use of spectacles.123 Of note, the only study that has examined the risk of pterygium in association with wavelengths of solar radiation other than those in the UV range (using retrospectively reconstructed exposure behaviour during working hours) found a stronger association with visible light, and a weaker but still significant association with UV-B radiation.127 Recent evidence suggests that a pterygium is not always benign: there is histological evidence of inflammatory and dysplastic changes in the epithelium and underlying connective tissue,128 and of neoplasia in 2–10% of excised pterygia.129,130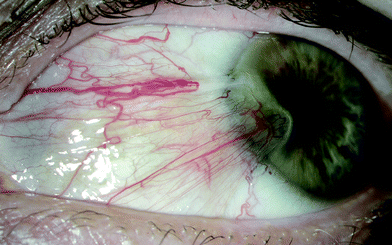 | ||
| Fig. 3 Pterygium of the eye; photograph provided by Dr David Mackey, University of Western Australia, Perth, Australia. | ||
Squamous cell carcinoma of the cornea and conjunctiva
Squamous cell carcinoma of the cornea and conjunctiva (SCCC) is rare; the annual incidence in the USA is estimated to be 0.84 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population.131 The incidence is higher in men, and in association with older age, residence at lower latitude, infection with HIV, and high exposure to UV radiation.131 Infection with HPV and exposure to UV radiation may be common risk factors for pterygium and SCCC.132
000 population.131 The incidence is higher in men, and in association with older age, residence at lower latitude, infection with HIV, and high exposure to UV radiation.131 Infection with HPV and exposure to UV radiation may be common risk factors for pterygium and SCCC.132
Cataract
In 2010, cataract was the leading cause of blindness worldwide.133 Of the three main types of age-related cataract – cortical, nuclear, and posterior subcapsular (Fig. 2) – UV radiation is primarily linked to an increased risk of cortical cataract.134The action spectrum for acute/short term cataract development is relatively consistent across animal models, with UV-B radiation of shorter wavelengths being most damaging.135 However, these models have limited relevance for cataract formation in humans because of wide inter-species variation in the dose of UV irradiation required,136 and the typical use of a single very high dose, rather than repeated lower doses. Melanin in pigmented irises may absorb UV radiation, leading to fewer cataracts in those with more pigmented, than less pigmented, irises.137 Lenses in older individuals may be more susceptible to UV-induced cataracts due to poorer defence against oxidative damage and decreased repair mechanisms.138–140
In the large Salisbury Eye Evaluation Study with participants aged 65–84 years, the incidence and progression of cortical cataract (but not nuclear cataract) were associated with higher levels of estimated exposure to UV-B radiation (calculated based on an empirical model incorporating self-reported time outdoors and use of protection from the sun).141 In contrast, in the 15 year follow-up of participants in the Beaver Dam Eye Study,142 there was no association between exposure to the sun (as measured by residential history) and cumulative incidence of any type of age-related cataract, after controlling for age and sex. However, the combined use of sun-sensitising medications and high exposure to the sun led to a significantly increased risk of cortical cataract.142
Ocular malignancies
There is strong evidence to support exposure to UV radiation as a risk factor for tumours of the eyelid and weaker evidence for ocular melanomas (reviewed in Yam and Kwok143). Over 90% of the malignancies of the eyelid are BCCs, particularly affecting the lower lid (50–65%), but also the medial canthus (inner corner of the eye, 25–30%), upper eyelid (15%) and lateral canthus (outer corner of the eye, 5%).143 SCC accounts for most of the remainder of the periocular cutaneous tumours.143Melanomas of the eye can involve the surface (i.e., the eyelid or conjunctiva), or occur at an intra-ocular location, affecting the elements of the uvea (i.e., the iris, ciliary body, and choroid; Fig. 2). Uveal melanoma is the most common primary intraocular malignancy (>90%) and the leading cause of death from intraocular cancer.144,145 The reported annual incidence varies from 0.53 to 1.09 cases per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population, and is stable or decreasing.144,145 Uveal melanoma is primarily a disease of white populations; light-coloured irises, blond hair, and fair skin are risk factors.146 There is mixed evidence implicating UV radiation as a risk factor for uveal melanoma: latitudinal variation in incidence is not consistently found;145 occupational exposure to UV radiation may have a protective effect, but intermittent exposure may increase risk (reviewed in Mallet et al.146). People with the disease xeroderma pigmentosum, in which there is impaired ability to repair UV-induced DNA damage (see discussion above), have a 58-fold increased risk of uveal melanoma.146,147 Genetic studies show that the mutation patterns of the most frequently mutated genes in CMM and uveal melanoma (i.e., BRAF vs. GNAQ, GNA11) are similar,146 and a mutation recently identified in CMM (RAC1) is also found in 20% of uveal melanoma cell lines.146 Given the strong evidence supporting a role for UV radiation as a cause of CMM, these studies also provide some evidence that exposure to UV radiation is a risk factor for uveal melanoma.
000 population, and is stable or decreasing.144,145 Uveal melanoma is primarily a disease of white populations; light-coloured irises, blond hair, and fair skin are risk factors.146 There is mixed evidence implicating UV radiation as a risk factor for uveal melanoma: latitudinal variation in incidence is not consistently found;145 occupational exposure to UV radiation may have a protective effect, but intermittent exposure may increase risk (reviewed in Mallet et al.146). People with the disease xeroderma pigmentosum, in which there is impaired ability to repair UV-induced DNA damage (see discussion above), have a 58-fold increased risk of uveal melanoma.146,147 Genetic studies show that the mutation patterns of the most frequently mutated genes in CMM and uveal melanoma (i.e., BRAF vs. GNAQ, GNA11) are similar,146 and a mutation recently identified in CMM (RAC1) is also found in 20% of uveal melanoma cell lines.146 Given the strong evidence supporting a role for UV radiation as a cause of CMM, these studies also provide some evidence that exposure to UV radiation is a risk factor for uveal melanoma.
Age-related macular degeneration
Age-related macular degeneration (AMD) was the cause of 7% of blindness worldwide in 2010,133 and was the most frequent cause of blindness in older (50–75 years) white populations in Europe.148 UV radiation had been discounted previously as a risk factor in AMD as it does not reach the retina. However, recognition of the considerable individual variability in the transmission of longer UV-B/shorter UV-A wavelengths (<320 nm) in older adults (60+ years) has led to reconsideration of a potential role of UV radiation. Possible mechanisms include: UV-induced oxidative damage to mitochondrial DNA, particularly in the macular region of the neural retina and the retinal pigment epithelium;149 and/or upregulation of inflammatory cytokines (e.g., IL-6) and transcription factors (e.g., STAT3). Higher vitamin D status is associated with lower risk of AMD in women <75 years, but a higher risk in women ≥75.150In a systematic review and meta-analysis of case-control and cross-sectional studies, higher exposure to the sun was associated with a 38% increase in the odds of having AMD.151 However, blue light (400–500 nm) may be more important than UV radiation as a risk factor for AMD.143,152,153
Other possible effects on the eye
There is a well-established association between spending less time outdoors and an increased risk of developing myopia in childhood.154–157 While early hypotheses focused on the importance of variation in focal length with a mix of indoor and outdoor activities, more recent work suggests the importance of exposure to light, possibly through increased secretion of dopamine in the retina, with effects on the growth of the eye.158 The wavelength dependence of this effect, and whether the pathways are mediated by vitamin D159 or not,157 are currently unknown.Evidence from animal studies suggests that UV irradiation of the eye can cause systemic immunosuppression,160,161 but the relevance of these findings for human health is unclear at present.
Effects of solar UV radiation on immune function and consequences for disease
Mechanisms
UV photons penetrate the epidermis and upper dermis162 and are absorbed by chromophores (Table 2), which then initiate a cascade leading to changes in immune responses.| Chromophore | Change in structure following irradiation | Effect on immune function |
|---|---|---|
| DNA | Cyclobutane pyrimidine dimers and reactive oxygen species-induced base oxidation after exposure to radiation in both the UV-A and UV-B wavelengths | Oxidative stress; up-regulation of several immunosuppressive mediators and down-regulation of some immunostimulatory mediators |
| trans-Urocanic acid | cis-Urocanic acid (peak effectiveness of isomerisation about 300 nm) | Oxidative damage; up-regulation of several immunosuppressive mediators; stimulation of neuropeptides; mast cell degranulation; cell growth arrest |
| Membrane phospholipids | Oxidative stress and lipid peroxidation | Clustering of receptors; activation of transcription factors; release of immune mediators |
| 7-Dehydrocholesterol | Previtamin D after UV-B irradiation, leading to vitamin D, then 25-hydroxyvitamin D and finally the active form, 1,25-dihydroxyvitamin D | Up-regulation of some antimicrobial responses and DNA repair; down-regulation of most acquired immune responses |
| Tryptophan | Activation of the arylhydrocarbon receptor following exposure to UV-B radiation | Clustering and internalisation of growth factor receptors |
While much of this information has been gathered from studies in vitro or in rodent models, less is known about humans. However, an action spectrum for the UV-induced suppression of the human immune response to a previously-encountered antigen (termed memory or recall immune responses) has been constructed: it has two peaks, one within the UV-B waveband at 300 nm and one at 370 nm in the UV-A waveband.164,165 There is also evidence from studies in both humans and mice that interactive and additive effects between wavebands can occur.166–168
Briefly, exposure to UV radiation causes up-regulation of some innate immune responses, and down-regulation of some acquired primary and memory immune responses, mainly through effects on T cell activity (reviewed in Gibbs & Norval,163 Schwarz & Schwarz,169 and Ullrich & Byrne170). The up-regulation includes the production of several antimicrobial peptides (AMPs) in the epidermis,171,172 possibly through a vitamin D pathway (see below). The AMPs provide immediate protection against a variety of pathogens (bacteria, fungi, and viruses having a viral envelope) and they are also involved in the promotion of cell growth, healing, and angiogenesis. In contrast to these stimulatory functions, exposure to UV radiation induces T regulatory cells (Tregs) and other cell types which contribute to immunosuppression and help to restore cutaneous homeostasis.172,173 Mediators such as platelet-activating factor, prostaglandin E2, histamine, and tumour necrosis factor-α are produced locally at the irradiated site. These alter the migration patterns and functions of various populations of immune cells. The end result is the generation of cell subsets with suppressive activity which are thought to remain for the life-time of the individual.174,175
The UV-induced alterations in the normal immune response can be beneficial for some human diseases and detrimental for others. Vitamin D, synthesised following exposure of the skin to UV-B radiation, also has positive and negative effects on immune-related diseases. Indeed, it is difficult to distinguish between immunoregulation by vitamin D and other mediators induced by UV radiation,176–180 since the downstream effects on immune parameters are similar. For clarity, the effects of UV radiation and those of vitamin D have been assessed separately in the sections below. We first focus on the effects of UV radiation on immunity, and address vitamin D-related effects on immune function in the section specifically on vitamin D.
Polymorphic light eruption
Polymorphic light eruption (PLE) is the commonest of the photodermatoses, with a prevalence of up to 20%.181 PLE manifests as an intermittent itchy red skin eruption which resolves without scarring after a few days to weeks. It occurs 2–3 times more frequently in women than in men, with onset typically in the first three decades of life,181 and is found predominantly in those with fair skin, although all skin types can be affected.181 A recent study of Indian patients with dark skin phototypes (IV and V) who suffered from various photodermatoses revealed that PLE was the commonest of these, affecting 60% of the group.182 The lesions occur most often in the spring and early summer or during a sunny holiday, following the first exposure to a large dose of sunlight. After repeated exposures, the lesions are less likely to occur. This process, called photohardening, is used therapeutically with good results. Recent investigations indicate that key events in photohardening include a decrease in the number of Langerhans cells in the epidermis and recruitment of mast cells into the dermis,183 together with changes in systemic cytokine levels.184PLE is immunologically-mediated as a result of a failure to establish the normal suppression of immune responses following exposure to UV radiation. The antigen involved has not been identified but is likely to be novel, induced by the DNA damaging properties of UV radiation. Various abnormalities in the cutaneous immune response following UV radiation have been demonstrated in people with PLE compared with controls.185,186 This disease therefore illustrates the positive evolutionary advantage of UV-induced immunosuppression in individuals who are not susceptible to PLE and what can happen if it is absent.
Asthma
Asthma comprises a group of diseases that evidence as wheeze, chest tightness, or shortness of breath, occurring as a result of obstruction of the airways and restriction of airflow that is usually reversible. The level of severity, frequency of symptoms, age of onset, main inflammatory phenotypes, and triggers and pathways are variable. This heterogeneity may explain the current lack of consistency in results from studies examining the relationship between UV radiation and the risk of asthma.There are anecdotal accounts that sunny holidays or living at high altitude decrease asthma symptoms. The prevalence of asthma was inversely associated with the intensity of UV radiation,187 or past personal exposure to solar UV radiation.188 However, in a study where different sub-types of asthma were considered, residence at latitudes closer to the equator (and with greater intensity of UV-B radiation) was associated with an increased risk of having asthma in atopic participants (with a history of allergic responses to specific antigens) but a decreased risk in those without atopy.189 These findings highlight the importance of differentiating between subtypes of asthma in examining associations with exposure to UV radiation. Nevertheless, individual-level exposure to UV radiation was not measured (only latitude and ambient UV radiation), so the results could reflect exposure to other latitude-associated factors such as temperature and indoor heating.
Infection and vaccination
Studies over the past 20 years have shown that exposure to solar UV radiation suppresses microbe-specific acquired immune responses in animal models of infection. This modulation can lead to an increased microbial load, reactivation from latency, and more severe symptoms, including death (reviewed in Norval et al.190). A recent study showed that spending 8 or more hours outdoors per week when the UV Index was ≥4 was associated with an increased risk of ocular recurrence of herpes simplex virus (HSV) infection resulting in eruptive lesions.191 UV radiation prior to vaccination causes a less effective immune response in several mouse models (reviewed in Norval & Woods192), but whether exposure to UV radiation adversely affects the course of infections and the efficacy of vaccination in humans remains an open question.Despite the paucity of new information, there remains the possibility that UV-induced immunosuppression could convert an asymptomatic infection into a symptomatic one, reactivate a range of persistent infections, increase the oncogenic potential of microbes, and reduce the memory immune response, for example after vaccination, so that it is no longer protective.
Autoimmune diseases
Many autoimmune diseases are considered to have both environmental and genetic risk factors. Evidence to support the importance of environmental exposures comes from geographical variation (changing incidence with changing latitude), temporal patterns (such as variations in incidence with season or season-of-birth) and results from observational epidemiological studies. Several studies show an inverse association between exposure to UV radiation and immune-mediated diseases, suggesting that the UV may be protective. In many cases, the assumed pathway has been through enhanced synthesis of vitamin D (see section on Vitamin D below). However, this evidence is now being re-evaluated in light of possible alternative pathways, including UV-induced immune modulation and altered susceptibility to relevant viral infections, and non-UV pathways such as changes in the secretion of melatonin (reviewed in Hart et al.193). While there have been suggestions that exposure to UV radiation may be important for conditions such as inflammatory bowel disease (for example, Nerich et al.194), type 1 diabetes,195 and rheumatic diseases (including rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis, and others),196 the strongest evidence is for multiple sclerosis.The role of UV-induced immune suppression in skin cancer
UV-induced vitamin D and its effect on health
Metabolism of vitamin D
Vitamin D can be synthesised in the skin or ingested in the diet or as a supplement. The pathway by which vitamin D is produced in the skin and metabolised to its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D) is shown in Fig. 4. Synthesis is initiated by absorption of UV-B radiation by 7-dehydrocholesterol. The enzymatic steps converting vitamin D to 1,25(OH)2D occur predominantly in the liver and the kidney but also in other tissues, including the skin.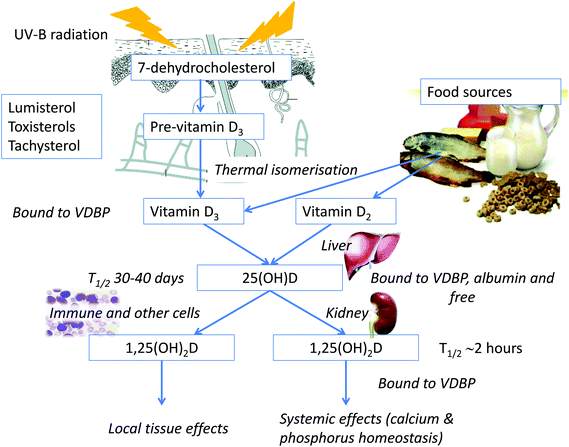 | ||
| Fig. 4 Synthesis of vitamin D and the vitamin D metabolic pathway. VDBP: vitamin D binding protein; 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; T1/2: half-life. | ||
Both pre-vitamin D3 and vitamin D3 can be converted to inactive photoproducts by continued UV-A or UV-B irradiation (discussed in Galkin & Terenetskaya215 and Norval et al.216). Once pre-vitamin D has formed and isomerization to vitamin D has occurred, there is preferential degradation of vitamin D compared with synthesis of pre-vitamin D at wavelengths of UV radiation between 300–330 nm.217 This may explain why vitamin D toxicity from exposure to solar UV radiation does not occur and may be of importance to public health messages about safe exposure to the sun – recurrent shorter periods of exposure to the sun are preferable to prolonged exposure to achieve vitamin D production while minimizing UV-induced damage of DNA.218
Vitamin D status – assessment and geographic variability
Vitamin D status is assessed by measuring the concentration of the intermediate metabolite, 25-hydroxyvitamin D (25(OH)D) (Fig. 4) in serum. Vitamin D deficiency is reportedly widespread globally, but it is important to note that there are considerable problems with the accuracy and reproducibility of vitamin D assays219 as well as a lack of consensus on the concentration of 25(OH)D that denotes deficient, insufficient, sufficient, or optimal vitamin D status.220 Common cut-off points are provided in Table 3.There is a striking lack of data on the vitamin D status of infants, children, and adolescents and an almost complete lack of data from Africa and South America.225–227
Variation in vitamin D status according to latitude is apparent when results arise from a single assay with good agreement across batches of samples,228 and is stronger where blood is taken in winter compared to summer.229 Comparisons across studies may not show a latitudinal gradient due to the analytical challenges and lack of standardisation of the season of blood collection.225–227 In addition, contributions to vitamin D status from dietary intake and sunny holidays may obscure latitudinal gradients that would otherwise occur. For example, a lower latitude holiday in the previous year with the purpose of sun-bathing was associated with higher 25(OH)D levels by 20–30 nmol L−1 in the following winter months in residents of Uppsala, Sweden.230
Skin pigmentation and vitamin D status
Within a given location, people with darker skin commonly have lower concentrations of 25(OH)D than those with fairer skin231,232 and this is usually attributed to the photoprotective properties of melanin. However, recent work highlights the importance of cultural practices, or personal preferences, leading to avoidance of the sun.233 In a recent study, however, black Americans (n = 2085) had lower concentrations of 25(OH)D, but also lower concentrations of vitamin D binding protein, than white Americans.231 The consequence of this was similar (calculated) levels of “free” (not bound to vitamin D binding protein) or loosely bound (to albumin), i.e., bioavailable, 25(OH)D. This observation may explain why black people with low total 25(OH)D have higher bone mineral density than white people with similar 25(OH)D concentrations. Furthermore, it may mean that vitamin D status should be defined by the concentration of bioavailable, rather than total, 25(OH)D.234,235Several recent experimental studies have examined the impact of pigmentation of the skin on synthesis of vitamin D. Most of these,236,237 although not all,238 show that for a specific dose of simulated solar UV irradiation there is a greater increase in the concentration of 25(OH)D in fairer-skinned than in darker-skinned participants. The lack of effect in the latter study has been attributed to the short wavelength UV-B output from the source lamp, resulting in penetration only into the superficial layers of the epidermis above the main concentration of melanin.239
Exposure to the sun and vitamin D status
In general, laboratory studies show an initial linear dose–response between exposure to UV radiation and change in concentration of 25(OH)D,240 but a plateau with continuing exposures over a longer period of time.218,241–243 Results suggest that the shape of the dose–response curve depends on the baseline concentration of 25(OH)D, with a greater response to UV irradiation238 and no plateau effect in those with a lower starting concentration (<50 nmol L−1),244 although conflicting results have also been obtained.245Understanding the relationship between the dose of UV radiation, the surface area of skin exposed and the production of vitamin D is important for the development of public health messages. Results from population-based epidemiological studies and experimental studies using artificial irradiation demonstrate that exposing a larger area of skin to UV radiation results in a greater increase in the concentration of 25(OH)D.246,247 Field studies have shown that increases in the concentrations of 25(OH)D are positively associated with DNA damage (assessed by concentration of CPDs in urine), suggesting that improved vitamin D status is always associated with some potentially mutagenic damage to DNA.248 However, production of vitamin D may be optimised, and skin DNA damage minimised, by increasing the body surface area exposed, and decreasing the UV-B-dose per unit area.
The World Health Organization's INTERSUN programme recommends sun protection when the UV Index is ≥3 and there have been concerns that there may be little or no vitamin D production when the UV Index is <3. However, recent computational work suggests that vitamin D could be synthesised at these lower levels of UV radiation, albeit more slowly.249 Webb and colleagues have shown that for the white-skinned population of Manchester, UK, a normal lifestyle with relatively short, regular exposures to summer sunlight in northern mid-latitudes could increase vitamin D enough at the end of summer to maintain sufficiency levels (>50 nmol L−1 or 20 ng ml−1) throughout the winter.218,250
Evidence of associations between vitamin D and human disease
The vital role of 1,25(OH)2D in maintaining the concentration of calcium in the blood within a narrow range is well-established; vitamin D deficiency causes rickets in children and osteomalacia in adults. In recent years, many protective functions have been attributed to vitamin D. However, two recent systematic reviews of observational and intervention studies cast doubt on the importance of vitamin D in decreasing the risk of many of these diseases.251,252Immune function, infections, autoimmune diseases and cancer
Many of the cell types involved in immune function are able to convert 25(OH)D to the active form, 1,25(OH)2D.253 The actions of 1,25(OH)2D are mediated through ligation with a nuclear vitamin D receptor (VDR) that regulates gene transcription, or via rapid-response membrane receptors. The VDR is expressed in many human cells, including those with immune functions. Polymorphisms in the VDR can affect the effectiveness of gene transcription, altering the action of the active hormone. Immunostimulatory and immunosuppressive pathways are induced by 1,25(OH)2D (reviewed in Hewison254,255 and Christakos et al.255). Immunostimulatory effects include the production of AMPs such as cathelicidin by macrophages, neutrophils, and epithelial cells, and the maturation of macrophages. Immunosuppressive effects include the inhibition of proinflammatory cytokines and the differentiation and maturation of dendritic cells and their ability to present antigens. Furthermore, 1,25(OH)2D can inhibit the differentiation and proliferation of B cells and their production of antibodies, and activate Treg cells that have suppressor activity. These multiple effects make it difficult to determine what role, if any, vitamin D has in protection against disorders of immunity. Studies of infectious diseases (using tuberculosis and respiratory viral infections as examples), autoimmune diseases (using multiple sclerosis as an example), and the risk of cancer incidence and progression are discussed below.Recent observational studies typically show that lower concentrations of 25(OH)D are associated with greater risk of having a respiratory tract infection (for example263–266). However, in most cases, study participants had disease symptoms when they were first assessed, so that low vitamin D status could be either a cause or a consequence of the infection. The results from supplementation trials with vitamin D are inconsistent, as indicated by the following studies. Separate trials in Japanese267 and Mongolian268 children showed reduced incidence of respiratory infection in the supplemented groups, and there is some evidence of benefit in postmenopausal women,269 young Finnish men270 and older Australian adults (using antibiotic use as a surrogate for infection).271 In contrast, trials of supplementation in adults from the United States272,273 and New Zealand274 failed to show any beneficial effect of vitamin D.
Reducing the risk of infectious diseases would have significant impacts on personal morbidity and global economies; however, the role of vitamin D in promoting this is unclear at this time.
Most studies, however, cannot distinguish between cause and effect; that is, does low vitamin D status cause the disease or does the disease cause the low vitamin D status? Indeed, there is evidence that concentrations of 25(OH)D are reduced by inflammation.276 Further, a review of randomised controlled trials of supplementation with vitamin D in people with MS found no evidence of improvement in clinical endpoints.251 Overall, the authors hypothesised that either uncontrolled confounding or reverse causality provided an explanation for the strong and consistent associations in observational studies and the lack of effect of supplementation.
It is also important to consider possible heterogeneity in MS, coupled with a lack of understanding of the timing of the onset of disease pathology and thus the most appropriate time to test vitamin D status or to give vitamin D supplementation. In addition, the risk factors for disease onset may differ from those of progression.277
It is particularly difficult to assess the effects of vitamin D on the risk of skin cancer, as UV radiation induces both. Production of 1,25(OH)2D in the skin enhances the repair of UV-induced DNA damage.279,280 Cohort studies have shown protective281,282 and adverse283 associations between concentrations of 25(OH)D and risk of NMSC and similar inconsistencies for the risk of CMM.283,284 Post-hoc analyses of the Women's Health Initiative trial did not show a protective effect of supplementation with vitamin D on the risk of NMSC or CMM, but in people with a history of NMSC, the incidence of CMM was reduced in those randomised to 400 IU of vitamin D per day.285
In summary, the evidence that higher vitamin D status is protective for cancer is weak and inconsistent, except possibly for colorectal cancer risk. If vitamin D deficiency is truly a risk factor, it is low concentrations of 25(OH)D (i.e., <30 nmol L−1) that are associated with increased risk with little additional benefit for concentrations >50 nmol L−1.286
Prospective studies have reported moderate to strong inverse correlations between concentrations of 25(OH)D and cardiovascular diseases, concentrations of serum lipids, inflammation, disorders of glucose metabolism, weight gain, mood disorders, declining cognitive function, dementia, Alzheimer's disease, impaired physical functioning, and mortality. In contrast, intervention trials show no effect of vitamin D supplementation on these outcomes.251
U-shaped associations between vitamin D metabolites and disease
Recent research shows that both high and low concentrations of 25(OH)D are associated with increased disease risk – a so-called U-shaped association. Table 4 provides a summary of studies showing this effect.| Disease/condition | Concentration (nmol L−1) of lowest risk, i.e. the turning point of the U-shaped dose response |
|---|---|
| All-cause mortality292–294 | 80–100 |
| Cardiovascular events295,296 | 50–100 |
| Cancer mortality297,298 | 100 (men only) |
| Prostate cancer299,[300] | 50 [≤55 (highest risk, 91–106)] |
| Pancreatic cancer301 | <100 |
| Allergen-specific IgE during childhood302 | 50–74.9 |
| Tuberculosis303 | 76–140 |
| Schizophrenia (neonatal vitamin D status)291 | 47 |
| Small-for-gestational-age births among white women304 | 60–70 |
| Physical frailty in older women305 | 50–74.9 |
An additional U-shaped association has been shown between concentration of 1,25(OH)2D and viral load in patients with HIV.306
There is a trend to advocate ever higher cut-offs to denote the concentration of 25(OH)D concentrations that is optimal to prevent disease.307 It is therefore important to continue to investigate possible disease risks at higher concentrations of 25(OH)D, especially if the aim is to maintain such levels over the long-term.
Other effects of solar UV radiation on human health
Chronic exposure to UV radiation has been weakly linked to a range of other adverse health outcomes, including: decreased epidermal308 and subcutaneous309 lipid synthesis resulting in weakening of the barrier function of the skin; hearing impairment, through oxidative stress pathways;310,311 lactase non-persistence;312 increased risk of prostate cancer;313 and acquired bilateral nevus-of-Ota-like macule, a common pigmentation disorder in Asian females.314In contrast, there is evidence of a protective effect of higher UV radiation on development of restless legs syndrome315 and keloid formation in scars.316 There are a growing number of studies examining the apparently beneficial effects of exposure to the sun. A recent study has shown that UV-A irradiation is effective in lowering blood pressure, possibly through UV-A-induced nitric oxide bioactivity.317 A reduced risk of cancer and particularly breast cancer has been reported in association with greater exposure to the sun in early life,318 as well as a decreased risk of myocardial infarction, and all-cause mortality, and hip fracture in those below age 90, where history of skin cancer was the measure of past exposure to the sun.319 In Chile the most frequent cases of food-related anaphylaxis (severe allergic reaction) occur at higher latitudes where there is lower solar radiation.320
Personal protection from solar UV radiation
The threat of increasing levels of UV-B radiation due to stratospheric ozone depletion, and rising skin cancer incidence rates, led to the development of sun protection programs and strategies. Despite the partial recovery of the ozone layer (reviewed in Bais et al.2), such strategies remain important because changes in climate are likely to alter both ambient UV radiation and behaviour that affects exposure to the sun.A sustained effort is required to change attitudes and behaviours in relation to exposure to the sun, especially in young people.321 Several recent surveys indicate that adults,322,323 adolescents,324 and children,325,326 still commonly report having been sunburned in the previous year, and this applies to both fair- and dark-skinned populations.327
While in some locations a majority of children use some form of photoprotection, particularly shade,325,326,328 this is not true of adults.323 Good knowledge of protection from the sun may not translate into attitudes and practices for reducing exposure.326,328,329 Further, more education about the specific needs for photoprotection in different situations may be required. In a sample of young German children, parental knowledge of appropriate use of shade, clothing, a sunhat, and sunscreen was considered to be adequate for summer holidays at the beach, but not for everyday outdoor activities.330 In a Danish study, travel to a sunny destination was common (almost 50% of those aged 15–59 years took such a holiday each year), with a high likelihood of sunburn and intentional tanning.331 There are limited data on strategies for photoprotection in tropical countries but one survey in adolescents living in Bangkok, Thailand, found that sunscreens, sun-protective clothing, and shade were seldom used, particularly in males, compared with Western countries.332 In a study in Philadelphia, USA, Hispanic adolescents and young adults who showed evidence of greater adoption of US culture were less likely to use sunscreen and more likely to deliberately expose themselves to the sun than those who retained their traditional culture.333 Barriers to personal protection from the sun in young Australians include peer pressure, lifestyle, fashion and social norms.334
The ultraviolet index (UVI) is routinely published in the media in some countries, available online,335 on a mobile phone,336 on mobile phone apps,337 or can be approximated using a compact disk as a sundial.338 However, its use to guide behaviour in relation to exposure to the sun remains limited.339 Furthermore, the specific guidance in relation to the UVI varies across different countries. For example, in Australia, photoprotection is recommended when the UV Index is ≥3.335 In New Zealand, a “UV Sun protection Alert Period” is provided daily, rather than the UVI, and is defined as the period of the day where the forecast clear-sky UVI is >3.340 The United States Environmental Protection Agency provides a UV Alert when the forecast UVI is ≥6, with advice to minimise time in the sun and use protection.341 As noted, exposure to the sun when the UVI is <3 is relatively ineffective for vitamin D production, whereas, for some fair-skinned individuals, even short exposures at a UVI of 6 may result in erythema. It is difficult to provide blanket recommendations, even according to the UVI and skin type, as there is wide variation in the minimal erythema dose (MED, the dose of UV radiation required to cause a slight reddening of the skin) even within a specific skin type, and different messages may be required for different regions.69
It is not generally realised that measures for protection against the sun may be required on cloudy days, in addition to clear days, due to diffuse solar UV radiation,342,343 and that some shade devices, such as umbrellas, provide incomplete protection from UV-B radiation.344
Sunscreens
Public health bodies have long advocated the use of sunscreens as a means of photoprotection. A long-term prospective study in Queensland, Australia, showed that daily use of sunscreen reduced the incidence of SCC345 with some evidence of reduction in the incidence of CMM.346 In addition, such use also reduced photoageing of the skin.347 However, a recent meta-analysis of the effectiveness of interventions promoting use of sunscreen in adults and children in recreational settings showed no reduction of sunburn in adults and only a modest effect in children.348Sunscreens are formulated and tested for their ability to prevent erythema in vivo and their index of efficacy is the sun protection factor (SPF).349 The labelled SPF is equal to: [MEDprotected skin]/[MEDunprotected skin], when tested under laboratory conditions with simulated solar radiation. The erythema action spectrum dictates that this is primarily an index of protection from UV-B radiation, although sunscreens are also required to have a measure of protection against UV-A radiation.350 Sunscreens with uniform absorption across the whole UV spectrum (broad-spectrum) provide photoprotection that is similar to shade or some types of clothing fabric351 (for more detail, see Andrady et al.352). Some UV radiation filters may also have anti-inflammatory properties,353 in which case the SPF may be more than a measure of optical filtering.354
One requirement of the SPF test is that sunscreen is applied at a coverage of 2 mg cm−2, but several studies have shown that people apply much less (for example, Petersen et al.355). The thickness of the application is also dependent on the formulation, with coverage of only 0.22 mg cm−2 achieved by children applying sunscreen with a roll-on.356 Use of a higher SPF sunscreen or two applications of sunscreen can achieve a greater level of protection.357,358 In addition to inadequate thickness of application, failure to apply sunscreen to all areas of exposed skin also limits the amount of photoprotection achieved. There are concerns that using sunscreen will decrease synthesis of vitamin D. Current evidence suggests that, if sunscreen is correctly applied, there may be no increase in concentrations of 25(OH)D following exposure to the sun.359 However, with usual applications, there is minimal impairment of the synthesis of vitamin D.282,360
Traditional topical sunscreens depend on the filtering or scattering of UV radiation, i.e., “passive” photoprotection. Table 5 summarises some recent developments in compounds providing “active” photoprotection at a topical and systemic level.361
| Class | Route and Effectiveness |
|---|---|
| α-Melanocyte stimulating hormone analogues – stimulate melanogenesis (tanning), e.g., afamelanotide | Sub-cutaneous; effective in some photosensitive patients362,363 |
| Natural anti-oxidants, e.g., vitamin A (retinol), vitamin C (ascorbic acid), vitamin E (tocopherol), pycnogenol (pine bark extract), carotenoids | Oral; not as effective as topical sunscreens361,364 |
| Nicotinamide (an amide form of vitamin B3) | Topical; enhances DNA repair, prevents UV-induced immunosuppression, reduces incidence of NMSC365 |
| Resveratrate, found in red wine, grapes, plums and peanuts | Topical; application immediately after exposure to the sun protected against erythema and sunburn cell formation366 |
| Flavonoids, e.g., luteolin (a) | Protects skin by a combination of UV-absorbing, DNA-protective, antioxidant and anti-inflammatory effects.367 |
| Lycopene, found in tomato paste | Oral; ingestion over a 12 week period reduced acute & chronic effects of photodamage368 |
| Green tea polyphenols | Oral; some evidence of modest effect369,370 |
| Diet rich in omega-3 fatty acids | Oral; may reduce risk of skin cancer371,372 |
| Liposomes containing natural endonucleases or photolyases | Topical; enhance nucleotide excision repair of UV-induced DNA damage; shown to reduce the incidence of solar keratoses and the severity of polymorphic light eruption.373–375 |
Overall, people are probably getting much less protection from the harmful effects of UV radiation than they believe when they use sunscreens, especially if their intention is to prolong their time in the sun. This is a public health issue that has to be addressed either by encouraging people not to go outdoors when the UVI is high, or to use appropriate clothing (see also Andrady et al.352) for sun protection.335 Alternatively, people need to apply sunscreen more thickly, or use sunscreen of a higher SPF to compensate for inadequate application.
Clothing and shade
Clothing modifies the skin surface area exposed to solar UV radiation. It offers good protection against sunburn, although this is dependent on the properties of the fabric such as colour, structure (e.g., woven vs. knit and tightness of the weave for woven fabrics), and wetness (see Andrady et al.352). Clothing typically strongly attenuates transmission of erythemally-effective UV radiation (i.e., weighted with the erythema action spectrum) to the skin. However, this blocking of UV-B radiation commonly leads to low vitamin D status in people who wear full body clothing,376 for example for religious or cultural reasons.377,378 It may be possible to design and manufacture clothing from fabrics that allows synthesis of vitamin D while preventing a visible erythema.379 However, it is likely that this will not protect from suberythemal damage, such as to the DNA of the epidermal cells.Shade-seeking is a well-recognised and effective way of reducing exposure to solar UV radiation, as evidenced by a lower level of sunburn380 and lower vitamin D status in adults from the USA who reported frequent use of shade on a sunny day, compared to those who used shade rarely.360 Provision of shade over playgrounds, particularly in sunny locations, is a relatively inexpensive method of mass protection from the sun. Nevertheless, a systematic review of 23 publications found no evidence that health promotion interventions had any effect in increasing shade-seeking in adults or children.348 Shade may be less effective at reducing exposure to diffuse UV radiation and this may account for about 80% of people's cumulative annual erythemal exposure.343 A recent study has used a manikin head (in a fixed position) to measure exposure to UV radiation under different conditions of shade, cloud cover and solar angle.381 This approach can be used to quantify the protective benefits of shade that are currently not well documented.
Preventing skin cancer versus ensuring adequate vitamin D status
There is no simple message to guide optimal levels of exposure to the sun. There is considerable variation between individuals in the doses of UV radiation that cause damage to DNA and induce synthesis of vitamin D. However, some broad recommendations can be made. Repeated short exposures to the sun are more efficient at vitamin D production than a single prolonged exposure.218 Levels of UV-B radiation are greatest at midday and vitamin D synthesis is thus most efficient at this time,382 although it is also the time when sunburn occurs most quickly.383 The amount of time in the sun that is needed for the synthesis of vitamin D varies according to location, time of day, and time of year. After controlling for the level of exposure to UV radiation, having more skin not covered by clothing is associated with higher concentrations of 25(OH)D in serum.246,247 As noted above, there are conflicting results on the effect of darker pigmentation of skin on the UV-induced production of vitamin D. In high latitude locations where UV-B levels are too low for vitamin D synthesis and a cooler climate may mean that little skin is exposed to the sun even during summer, greater intake of vitamin D may be required to avoid vitamin D deficiency.Protection of the eye
One of the cheapest and most practical methods of protecting the eye from exposure to UV-B radiation is wearing a hat with a brim of at least 6–7 cm.335,384 Sunglasses provide variable protection, and standards are more rigorous for UV-B than for UV-A radiation. For UV-B radiation the upper transmission limits range from 1.0% to 12.5% depending on the international jurisdiction and the type of use. For UV-A radiation, the limit is either a maximum of 50% of visible transmittance or is unspecified. The size of the frame and design can influence eye protection and some standards for sunglasses incorporate a minimum size limit.385 Wrap-around designs are most protective and are especially important when in highly reflective conditions, for example when skiing.385 Many ordinary eye-glass lenses have UV filters in them.The American National Standards Institute (ANSI) requires that contact lenses absorb at least 95% of UV-B radiation and 70% of UV-A radiation for a “UV blocking” claim. A recent survey on a selection of lenses showed compliance with claims for photoprotection.386
Studies using manikin heads387,388 have shown that, although the ambient UV radiation is greatest at solar noon, the highest dose of UV radiation was received by the eye at 4 hours before and after noon, when the solar elevation angle was lower. Thus, photoprotection of the eyes during outdoor activities is important not only at noon, but also at other times, and during winter.389 In some occupations, e.g., working on a building site, the reflectivity of the building materials may influence the amount of UV radiation received by the eye390 and should be considered when wearing eye-protection.
Protection of the eyes from the sun should reduce the risk of pterygium; UV absorbing contact lenses that cover most of the cornea can protect against UV-induced damage.385,391 Eye drops for the prevention of pterygium and photokeratitis/photoconjunctivitis through anti-oxidant and anti-inflammatory pathways have been effective in animal models, but are not commonly used in humans.119,392
Effects of interactions between solar UV radiation and the environment
Environmental contaminants may interact synergistically with UV radiation to harm human health. For example, in the presence of UV-B radiation, chrysene,393 a common environmental contaminant produced by incomplete burning of fossil fuels, and some pesticides394 have adverse effects on human health, including through damage to DNA.395 Topical corticosteroids are unstable under UV-B irradiation, possibly causing skin damage as well as loss of therapeutic effect.396 Engineered nanoparticles (NPs) are increasingly incorporated into sporting equipment, sunscreens, clothing and cosmetics (see also Andrady et al.352). Concerns have been raised about possible health risks of NPs.397,398 A recent review of the evidence on NPs in sunscreens concluded that “on current evidence, neither TiO2 nor ZnO NPs are likely to cause harm when used as ingredients in sunscreens”.399 Nevertheless, exposure of skin to UV radiation may enhance the penetration of engineered NPs,400 and the formulations using these particles are often used around the time of irradiation when skin damage may occur. Further, UV-induced immunosuppression could potentially impair an immune protective response induced by engineered NPs applied to the skin.400On the positive side, UV radiation is a potent environmental disinfectant able to inactivate viruses in clear water (for further discussion see Häder et al.401). This property is used in the SODIS (solar disinfection) technique, where exposure of surface water within a transparent bottle to sunlight effectively disinfects the water, decreasing the incidence of diarrhoeal diseases (reviewed in McGuigan et al.402).
Health implications of interactions between ozone depletion and climate change
Past studies have estimated the health gains in terms of skin cancers avoided through the implementation of the Montreal Protocol and its amendments.403 A recent update of this work that also integrated coupled climate-chemistry models has estimated that the world-wide incidence of skin cancer would have been 14% greater (2 million people) by 2030 without implementation of the Montreal Protocol and its amendments,404 with the largest effects in the South West USA and in Australia (see Bais et al.2 for further details).Model estimates405 suggest that by 2050, any increases in erythemal UV radiation above present levels will be small and confined to the tropical region (reviewed in Bais et al.2). These should have only a small effect on the incidence of skin cancer406 but may impair immune responses to some vaccinations. However, outside the tropical region, erythemal UV radiation is projected to be lower, especially in winter (reviewed Bais et al.2), which could be detrimental for the vitamin D status of populations in these regions, as well as for diseases that may be modulated by exposure to UV radiation, such as some of the autoimmune diseases discussed above. Estimates of exposure times for erythema and vitamin D synthesis in Europe, taking account of ozone recovery and interactions with different concentrations of greenhouse gases (GHG),406 suggest that there will be very little change in the exposure time for both endpoints for Southern Europe. However, an increased exposure time of about 30% for vitamin D production would be required in a worst case scenario in Stockholm in spring with high levels of GHGs.406
A major determinant of the received dose of UV radiation is behaviour in relation to exposure to the sun.407 Ambient temperature is likely to influence time spent outdoors. In temperate parts of Australia, the increase in temperature is likely to increase skin cancer incidence because people will spend more time outside with less clothing.408,409 However, with temperature increases in already warm climates, people will be more likely to stay indoors or to seek shade.229 Warmer ambient temperatures may have direct physiological effects to accelerate both skin cancer development410 and vitamin D production.411
In addition to these direct effects of interactions between climate change and ozone depletion and/or UV radiation for human health, there are potential indirect effects that may become important, but at this stage remain ill-defined.412 Concurrent changes in climate and levels of ambient UV radiation will influence aquatic and terrestrial ecosystems (see also Häder et al.401 and Bornman et al.413), which may have consequences for food safety, quality and supply (reviewed in412).
Migration of populations, often with dark skin pigmentation, from low-lying tropical regions because of rising sea levels, to higher latitude regions may increase the diseases associated with vitamin D deficiency.
Higher temperatures should foster microbial growth in surface waters, and this will be more pronounced in the presence of lower levels of disinfecting UV-B radiation, or where increases in colour due to dissolved organic matter limit penetration of UV-B radiation (for more detail, see Häder et al.,401 Bornman et al.,413 and Erickson et al.414). However, there has been little research to date that allows prediction or quantification of the risks to human health that might arise from these interactions.
Gaps in our knowledge
Considerable evidence from animal models suggests that UV-induced immunosuppression may increase the risk of some infections and decrease the protection offered by vaccination (reviewed in Norval & Halliday212). Studies in humans are required to determine whether any increased risk is clinically relevant, for example, requiring changes in vaccination protocols. Although some animal studies similarly suggest that vitamin D status affects the outcomes of vaccination or immune responses to infection, results from clinical trials mostly show no effect.415,416There is currently considerable controversy about which health conditions are influenced by vitamin D. Large-scale vitamin D supplementation trials that are in progress will provide some answers (see Table 1 in Byrne417). While there is consensus that vitamin D is important for bone health, there is lack of agreement about the concentration of 25(OH)D required. In addition, there is substantial individual variability in the change in concentration in 25(OH)D in response to UV irradiation and vitamin D supplementation, and in the clinical effects associated with different concentrations of 25(OH)D. The reasons for this are poorly understood, but are likely to depend on variation in the genes encoding the vitamin D binding protein and/or the vitamin D receptor.
Production of vitamin D occurs readily at sub-erythemal doses, but repeated sub-erythemal exposures can also cause accumulation of CPDs that repair only slowly (over 24–36 hours) and thus may increase the risk of skin cancer.418 Until there is a better understanding of the numbers of CPDs that accrue during brief sun exposures, and their importance in determining the risk of subsequent skin cancer, it is difficult to recommend safe exposures that would result in sufficient production of vitamin D.
There is a lack of data on the vitamin D status of infants, children, and adolescents and for populations in Africa and South America. Further, the lack, until recently, of accurate and precise assays has limited our ability to examine variability in vitamin D status across countries (for example those with and without fortification of food) and over time. The advent of the standardised vitamin D assays419 means that this is now possible. Development of a less invasive sampling method, for example using saliva, would allow more widespread assessment of the vitamin D status of infants and children.
Protection from the sun is currently recommended by the World Health Organization when the UVI is ≥3. The corollary of this message is that photoprotection is not required when the UVI is <3. At these low UVI values, there is little UV-B radiation (and thus little vitamin D synthesis), but, with prolonged exposure, there may be a relatively high dose of UV-A radiation. With recognition that UV-A radiation can induce immune suppression164 and is involved in the initiation of CMM, but may also have beneficial effects on blood pressure, the doses received and potential health effects need to be better defined.
The action spectra for a number of health outcomes have not been determined. These include cataract from protracted exposure, myopia, carcinoma in deeply pigmented skin, melanoma and production of pre-vitamin D from a polychromatic source (the sun) and in both dark and fair skin types. However, the relevance of the currently available animal models is uncertain, so obtaining these action spectra with definite relevance to humans is very difficult.
There is emerging evidence that exposure to the sun may have beneficial effects independently of vitamin D. The lack of a strong evidence-base challenges our ability to provide accurate guidance to the public regarding exposure to the sun.
As noted above, there is much uncertainty about the effect of the potential indirect interactions of climate change and ozone depletion on human health. Effects on disinfection of surface water and on supply and security of food could become important sources of risks to health, particularly in some regions of the world. Modelling now may be able to identify those areas at greatest risk, and allow forward planning to mitigate the risks.
Acknowledgements
We would like to acknowledge the following people and organizations for their support in the preparation of this paper. Prof Robyn Lucas' participation in the Panel was supported through funding from the Australian Government's Ozone Science Strategy. A/Prof Rachel Neale was supported by the QIMR Berghofer Institute for Medical Research. Prof Yukio Takizawa was sponsored by the Japanese Ministry of the Environment. Ms Tammy Gibbs provided support with the figures in this paper.References
- P. Rettberg, U. Eschweiler, K. Strauch, G. Reitz, G. Horneck, H. Wanke, A. Brack and B. Barbier, Survival of microorganisms in space protected by meteorite material: results of the experiment ‘EXOBIOLOGIE’ of the PERSEUS mission, Adv. Space Res., 2002, 30, 1539–1545 CrossRef CAS.
- A. F. Bais, R. L. McKenzie, P. J. Aucamp, M. Ilyas, S. Madronich, G. Bernhard and K. Tourpali, Ozone depletion and climate change: Impacts on UV radiation, Photochem. Photobiol. Sci., 2015, 14 10.1039/c4pp90032d , this issue.
- WHO, Environmental Health Criteria 160 - Ultraviolet Radiation, World Health Organization, 1994 Search PubMed.
- M. R. Albert and K. G. Ostheimer, The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 2, J. Am. Acad. Dermatol., 2003, 48, 909–918 CrossRef PubMed.
- A. M. Eggermont, A. Spatz and C. Robert, Cutaneous melanoma, Lancet, 2013, 383, 816–827 CrossRef.
- J. E. Frangos, L. M. Duncan, A. Piris, R. M. Nazarian, M. C. Mihm, Jr., M. P. Hoang, B. Gleason, T. J. Flotte, H. R. Byers, R. L. Barnhill and A. B. Kimball, Increased diagnosis of thin superficial spreading melanomas: A 20-year study, J. Am. Acad. Dermatol., 2012, 67, 387–394 CrossRef PubMed.
- IARC, Globocan 2012. Estimated cancer incidence, mortality and prevalence worldwide in 2012, International Agency for Research on Cancer, http://globocan.iarc.fr/Pages/summary_table_site_sel.aspx, accessed 28 June 2014.
- R. Laishram, A. Banerjee, P. Punyabati, L. Chandra and A. Sharma, Pattern of skin malignancies in Manipur, India: A 5-year histopathological review, J. Pak. Assoc. Dermatol., 2010, 20, 128–132 Search PubMed.
- F. Erdmann, J. Lortet-Tieulent, J. Schuz, H. Zeeb, R. Greinert, E. W. Breitbart and F. Bray, International trends in the incidence of malignant melanoma 1953–2008 – are recent generations at higher or lower risk?, Int. J. Cancer, 2013, 132, 385–400 CrossRef CAS PubMed.
- M. Arnold, C. Holterhues, L. M. Hollestein, J. W. Coebergh, T. Nijsten, E. Pukkala, B. Holleczek, L. Tryggvadottir, H. Comber, M. J. Bento, S. Diba Ch, R. Micallef, M. Primic-Zakelj, M. I. Izarzugaza, J. Perucha, R. Marcos-Gragera, J. Galceran, E. Ardanaz, R. Schaffar, A. Pring and E. de Vries, Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015, J. Eur. Acad. Dermatol. Venereol., 2014, 28, 1170–1178 CrossRef CAS PubMed.
- J. Ferlay, F. Bray, P. Pisani and D. Parkin, GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, IARC Cancer Base No. 5. Lyon, 2001.
- V. Nikolaou and A. J. Stratigos, Emerging trends in the epidemiology of melanoma, Br. J. Dermatol., 2014, 170, 11–19 CrossRef CAS PubMed.
- A. C. Geller, R. W. Clapp, A. J. Sober, L. Gonsalves, L. Mueller, C. L. Christiansen, W. Shaikh and D. R. Miller, Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry, J. Clin. Oncol., 2013, 31, 4172–4178 CrossRef PubMed.
- P. T. Bradford, W. F. Anderson, M. P. Purdue, A. M. Goldstein and M. A. Tucker, Rising melanoma incidence rates of the trunk among younger women in the United States, Cancer Epidemiol. Biomarkers Prev., 2010, 19, 2401–2406 CrossRef PubMed.
- P. D. Baade, A. C. Green, B. M. Smithers and J. F. Aitken, Trends in melanoma incidence among children: possible influence of sun-protectionprograms, Expert. Rev. Anticancer Ther., 2011, 11, 661–664 CrossRef PubMed.
- A. Stang, S. Valiukeviciene, B. Aleknaviciene and J. Kurtinaitis, Time trends of incidence, mortality, and relative survival of invasive skin melanoma in Lithuania, Eur. J. Cancer Prev., 2006, 42, 660–667 CrossRef PubMed.
- N. Duschek, H. Skvara, H. Kittler, G. Delir, A. Fink, A. Pinkowicz and T. Waldhor, Melanoma epidemiology of Austria reveals gender-related differences, Eur. J. Dermatol., 2013, 23, 872–878 Search PubMed.
- L. Tryggvadottir, M. Gislum, T. Hakulinen, A. Klint, G. Engholm, H. H. Storm and F. Bray, Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964–2003 followed up to the end of 2006, Acta Oncol., 2010, 49, 665–672 CrossRef PubMed.
- M. J. Sneyd and B. Cox, A comparison of trends in melanoma mortality in New Zealand and Australia: the two countries with the highest melanoma incidence and mortality in the world, BMC Cancer, 2013, 13, 372 CrossRef PubMed.
- A. Katalinic, A. Waldmann, M. A. Weinstock, A. C. Geller, N. Eisemann, R. Greinert, B. Volkmer and E. Breitbart, Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening, Cancer, 2012, 118, 5395–5402 CrossRef PubMed.
- A. E. Cust, M. A. Jenkins, C. Goumas, B. K. Armstrong, H. Schmid, J. F. Aitken, G. G. Giles, R. F. Kefford, J. L. Hopper and G. J. Mann, Early-life sun exposure and risk of melanoma before age 40 years, Cancer Causes Control, 2011, 22, 885–897 CrossRef PubMed.
- S. C. Wallingford, R. D. Alston, J. M. Birch and A. C. Green, Increases in invasive melanoma in England, 1979–2006, by anatomical site, Br. J. Dermatol., 2011, 165, 859–864 CrossRef CAS PubMed.
- N. B. Fuglede, U. O. Brinck-Claussen, I. Deltour, E. H. Boesen, S. O. Dalton and C. Johansen, Incidence of cutaneous malignant melanoma in Denmark, 1978–2007, Br. J. Dermatol., 2011, 165, 349–353 CrossRef CAS PubMed.
- B. Diffey, A behavioral model for estimating population exposure to solar ultraviolet radiation, Photochem. Photobiol., 2008, 84, 371–375 CrossRef CAS PubMed.
- P. Rouhani, S. Hu and R. S. Kirsner, Melanoma in Hispanic and black Americans, Cancer Control, 2008, 15, 248–253 Search PubMed.
- M. Clairwood, J. Ricketts, J. Grant-Kels and L. Gonsalves, Melanoma in skin of color in Connecticut: an analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks, non-Hispanic whites, and Hispanics, Int. J. Dermatol., 2013, 53, 425–433 CrossRef PubMed.
- M. G. Cockburn, J. Zadnick and D. Deapen, Developing epidemic of melanoma in the Hispanic population of California, Cancer, 2006, 106, 1162–1168 CrossRef PubMed.
- R. A. Schmerling, D. Loria, G. Cinat, W. E. Ramos, A. F. Cardona, J. L. Sanchez, H. Martinez-Said and A. C. Buzaid, Cutaneous melanoma in Latin America: the need for more data, Rev. Panam. Salud. Pacific., 2011, 30, 431–438 CrossRef PubMed.
- P. M. Nthumba, P. C. Cavadas and L. Landin, Primary cutaneous malignancies in sub-Saharan Africa, Ann. Plast. Surg., 2011, 66, 313–320 CrossRef CAS PubMed.
- M. Norval, P. Kellett and C. Y. Wright, The Incidence and Body Site of Skin Cancers in the Population Groups of South Africa, Photodermatol. Photoimmunol. Photomed., 2014, 30(5), 262–265 CrossRef PubMed.
- O. N. Agbai, K. Buster, M. Sanchez, C. Hernandez, R. V. Kundu, M. Chiu, W. E. Roberts, Z. D. Draelos, R. Bhushan, S. C. Taylor and H. W. Lim, Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public, J. Am. Acad. Dermatol, 2014, 70, 748–762 CrossRef PubMed.
- S. W. Lin, D. C. Wheeler, Y. Park, E. K. Cahoon, A. R. Hollenbeck, D. M. Freedman and C. C. Abnet, Prospective study of ultraviolet radiation exposure and risk of cancer in the United States, Int. J. Cancer, 2012, 131, E1015–E1023 CrossRef CAS PubMed.
- H. E. Kanavy and M. R. Gerstenblith, Ultraviolet radiation and melanoma, Semin. Cutaneous Med. Surg., 2011, 30, 222–228 CrossRef CAS PubMed.
- H. Levine, A. Afek, A. Shamiss, E. Derazne, D. Tzur, N. Astman, L. Keinan-Boker, D. Mimouni and J. D. Kark, Country of origin, age at migration and risk of cutaneous melanoma: a migrant cohort study of 1,100,000 Israeli men, Int. J. Cancer, 2013, 133, 486–494 CrossRef CAS PubMed.
- L. Le Marchand, B. S. Saltzman, J. H. Hankin, L. R. Wilkens, A. A. Franke, S. J. Morris and L. N. Kolonel, Sun exposure, diet, and melanoma in Hawaii Caucasians, Am. J. Epidemiol., 2006, 164, 232–245 CrossRef PubMed.
- F. P. Noonan, M. R. Zaidi, A. Wolnicka-Glubisz, M. R. Anver, J. Bahn, A. Wielgus, J. Cadet, T. Douki, S. Mouret, M. A. Tucker, A. Popratiloff, G. Merlino and E. C. De Fabo, Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment, Nat. Commun., 2012, 3, 884 CrossRef PubMed.
- D. L. Mitchell and A. A. Fernandez, Different types of DNA damage play different roles in the etiology of sunlight-induced melanoma, Pigm. Cell Melanoma. Res., 2011, 24, 119–124 CrossRef CAS PubMed.
- N. O. Basta, P. W. James, A. W. Craft and R. J. McNally, Seasonal variation in the month of birth in teenagers and young adults with melanoma suggests the involvement of early-life UV exposure, Pigm. Cell Melanoma. Res., 2011, 24, 250–253 CrossRef PubMed.
- C. Crump, K. Sundquist, W. Sieh, M. A. Winkleby and J. Sundquist, Season of birth and other perinatal risk factors for melanoma, Int. J. Epidemiol., 2014, 43(3), 793–801 CrossRef PubMed.
- S. L. Harrison, P. G. Buettner and R. MacLennan, Why do mothers still sun their infants?, J. Pediatr. Child Health, 1999, 35, 296–299 CrossRef CAS.
- S. Harrison, M. Nowak, S. Devine, V. Saunders, A. Smith and P. Buettner, An intervention to discourage Australian mothers from unnecessarily exposing their babies to the sun for therapeutic reasons, J. Trop. Pediatr., 2013, 59, 403–406 CrossRef PubMed.
- A. S. Paller, J. L. Hawk, P. Honig, Y. C. Giam, S. Hoath, M. C. Mack and G. N. Stamatas, New insights about infant and toddler skin: implications for sun protection, Pediatrics, 2011, 128, 92–102 CrossRef PubMed.
- J. Reichrath, Sunlight, Vitamin D and Skin Cancer, in Advances in Experimental Medicine and Biology, ed. N. Back, I. Cohen, A. Lajtha, J. Lambris and R. Poaletti, Landes Bioscience/Springer Science+Business media, 2014, 2nd edn Search PubMed.
- I. Chaillol, M. Boniol, R. Middleton, J. F. Dore, P. Autier and A. Gavin, Seasonality of cutaneous melanoma diagnosis in Northern Ireland with a review, Melanoma Res., 2011, 21, 144–151 CrossRef PubMed.
- C. M. Olsen, H. J. Carroll and D. C. Whiteman, Familial melanoma: a meta-analysis and estimates of attributable fraction, Cancer Epidemiol. Biomarkers Prev., 2010, 19, 65–73 CrossRef PubMed.
- V. K. Hill, J. J. Gartner, Y. Samuels and A. M. Goldstein, The genetics of melanoma: recent advances, Annu. Rev. Genomics Hum. Genet., 2013, 14, 257–279 CrossRef CAS PubMed.
- E. Hodis, I. R. Watson, G. V. Kryukov, S. T. Arold, M. Imielinski, J. P. Theurillat, E. Nickerson, D. Auclair, L. Li, C. Place, D. Dicara, A. H. Ramos, M. S. Lawrence, K. Cibulskis, A. Sivachenko, D. Voet, G. Saksena, N. Stransky, R. C. Onofrio, W. Winckler, K. Ardlie, N. Wagle, J. Wargo, K. Chong, D. L. Morton, K. Stemke-Hale, G. Chen, M. Noble, M. Meyerson, J. E. Ladbury, M. A. Davies, J. E. Gershenwald, S. N. Wagner, D. S. Hoon, D. Schadendorf, E. S. Lander, S. B. Gabriel, G. Getz, L. A. Garraway and L. Chin, A landscape of driver mutations in melanoma, Cell, 2012, 150, 251–263 CrossRef CAS PubMed.
- A. Viros, B. Sanchez-Laorden, M. Pedersen, S. J. Furney, J. Rae, K. Hogan, S. Ejiama, M. R. Girotti, M. Cook, N. Dhomen and R. Marais, Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53, Nature, 2014, 511, 478–482 CrossRef CAS PubMed.
- M. F. Berger, E. Hodis, T. P. Heffernan, Y. L. Deribe, M. S. Lawrence, A. Protopopov, E. Ivanova, I. R. Watson, E. Nickerson, P. Ghosh, H. Zhang, R. Zeid, X. Ren, K. Cibulskis, A. Y. Sivachenko, N. Wagle, A. Sucker, C. Sougnez, R. Onofrio, L. Ambrogio, D. Auclair, T. Fennell, S. L. Carter, Y. Drier, P. Stojanov, M. A. Singer, D. Voet, R. Jing, G. Saksena, J. Barretina, A. H. Ramos, T. J. Pugh, N. Stransky, M. Parkin, W. Winckler, S. Mahan, K. Ardlie, J. Baldwin, J. Wargo, D. Schadendorf, M. Meyerson, S. B. Gabriel, T. R. Golub, S. N. Wagner, E. S. Lander, G. Getz, L. Chin and L. A. Garraway, Melanoma genome sequencing reveals frequent PREX2 mutations, Nature, 2012, 485, 502–506 CAS.
- M. Krauthammer, Y. Kong, B. H. Ha, P. Evans, A. Bacchiocchi, J. P. McCusker, E. Cheng, M. J. Davis, G. Goh, M. Choi, S. Ariyan, D. Narayan, K. Dutton-Regester, A. Capatana, E. C. Holman, M. Bosenberg, M. Sznol, H. M. Kluger, D. E. Brash, D. F. Stern, M. A. Materin, R. S. Lo, S. Mane, S. Ma, K. K. Kidd, N. K. Hayward, R. P. Lifton, J. Schlessinger, T. J. Boggon and R. Halaban, Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma, Nat. Genet., 2012, 44, 1006–1014 CrossRef CAS PubMed.
- S. C. Flohil, C. M. Proby, A. D. Forrest, S. van Tiel, O. Saksela, S. Pitkanen, T. Ahti, R. Micallef and E. de Vries, Basal cell carcinomas without histological confirmation and their treatment: an audit in four European regions, Br. J. Dermatol., 2012, 167(Suppl 2), 22–28 CrossRef PubMed.
- F. Xiang, R. Lucas, S. Hales and R. E. Neale, Incidence of Nonmelanoma Skin Cancer in Relation to Ambient UV Radiation in White Populations, 1978-2012: Empirical Relationships, JAMA Dermatol., 2014, 150(10), 1063–71 CrossRef PubMed.
- A. Lomas, J. Leonardi-Bee and F. Bath-Hextall, A systematic review of worldwide incidence of nonmelanoma skin cancer, Br. J. Dermatol., 2012, 166, 1069–1080 CrossRef CAS PubMed.
- National Cancer Registry of South Africa, Cancer in South Africa, http://www.nioh.ac.za/?page=national_cancer_registry&id=41, accessed 29 July, 2014.
- S. C. Flohil, I. Seubring, M. M. van Rossum, J. W. Coebergh, E. de Vries and T. Nijsten, Trends in Basal cell carcinoma incidence rates: a 37-year Dutch observational study, J. Invest. Dermatol., 2013, 133, 913–918 CrossRef CAS PubMed.
- H. W. Rogers, M. A. Weinstock, A. R. Harris, M. R. Hinckley, S. R. Feldman, A. B. Fleischer and B. M. Coldiron, Incidence estimate of nonmelanoma skin cancer in the United States, 2006, Arch. Dermatol., 2010, 146, 283–287 Search PubMed.
- S. Franceschi, F. Levi, L. Randimbison and C. La Vecchia, Site distribution of different types of skin cancer: new aetiological clues, Int. J. Cancer, 1996, 67, 24–28 CrossRef CAS.
- B. A. Raasch, P. G. Buettner and C. Garbe, Basal cell carcinoma: histological classification and body-site distribution, Br. J. Dermatol., 2006, 155, 401–407 CrossRef CAS PubMed.
- M. P. Staples, M. Elwood, R. C. Burton, J. L. Williams, R. Marks and G. G. Giles, Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985, Med. J. Aust., 2006, 184, 6–10 Search PubMed.
- A. Kricker, B. K. Armstrong, D. R. English and P. J. Heenan, Pigmentary and cutaneous risk factors for non-melanocytic skin cancer–a case-control study, Int. J. Cancer, 1991, 48, 650–662 CrossRef CAS.
- C. Dessinioti, C. Antoniou, A. Katsambas and A. J. Stratigos, Basal cell carcinoma: what's new under the sun, Photochem. Photobiol., 2010, 86, 481–491 CrossRef CAS PubMed.
- A. Bauer, T. L. Diepgen and J. Schmitt, Is occupational solar ultraviolet irradiation a relevant risk factor for basal cell carcinoma? A systematic review and meta-analysis of the epidemiological literature, Br. J. Dermatol., 2011, 165, 612–625 CAS.
- M. Khalesi, D. C. Whiteman, S. A. Doi, J. Clark, M. G. Kimlin and R. E. Neale, Cutaneous markers of photo-damage and risk of Basal cell carcinoma of the skin: a meta-analysis, Cancer Epidemiol. Biomarkers Prev., 2013, 22, 1483–1489 CrossRef PubMed.
- M. R. Iannacone, W. Wang, H. G. Stockwell, K. O'Rourke, A. R. Giuliano, V. K. Sondak, J. L. Messina, R. G. Roetzheim, B. S. Cherpelis, N. A. Fenske and D. E. Rollison, Patterns and timing of sunlight exposure and risk of basal cell and squamous cell carcinomas of the skin–a case-control study, BMC Cancer, 2012, 12, 417 CrossRef PubMed.
- R. E. Neale, M. Davis, N. Pandeya, D. C. Whiteman and A. C. Green, Basal cell carcinoma on the trunk is associated with excessive sun exposure, J. Am. Acad. Dermatol., 2007, 56, 380–386 CrossRef PubMed.
- J. R. Rees, M. S. Zens, J. Gui, M. O. Celaya, B. L. Riddle and M. R. Karagas, Non melanoma skin cancer and subsequent cancer risk, PLoS One, 2014, 9, e99674 Search PubMed.
- R. M. Halder and S. Bridgeman-Shah, Skin cancer in African Americans, Cancer, 1995, 75, 667–673 CrossRef CAS.
- H. M. Gloster, Jr. and K. Neal, Skin cancer in skin of color, J. Am. Acad. Dermatol., 2006, 55, 741–760 CrossRef PubMed ; quiz 761–744.
- F. Zaratti, R. D. Piacentini, H. A. Guillen, S. H. Cabrera, J. B. Liley and R. L. McKenzie, Proposal for a modification of the UVI risk scale, Photochem. Photobiol. Sci., 2014, 13, 980–985 CAS.
- H. Nan, M. Xu, P. Kraft, A. A. Qureshi, C. Chen, Q. Guo, F. B. Hu, G. Curhan, C. I. Amos, L. E. Wang, J. E. Lee, Q. Wei, D. J. Hunter and J. Han, Genome-wide association study identifies novel alleles associated with risk of cutaneous basal cell carcinoma and squamous cell carcinoma, Hum. Mol. Genet., 2011, 20, 3718–3724 CrossRef CAS PubMed.
- S. N. Stacey, D. F. Gudbjartsson, P. Sulem, J. T. Bergthorsson, R. Kumar, G. Thorleifsson, A. Sigurdsson, M. Jakobsdottir, B. Sigurgeirsson, K. R. Benediktsdottir, K. Thorisdottir, R. Ragnarsson, D. Scherer, P. Rudnai, E. Gurzau, K. Koppova, V. Hoiom, R. Botella-Estrada, V. Soriano, P. Juberias, M. Grasa, F. J. Carapeto, P. Tabuenca, Y. Gilaberte, J. Gudmundsson, S. Thorlacius, A. Helgason, T. Thorlacius, A. Jonasdottir, T. Blondal, S. A. Gudjonsson, G. F. Jonsson, J. Saemundsdottir, K. Kristjansson, G. Bjornsdottir, S. G. Sveinsdottir, M. Mouy, F. Geller, E. Nagore, J. I. Mayordomo, J. Hansson, T. Rafnar, A. Kong, J. H. Olafsson, U. Thorsteinsdottir and K. Stefansson, Common variants on 1p36 and 1q42 are associated with cutaneous basal cell carcinoma but not with melanoma or pigmentation traits, Nat. Genet., 2008, 40, 1313–1318 CrossRef CAS PubMed.
- J. J. DiGiovanna and K. H. Kraemer, Shining a light on xeroderma pigmentosum, J. Invest. Dermatol., 2012, 132, 785–796 CrossRef CAS PubMed.
- S. Durinck, C. Ho, N. J. Wang, W. Liao, L. R. Jakkula, E. A. Collisson, J. Pons, S. W. Chan, E. T. Lam, C. Chu, K. Park, S. W. Hong, J. S. Hur, N. Huh, I. M. Neuhaus, S. S. Yu, R. C. Grekin, T. M. Mauro, J. E. Cleaver, P. Y. Kwok, P. E. LeBoit, G. Getz, K. Cibulskis, J. C. Aster, H. Huang, E. Purdom, J. Li, L. Bolund, S. T. Arron, J. W. Gray, P. T. Spellman and R. J. Cho, Temporal dissection of tumorigenesis in primary cancers, Cancer Discovery, 2011, 1, 137–143 CrossRef CAS PubMed.
- S. S. Jayaraman, D. J. Rayhan, S. Hazany and M. S. Kolodney, Mutational landscape of basal cell carcinomas by whole-exome sequencing, J. Invest. Dermatol., 2014, 134, 213–220 CrossRef CAS PubMed.
- D. E. Brash, A. Ziegler, A. S. Jonason, J. A. Simon, S. Kunala and D. J. Leffell, Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion, J. Invest. Dermatol. Symp. Proc., 1996, 1, 136–142 CAS.
- A. Ziegler, A. S. Jonason, D. J. Leffell, J. A. Simon, H. W. Sharma, J. Kimmelman, L. Remington, T. Jacks and D. E. Brash, Sunburn and p53 in the onset of skin cancer, Nature, 1994, 372, 773–776 CrossRef CAS PubMed.
- E. H. Epstein, Basal cell carcinomas: attack of the hedgehog, Nat. Rev. Cancer, 2008, 8, 743–754 CrossRef CAS PubMed.
- P. Manga, R. Kerr, M. Ramsay and J. G. Kromberg, Biology and genetics of oculocutaneous albinism and vitiligo - common pigmentation disorders in southern Africa, S. Afr. Med. J., 2013, 103, 984–988 CrossRef PubMed.
- K. Gronskov, J. Ek and K. Brondum-Nielsen, Oculocutaneous albinism, Orphanet J. Rare. Dis., 2007, 2, 43 CrossRef PubMed.
- C. V. David, Oculocutaneous albinism, Cutis, 2013, 91, E1–E4 Search PubMed.
- J. G. Kromberg and T. Jenkins, Prevalence of albinism in the South African negro, S. Afr. Med. J., 1982, 61, 383–386 CAS.
- C. G. Summers, Albinism: classification, clinical characteristics, and recent findings, Optom. Visual Sci., 2009, 86, 659–662 CrossRef PubMed.
- E. S. Hong, H. Zeeb and M. H. Repacholi, Albinism in Africa as a public health issue, BMC Public Health, 2006, 6, 212 CrossRef PubMed.
- J. Luande, C. I. Henschke and N. Mohammed, The Tanzanian human albino skin. Natural history, Cancer, 1985, 55, 1823–1828 CrossRef CAS.
- J. B. Mabula, P. L. Chalya, M. D. McHembe, H. Jaka, G. Giiti, P. Rambau, N. Masalu, E. Kamugisha, S. Robert and J. M. Gilyoma, Skin cancers among Albinos at a University teaching hospital in Northwestern Tanzania: a retrospective review of 64 cases, BMC Dermatol., 2012, 12, 5 CrossRef PubMed.
- S. K. Kiprono, B. M. Chaula and H. Beltraminelli, Histological review of skin cancers in African Albinos: a 10-year retrospective review, BMC Cancer, 2014, 14, 157 CrossRef PubMed.
- P. K. Perry and N. B. Silverberg, Cutaneous malignancy in albinism, Cutis, 2001, 67, 427–430 CAS.
- R. Aquaron, Oculocutaneous albinism in Cameroon. A 15-year follow-up study, Ophthalmic. Paediatr. Genet., 1990, 11, 255–263 CrossRef CAS.
- M. P. Hughes, M. E. Hardee, L. A. Cornelius, L. F. Hutchins, J. C. Becker and L. Gao, Merkel Cell Carcinoma: Epidemiology, Target, and Therapy, Curr. Dermatol. Rep., 2014, 3, 46–53 CrossRef PubMed.
- J. Girschik, K. Thorn, T. W. Beer, P. J. Heenan and L. Fritschi, Merkel cell carcinoma in Western Australia: a population-based study of incidence and survival, Br. J. Dermatol., 2011, 165, 1051–1057 CrossRef CAS PubMed.
- R. W. Miller and C. S. Rabkin, Merkel cell carcinoma and melanoma: etiological similarities and differences, Cancer Epidemiol. Biomarkers Prev., 1999, 8, 153–158 CAS.
- N. C. Hodgson, Merkel cell carcinoma: changing incidence trends, J. Surg. Oncol., 2005, 89, 1–4 CrossRef PubMed.
- R. A. Wolfe, E. C. Roys and R. M. Merion, Trends in organ donation and transplantation in the United States, 1999–2008, Am. J. Transplant., 2010, 10, 961–972 CrossRef CAS PubMed.
- R. Arora, Y. Chang and P. S. Moore, MCV and Merkel cell carcinoma: a molecular success story, Curr. Opin. Virol, 2012, 2, 489–498 CrossRef CAS PubMed.
- S. K. Demetriou, K. Ona-Vu, E. M. Sullivan, T. K. Dong, S. W. Hsu and D. H. Oh, Defective DNA repair and cell cycle arrest in cells expressing Merkel cell polyomavirus T antigen, Int. J. Cancer, 2012, 131, 1818–1827 CrossRef CAS PubMed.
- A. Mogha, A. Fautrel, N. Mouchet, N. Guo, S. Corre, H. Adamski, E. Watier, L. Misery and M. D. Galibert, Merkel cell polyomavirus small T antigen mRNA level is increased following in vivo UV-radiation, PLoS One, 2010, 5, e11423 Search PubMed.
- S. Bhatia, O. Afanasiev and P. Nghiem, Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer, Curr. Oncol. Rep., 2011, 13, 488–497 CrossRef CAS PubMed.
- H. U. Bernard, R. D. Burk, Z. Chen, K. van Doorslaer, H. zur Hausen and E. M. de Villiers, Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments, Virology, 2010, 401, 70–79 CrossRef CAS PubMed.
- S. F. Farzan, T. Waterboer, J. Gui, H. H. Nelson, Z. Li, K. M. Michael, A. E. Perry, S. K. Spencer, E. Demidenko, A. C. Green, M. Pawlita and M. R. Karagas, Cutaneous alpha, beta and gamma human papillomaviruses in relation to squamous cell carcinoma of the skin: a population-based study, Int. J. Cancer, 2013, 133, 1713–1720 CrossRef CAS PubMed.
- C. M. Proby, C. A. Harwood, R. E. Neale, A. C. Green, S. Euvrard, L. Naldi, G. Tessari, M. C. Feltkamp, M. N. de Koning, W. G. Quint, T. Waterboer, M. Pawlita, S. Weissenborn, U. Wieland, H. Pfister, E. Stockfleth, I. Nindl, D. Abeni, J. T. Schegget and J. N. Bouwes Bavinck, A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients, Am. J. Transplant., 2011, 11, 1498–1508 CrossRef CAS PubMed.
- K. Andersson, K. M. Michael, T. Luostarinen, T. Waterboer, R. Gislefoss, T. Hakulinen, O. Forslund, M. Pawlita and J. Dillner, Prospective study of human papillomavirus seropositivity and risk of nonmelanoma skin cancer, Am. J. Epidemiol., 2012, 175, 685–695 CrossRef PubMed.
- R. E. Neale, S. Weissenborn, D. Abeni, J. N. Bavinck, S. Euvrard, M. C. Feltkamp, A. C. Green, C. Harwood, M. de Koning, L. Naldi, I. Nindl, M. Pawlita, C. Proby, W. G. Quint, T. Waterboer, U. Wieland and H. Pfister, Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma, Cancer Epidemiol. Biomarkers Prev., 2013, 22, 719–727 CrossRef CAS PubMed.
- J. N. Bouwes Bavinck, M. Feltkamp, L. Struijk and J. ter Schegget, Human papillomavirus infection and skin cancer risk in organ transplant recipients, J. Invest. Dermatol. Symp. Proc., 2001, 6, 207–211 CrossRef CAS PubMed.
- N. J. Lowe, D. P. Meyers, J. M. Wieder, D. Luftman, T. Borget, M. D. Lehman, A. W. Johnson and I. R. Scott, Low doses of repetitive ultraviolet A induce morphologic changes in human skin, J. Invest. Dermatol., 1995, 105, 739–743 CAS.
- E. C. Naylor, R. E. Watson and M. J. Sherratt, Molecular aspects of skin ageing, Maturitas, 2011, 69, 249–256 CrossRef CAS PubMed.
- A. Tewari, C. Lahmann, R. Sarkany, J. Bergemann and A. R. Young, Human erythema and matrix metalloproteinase-1 mRNA induction, in vivo, share an action spectrum which suggests common chromophores, Photochem. Photobiol. Sci., 2012, 11, 216–223 CAS.
- V. M. Sheth and A. G. Pandya, Melasma: a comprehensive update: part I, J. Am. Acad. Dermatol, 2011, 65, 689–697 CrossRef PubMed ; quiz 698.
- R. Jobanputra and M. Bachmann, The effect of skin diseases on quality of life in patients from different social and ethnic groups in Cape Town, South Africa, Int. J. Cancer, 2000, 39, 826–831 CAS.
- A. C. Handel, P. B. Lima, V. M. Tonolli, L. D. Miot and H. A. Miot, Risk factors for facial melasma in women: a case-control study, Br. J. Dermatol., 2014, 171(3), 588–94 CrossRef CAS PubMed.
- M. de Paula Correa and L. C. Pires, Doses of erythemal ultraviolet radiation observed in Brazil, Int. J. Dermatol., 2013, 52, 966–973 CrossRef PubMed.
- A. Sivayathorn, Melasma in Orientals, Clin. Drug Invest., 1995, 10, 34–40 CrossRef PubMed.
- C. Guinot, S. Cheffai, J. Latreille, M. A. Dhaoui, S. Youssef, K. Jaber, O. Nageotte and N. Doss, Aggravating factors for melasma: a prospective study in 197 Tunisian patients, J. Eur. Acad. Dermatol. Venereol., 2010, 24, 1060–1069 CAS.
- W. C. Chou, M. Takeo, P. Rabbani, H. Hu, W. Lee, Y. R. Chung, J. Carucci, P. Overbeek and M. Ito, Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling, Nat. Med., 2013, 19, 924–929 CrossRef CAS PubMed.
- R. Hernandez-Barrera, B. Torres-Alvarez, J. P. Castanedo-Cazares, C. Oros-Ovalle and B. Moncada, Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma, Clin. Exp. Dermatol., 2008, 33, 305–308 CrossRef CAS PubMed.
- M. B. Rozanowska, Light-induced damage to the retina: current understanding of the mechanisms and unresolved questions: a symposium-in-print, Photochem. Photobiol., 2012, 88, 1303–1308 CrossRef CAS PubMed.
- J. M. Artigas, A. Felipe, A. Navea, A. Fandino and C. Artigas, Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light, Invest. Ophthalmol. Visual. Sci., 2012, 53, 4076–4084 Search PubMed.
- A. P. Cullen, Photokeratitis and other phototoxic effects on the cornea and conjunctiva, Int. J. Toxicol., 2002, 21, 455–464 CrossRef CAS PubMed.
- C. Cejka, J. Luyckx and J. Cejkova, Central corneal thickness considered an index of corneal hydration of the UVB irradiated rabbit cornea as influenced by UVB absorber, Physiol. Res., 2012, 61, 299–306 CAS.
- J. Cejkova, C. Cejka and J. Luyckx, Trehalose treatment accelerates the healing of UVB-irradiated corneas. Comparative immunohistochemical studies on corneal cryostat sections and corneal impression cytology, Histol. Histopathol., 2012, 27, 1029–1040 CAS.
- A. T. Black, M. K. Gordon, D. E. Heck, M. A. Gallo, D. L. Laskin and J. D. Laskin, UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells, Biochem. Pharmacol., 2011, 81, 873–880 CrossRef CAS PubMed.
- J. J. Doutch, A. J. Quantock, N. C. Joyce and K. M. Meek, Ultraviolet light transmission through the human corneal stroma is reduced in the periphery, Biophys. J., 2012, 102, 1258–1264 CrossRef CAS PubMed.
- J. C. Sherwin, A. W. Hewitt, L. S. Kearns, L. R. Griffiths, D. A. Mackey and M. T. Coroneo, The association between pterygium and conjunctival ultraviolet autofluorescence: The Norfolk Island Eye Study, Acta Ophthalmol., 2013, 91(4), 363–370 CrossRef PubMed.
- R. Asokan, R. S. Venkatasubbu, L. Velumuri, V. Lingam and R. George, Prevalence and associated factors for pterygium and pinguecula in a South Indian population, Ophthalmic Physiol. Opt., 2012, 32, 39–44 CrossRef PubMed.
- J. Landers, T. Henderson and J. Craig, Prevalence of pterygium in indigenous Australians within central Australia: the Central Australian Ocular Health Study, Clin. Exp. Ophthalmol., 2011, 39, 604–606 Search PubMed.
- J. C. Sherwin, A. W. Hewitt, L. S. Kearns, L. R. Griffiths, D. A. Mackey and M. T. Coroneo, The association between pterygium and conjunctival ultraviolet autofluorescence: the Norfolk Island Eye Study, Acta Ophthalmol., 2013, 91, 363–370 CrossRef PubMed.
- D. M. Taylor, D. Bennett, M. Carter, D. Garewal and C. F. Finch, Acute injury and chronic disability resulting from surfboard riding, J. Sci. Med. Sport., 2004, 7, 429–437 CrossRef.
- H. R. Taylor, S. West, B. Munoz, F. S. Rosenthal, S. B. Bressler and N. M. Bressler, The long-term effects of visible light on the eye, Arch. Ophthalmol., 1992, 110, 99–104 CrossRef CAS.
- T. Golu, L. Mogoanta, C. T. Streba, D. N. Pirici, D. Malaescu, G. O. Mateescu and G. Mutiu, Pterygium: histological and immunohistochemical aspects, Rom. J. Morphol. Embryol., 2011, 52, 153–158 CAS.
- P. Artornsombudh, A. Sanpavat, U. Tinnungwattana, V. Tongkhomsai, L. Sansopha and W. Tulvatana, Prevalence and clinicopathologic findings of conjunctival epithelial neoplasia in pterygia, Ophthalmol., 2013, 120, 1337–1340 CrossRef PubMed.
- P. Oellers, C. L. Karp, A. Sheth, A. A. Kao, A. Abdelaziz, J. L. Matthews, S. R. Dubovy and A. Galor, Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium, Ophthalmol., 2013, 120, 445–450 CrossRef PubMed.
- B. Emmanuel, E. Ruder, S. W. Lin, C. Abnet, A. Hollenbeck and S. Mbulaiteye, Incidence of squamous-cell carcinoma of the conjunctiva and other eye cancers in the NIH-AARP Diet and Health Study, Ecancermedicalscience, 2012, 6, 254 CAS.
- N. Di Girolamo, Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia, Eye, 2012, 26, 202–211 CrossRef CAS PubMed.
- R. Bourne, G. A. Stevens, R. White, J. Smith, S. Flaxman, H. Price, J. B. Jonas, J. Keeffe, J. Leasher, K. Naidoo, K. Pesudovs, S. Resnikoff, H. Taylor and Vision Loss Expert Group, Causes of vision loss worldwide, 1990–2010: a systematic analysis, Lancet Global. Health, 2013, 1, e339–e349 CrossRef.
- H. R. Taylor, S. K. West, F. S. Rosenthal, B. Munoz, H. S. Newland, H. Abbey and E. A. Emmett, Effect of ultraviolet radiation on cataract formation, N. Engl. J. Med., 1988, 319, 1429–1433 CrossRef CAS PubMed.
- T. Okuno, T. Nakanishi-Ueda, T. Ueda, H. Yasuhara and R. Koide, Ultraviolet action spectrum for cell killing of primary porcine lens epithelial cells, J. Occup. Health, 2012, 54, 181–186 CrossRef CAS.
- V. C. Mody, Jr., M. Kakar, P. G. Soderberg and S. Lofgren, High lenticular tolerance to ultraviolet radiation-B by pigmented guinea-pig; application of a safety limit strategy for UVR-induced cataract, Acta Ophthalmol., 2012, 90, 226–230 CrossRef PubMed.
- S. Lofgren, R. Michael and P. G. Soderberg, Impact of iris pigment and pupil size in ultraviolet radiation cataract in rat, Acta Ophthalmol., 2012, 90, 44–48 CrossRef PubMed.
- Y. Ishikawa, K. Hashizume, S. Kishimoto, Y. Tezuka, H. Nishigori, N. Yamamoto, Y. Kondo, N. Maruyama, A. Ishigami and D. Kurosaka, Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice, Exp. Eye Res., 2012, 94, 85–89 CrossRef CAS PubMed.
- M. Kronschlager, K. Galichanin, J. Ekstrom, M. F. Lou and P. G. Soderberg, Protective effect of the thioltransferase gene on in vivo UVR-300 nm-induced cataract, Invest. Ophthalmol. Visual Sci., 2012, 53, 248–252 Search PubMed.
- J. Zhang, H. Yan, S. Lofgren, X. Tian and M. F. Lou, Ultraviolet radiation-induced cataract in mice: the effect of age and the potential biochemical mechanism, Invest. Ophthalmol. Visual Sci., 2012, 53, 7276–7285 CAS.
- P. Storey, B. Munoz, D. Friedman and S. West, Racial differences in lens opacity incidence and progression: the Salisbury Eye Evaluation (SEE) study, Invest. Ophthalmol. Visual Sci., 2013, 54, 3010–3018 Search PubMed.
- B. E. Klein, K. E. Lee, L. G. Danforth, T. M. Schaich, K. J. Cruickshanks and R. Klein, Selected sun-sensitizing medications and incident cataract, Arch. Ophthalmol., 2010, 128, 959–963 CrossRef PubMed.
- J. C. Yam and A. K. Kwok, Ultraviolet light and ocular diseases, Int. Ophthalmol., 2014, 34, 383–400 CrossRef PubMed.
- I. Franco-Lie, T. Iversen, T. E. Robsahm and M. Abdelnoor, Incidence trends of melanoma of the skin compared with other localisations, in the Norwegian population, 1956–2005, Ann. Oncol., 2011, 22, 1443–1450 CrossRef CAS PubMed.
- V. P. Papastefanou and V. M. Cohen, Uveal melanoma, J. Skin Cancer, 2011, 2011, 573974 Search PubMed.
- J. D. Mallet, S. P. Gendron, M. C. Drigeard Desgarnier and P. J. Rochette, Implication of ultraviolet light in the etiology of uveal melanoma: a review, Photochem. Photobiol., 2014, 90(1), 15–21 CrossRef CAS PubMed.
- B. P. Brooks, A. H. Thompson, R. J. Bishop, J. A. Clayton, C. C. Chan, E. T. Tsilou, W. M. Zein, D. Tamura, S. G. Khan, T. Ueda, J. Boyle, K. S. Oh, K. Imoto, H. Inui, S. Moriwaki, S. Emmert, N. T. Iliff, P. Bradford, J. J. Digiovanna and K. H. Kraemer, Ocular Manifestations of Xeroderma Pigmentosum: Long-Term Follow-up Highlights the Role of DNA Repair in Protection from Sun Damage, Ophthalmol., 2013, 120, 1324–1336 CrossRef PubMed.
- E. Prokofyeva and E. Zrenner, Epidemiology of major eye diseases leading to blindness in Europe: a literature review, Ophthalmic. Res., 2012, 47, 171–188 CrossRef PubMed.
- S. P. Gendron, N. Bastien, J. D. Mallet and P. J. Rochette, The 3895-bp mitochondrial DNA deletion in the human eye: a potential involvement in corneal ageing and macular degeneration, Mutagenesis, 2013, 28, 197–204 CrossRef CAS PubMed.
- A. E. Millen, R. Voland, S. A. Sondel, N. Parekh, R. L. Horst, R. B. Wallace, G. S. Hageman, R. Chappell, B. A. Blodi, M. L. Klein, K. M. Gehrs, G. E. Sarto and J. A. Mares, Vitamin D status and early age-related macular degeneration in postmenopausal women, Arch. Ophthalmol., 2011, 129, 481–489 CrossRef CAS PubMed.
- G. Y. Sui, G. C. Liu, G. Y. Liu, Y. Y. Gao, Y. Deng, W. Y. Wang, S. H. Tong and L. Wang, Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis, Br. J. Opthalmol., 2013, 97, 389–394 CrossRef PubMed.
- T. H. Margrain, M. Boulton, J. Marshall and D. H. Sliney, Do blue light filters confer protection against age-related macular degeneration?, Prog. Retinal Eye Res., 2004, 23, 523–531 CrossRef CAS PubMed.
- S. K. West, F. S. Rosenthal, N. M. Bressler, S. B. Bressler, B. Munoz, S. L. Fine and H. R. Taylor, Exposure to sunlight and other risk factors for age-related macular degeneration, Arch. Ophthalmol., 1989, 107, 875–879 CrossRef CAS.
- J. A. Guggenheim, K. Northstone, G. McMahon, A. R. Ness, K. Deere, C. Mattocks, B. S. Pourcain and C. Williams, Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study, Invest. Ophthalmol. Visual Sci., 2012, 53, 2856–2865 Search PubMed.
- Y. Guo, L. J. Liu, L. Xu, P. Tang, Y. Y. Lv, Y. Feng, M. Meng and J. B. Jonas, Myopic Shift and Outdoor Activity among Primary School Children: One-Year Follow-Up Study in Beijing, PLoS One, 2013, 8, e75260 CAS.
- Y. Guo, L. J. Liu, L. Xu, Y. Y. Lv, P. Tang, Y. Feng, M. Meng and J. B. Jonas, Outdoor activity and myopia among primary students in rural and urban regions of Beijing, Ophthalmol., 2013, 120, 277–283 CrossRef PubMed.
- J. C. Sherwin, A. W. Hewitt, M. T. Coroneo, L. S. Kearns, L. R. Griffiths and D. A. Mackey, The association between time spent outdoors and myopia using a novel biomarker of outdoor light exposure, Invest. Ophthalmol. Visual Sci., 2012, 53, 4363–4370 Search PubMed.
- M. Nebbioso, A. M. Plateroti, B. Pucci and N. Pescosolido, Role of the Dopaminergic System in the Development of Myopia in Children and Adolescents, J. Child Neurol., 2014 Search PubMed , pii: 0883073814538666.
- S. Yazar, A. W. Hewitt, L. J. Black, C. M. McKnight, J. A. Mountain, J. C. Sherwin, W. H. Oddy, M. T. Coroneo, R. M. Lucas and D. A. Mackey, Myopia is associated with lower vitamin D status in young adults, Invest. Ophthalmol. Visual Sci., 2014, 55, 4552–4559 Search PubMed.
- K. Hiramoto, Y. Yamate, H. Kobayashi and M. Ishii, Long-term ultraviolet A irradiation of the eye induces photoaging of the skin in mice, Arch. Dermatol. Res., 2012, 304, 39–45 CrossRef CAS PubMed.
- K. Hiramoto, Y. Yamate, H. Kobayashi, M. Ishii, E. F. Sato and M. Inoue, Ultraviolet B irradiation of the mouse eye induces pigmentation of the skin more strongly than does stress loading, by increasing the levels of prohormone convertase 2 and alpha-melanocyte-stimulating hormone, Clin. Exp. Dermatol., 2013, 38, 71–76 CrossRef CAS PubMed.
- A. Tewari, M. M. Grage, G. I. Harrison, R. Sarkany and A. R. Young, UVA1 is skin deep: molecular and clinical implications, Photochem. Photobiol. Sci., 2013, 12, 95–103 CAS.
- N. K. Gibbs and M. Norval, Photoimmunosuppression: a brief overview, Photodermatol. Photoimmunol. Photomed., 2013, 29, 57–64 CrossRef CAS PubMed.
- D. L. Damian, Y. J. Matthews, T. A. Phan and G. M. Halliday, An action spectrum for ultraviolet radiation-induced immunosuppression in humans, Br. J. Dermatol., 2011, 164, 657–659 CAS.
- Y. J. Matthews, G. M. Halliday, T. A. Phan and D. L. Damian, Wavelength dependency for UVA-induced suppression of recall immunity in humans, J. Dermatol. Sci., 2010, 59, 192–197 CrossRef CAS PubMed.
- T. S. Poon, R. S. Barnetson and G. M. Halliday, Sunlight-induced immunosuppression in humans is initially because of UVB, then UVA, followed by interactive effects, J. Invest. Dermatol., 2005, 125, 840–846 CrossRef CAS PubMed.
- Y. Ibuki, M. Allanson, K. M. Dixon and V. E. Reeve, Radiation sources providing increased UVA/UVB ratios attenuate the apoptotic effects of the UVB waveband UVA-dose-dependently in hairless mouse skin, J. Invest. Dermatol., 2007, 127, 2236–2244 CrossRef CAS PubMed.
- V. E. Reeve, D. Domanski and M. Slater, Radiation sources providing increased UVA/UVB ratios induce photoprotection dependent on the UVA dose in hairless mice, Photochem. Photobiol., 2006, 82, 406–411 CrossRef CAS PubMed.
- T. Schwarz and A. Schwarz, Molecular mechanisms of ultraviolet radiation-induced immunosuppression, Eur. J. Cell Biol., 2011, 90, 560–564 CrossRef CAS PubMed.
- S. E. Ullrich and S. N. Byrne, The immunologic revolution: photoimmunology, J. Invest. Dermatol., 2012, 132, 896–905 CrossRef CAS PubMed.
- R. Glaser, F. Navid, W. Schuller, C. Jantschitsch, J. Harder, J. M. Schroder, A. Schwarz and T. Schwarz, UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo, Invest. Ophthalmol. Visual Sci., 2009, 123, 1117–1123 Search PubMed.
- M. K. Crispin, J. Fuentes-Duculan, N. Gulati, L. M. Johnson-Huang, T. Lentini, M. Sullivan-Whalen, P. Gilleaudeau, I. Cueto, M. Suarez-Farinas, M. A. Lowes and J. G. Krueger, Gene profiling of narrowband UVB-induced skin injury defines cellular and molecular innate immune responses, J. Invest. Dermatol., 2013, 133, 692–701 CrossRef PubMed.
- F. Navid, M. Boniotto, C. Walker, K. Ahrens, E. Proksch, T. Sparwasser, W. Muller, T. Schwarz and A. Schwarz, Induction of regulatory T cells by a murine beta-defensin, J. Immunol., 2012, 188, 735–743 CrossRef CAS PubMed.
- R. L. Ng, N. M. Scott, D. H. Strickland, S. Gorman, M. A. Grimbaldeston, M. Norval, J. Waithman and P. H. Hart, Altered immunityand dendritic cell activity in the periphery of mice after long-term engraftment with bone marrow from ultraviolet-irradiated mice, J. Immunol., 2013, 190, 5471–5484 CrossRef CAS PubMed.
- H. M. McGee, R. C. Malley, H. K. Muller and G. M. Woods, Neonatal exposure to UVR alters skin immune system development, and suppresses immunity in adulthood, Immunol. Cell. Biol., 2011, 89, 767–776 CrossRef CAS PubMed.
- P. H. Hart and S. Gorman, Exposure to UV Wavelengths in Sunlight Suppresses Immunity. To What Extent is UV-induced Vitamin D3 the Mediator Responsible?, Clin. Biochem. Rev., 2013, 34, 3–13 Search PubMed.
- A. Schwarz, F. Navid, T. Sparwasser, B. E. Clausen and T. Schwarz, 1,25-dihydroxyvitamin D exerts similar immunosuppressive effects as UVR but is dispensable for local UVR-induced immunosuppression, J. Invest. Dermatol., 2012, 132, 2762–2769 CrossRef CAS PubMed.
- M. Baarnhielm, A. K. Hedstrom, I. Kockum, E. Sundqvist, S. A. Gustafsson, J. Hillert, T. Olsson and L. Alfredsson, Sunlight is associated with decreased multiple sclerosis risk: no interaction with human leukocyte antigen-DRB1*15, Eur. J. Neurol., 2012, 19, 955–962 CrossRef CAS PubMed.
- H. van der Rhee, J. W. Coebergh and E. de Vries, Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies, Eur. J. Cancer. Prev., 2013, 49, 1422–1436 CrossRef CAS PubMed.
- S. V. Milliken, H. Wassall, B. J. Lewis, J. Logie, R. N. Barker, H. Macdonald, M. A. Vickers and A. D. Ormerod, Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function, J. Allergy Clin. Immunol., 2012, 129, 1554–1561 CrossRef CAS PubMed.
- V. K. Sharma, K. Sahni and A. R. Wadhwani, Photodermatoses in pigmented skin, Photochem. Photobiol. Sci., 2013, 12, 65–77 CAS.
- A. R. Wadhwani, V. K. Sharma, M. Ramam and B. K. Khaitan, A clinical study of the spectrum of photodermatoses in dark-skinned populations, Clin. Exp. Dermatol., 2013, 38, 823–829 CrossRef CAS PubMed.
- P. Wolf, A. Gruber-Wackernagel, I. Bambach, U. Schmidbauer, G. Mayer, M. Absenger, E. Frohlich and S. N. Byrne, Photohardening of polymorphic light eruption patients decreases baseline epidermal Langerhans cell density while increasing mast cell numbers in the papillary dermis, Exp. Dermatol., 2014, 23, 428–430 CrossRef CAS PubMed.
- P. Wolf, A. Gruber-Wackernagel, B. Rinner, A. Griesbacher, K. Eberhard, A. Groselj-Strele, G. Mayer, R. E. Stauber and S. N. Byrne, Phototherapeutic hardening modulates systemic cytokine levels in patients with polymorphic light eruption, Photochem. Photobiol. Sci., 2013, 12, 166–173 CAS.
- T. Gambichler, S. Terras, P. Kampilafkos, A. Kreuter and M. Skrygan, T regulatory cells and related immunoregulatory factors in polymorphic light eruption following ultraviolet A1 challenge, Br. J. Dermatol., 2013, 169, 1288–1294 CrossRef CAS PubMed.
- A. Gruber-Wackernagel, S. N. Byrne and P. Wolf, Polymorphous light eruption: clinic aspects and pathogenesis, Dermatol. Clin., 2014, 32, 315–334 CrossRef CAS PubMed.
- G. Krstic, Asthma prevalence associated with geographical latitude and regional insolation in the United States of America and Australia, PLoS One, 2011, 6, e18492 CAS.
- A. M. Hughes, R. M. Lucas, A. L. Ponsonby, C. Chapman, A. Coulthard, K. Dear, T. Dwyer, T. J. Kilpatrick, A. J. McMichael, M. P. Pender, B. V. Taylor, P. Valery, I. A. van der Mei and D. Williams, The role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: an Australian multicenter study, Pediatr. Allergy Immunol., 2011, 22, 327–333 CrossRef PubMed.
- V. Oktaria, S. C. Dharmage, J. A. Burgess, J. A. Simpson, S. Morrison, G. G. Giles, M. J. Abramson, E. H. Walters and M. C. Matheson, Association between latitude and allergic diseases: a longitudinal study from childhood to middle-age, Ann. Allergy Asthma Immunol., 2013, 110, 80–85.e1 CrossRef PubMed.
- M. Norval, J. Garssen, H. Van Loveren and A. A. el-Ghorr, UV-induced changes in the immune response to microbial infections in human subjects and animal models, J. Epidemiol, 1999, 9, S84–S92 CrossRef CAS.
- C. Ludema, S. R. Cole, C. Poole, J. S. Smith, V. J. Schoenbach and K. R. Wilhelmus, Association Between Unprotected Ultraviolet Radiation Exposure and Recurrence of Ocular Herpes Simplex Virus, Am. J. Epidemiol., 2013, 179(2), 208–215 CrossRef PubMed.
- M. Norval and G. M. Woods, UV-induced immunosuppression and the efficacy of vaccination, Photochem. Photobiol. Sci., 2011, 10, 1267–1274 CAS.
- P. H. Hart, S. Gorman and J. J. Finlay-Jones, Modulation of the immune system by UV radiation: more than just the effects of vitamin D?, Nat. Rev. Immunol., 2011, 11, 584–596 CrossRef CAS PubMed.
- V. Nerich, P. Jantchou, M. C. Boutron-Ruault, E. Monnet, A. Weill, V. Vanbockstael, G. R. Auleley, C. Balaire, P. Dubost, S. Rican, H. Allemand and F. Carbonnel, Low exposure to sunlight is a risk factor for Crohn's disease, Aliment. Pharmacol. Ther., 2011, 33, 940–945 CrossRef CAS PubMed.
- A. L. Ponsonby, R. M. Lucas and I. A. van der Mei, UVR, Vitamin D and three autoimmune diseases - Multiple Sclerosis, Type 1 Diabetes, Rheumatoid Arthritis, Photochem. Photobiol., 2005, 81, 1267–1275 CrossRef CAS PubMed.
- P. Gatenby, R. Lucas and A. Swaminathan, Vitamin D deficiency and risk for rheumatic diseases: an update, Curr. Opin. Rheumatol., 2013, 25, 184–191 CrossRef CAS PubMed.
- S. Hewer, R. Lucas, I. van der Mei and B. V. Taylor, Vitamin D and multiple sclerosis, J. Clin. Neurosci., 2013, 20, 634–641 CrossRef CAS PubMed.
- M. A. Hernan, M. J. Olek and A. Ascherio, Geographic variation of MS incidence in two prospective studies of US women, Neurology, 1999, 53, 1711–1718 CrossRef CAS.
- A. Ascherio and K. L. Munger, Environmental risk factors for multiple sclerosis. Part I: the role of infection, Ann. Neurol., 2007, 61, 288–299 CrossRef PubMed.
- C. J. Willer, D. A. Dyment, A. D. Sadovnick, P. M. Rothwell, T. J. Murray and G. C. Ebers, Timing of birth and risk of multiple sclerosis: population based study, Br. Med. J., 2005, 330, 120 CrossRef PubMed.
- J. Staples, A. L. Ponsonby and L. Lim, Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: longitudinal analysis, Br. Med. J., 2010, 340, c1640 Search PubMed.
- B. R. Becklund, K. S. Severson, S. V. Vang and H. F. Deluca, UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6418–6423 CrossRef CAS PubMed.
- R. M. Lucas, A. L. Ponsonby, K. Dear, P. C. Valery, M. P. Pender, B. V. Taylor, T. J. Kilpatrick, T. Dwyer, A. Coulthard, C. Chapman, I. van der Mei, D. Williams and A. J. McMichael, Sun exposure and vitamin D are independent risk factors for CNS demyelination, Neurology, 2011, 76, 540–548 CrossRef CAS PubMed.
- R. Zivadinov, C. N. Treu, B. Weinstock-Guttman, C. Turner, N. Bergsland, K. O'Connor, M. G. Dwyer, E. Carl, D. P. Ramasamy, J. Qu and M. Ramanathan, Interdependence and contributions of sun exposure and vitamin D to MRI measures in multiple sclerosis, J. Neurol. Neurosurg. Psychiatry, 2013 Search PubMed.
- M. T. van Leeuwen, A. C. Webster, M. R. McCredie, J. H. Stewart, S. P. McDonald, J. Amin, J. M. Kaldor, J. R. Chapman, C. M. Vajdic and A. E. Grulich, Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study, Br. Med. J., 2010, 340, c570 CrossRef PubMed.
- M. R. Zaidi, E. C. De Fabo, F. P. Noonan and G. Merlino, Shedding light on melanocyte pathobiology in vivo, Cancer Res., 2012, 72, 1591–1595 CrossRef CAS PubMed.
- H. Y. Handoko, M. P. Rodero, G. M. Boyle, B. Ferguson, C. Engwerda, G. Hill, H. K. Muller, K. Khosrotehrani and G. J. Walker, UVB-induced melanocyte proliferation in neonatal mice driven by CCR2-independent recruitment of Ly6c(low)MHCII(hi) macrophages, J. Invest. Dermatol., 2013, 133, 1803–1812 CAS.
- M. R. Zaidi, S. Davis, F. P. Noonan, C. Graff-Cherry, T. S. Hawley, R. L. Walker, L. Feigenbaum, E. Fuchs, L. Lyakh, H. A. Young, T. J. Hornyak, H. Arnheiter, G. Trinchieri, P. S. Meltzer, E. C. De Fabo and G. Merlino, Interferon-gamma links ultraviolet radiation to melanomagenesis in mice, Nature, 2011, 469, 548–553 CrossRef CAS PubMed.
- T. Bald, T. Quast, J. Landsberg, M. Rogava, N. Glodde, D. Lopez-Ramos, J. Kohlmeyer, S. Riesenberg, D. van den Boorn-Konijnenberg, C. Homig-Holzel, R. Reuten, B. Schadow, H. Weighardt, D. Wenzel, I. Helfrich, D. Schadendorf, W. Bloch, M. E. Bianchi, C. Lugassy, R. L. Barnhill, M. Koch, B. K. Fleischmann, I. Forster, W. Kastenmuller, W. Kolanus, M. Holzel, E. Gaffal and T. Tuting, Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma, Nature, 2014, 507, 109–113 CrossRef CAS PubMed.
- G. Erdag, J. T. Schaefer, M. E. Smolkin, D. H. Deacon, S. M. Shea, L. T. Dengel, J. W. Patterson and C. L. Slingluff, Jr., Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma, Cancer Res., 2012, 72, 1070–1080 CrossRef CAS PubMed.
- F. J. Moloney, H. Comber, P. O'Lorcain, P. O'Kelly, P. J. Conlon and G. M. Murphy, A population-based study of skin cancer incidence and prevalence in renal transplant recipients, Br. J. Dermatol., 2006, 154, 498–504 CrossRef CAS PubMed.
- M. Norval and G. M. Halliday, The consequences of UV-induced immunosuppression for human health, Photochem. Photobiol., 2011, 87, 965–977 CrossRef CAS PubMed.
- S. M. Thanos, G. M. Halliday and D. L. Damian, Nicotinamide reduces photodynamic therapy-induced immunosuppression in humans, Br. J. Dermatol., 2012, 167, 631–636 CrossRef CAS PubMed.
- D. Surjana, G. M. Halliday, A. J. Martin, F. J. Moloney and D. L. Damian, Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials, J. Invest. Dermatol., 2012, 132, 1497–1500 CrossRef CAS PubMed.
- O. N. Galkin and I. P. Terenetskaya, ‘Vitamin D’ biodosimeter: basic characteristics and potential applications, J. Photochem. Photobiol., B., 1999, 53, 12–19 CrossRef CAS.
- M. Norval, L. O. Bjorn and F. R. de Gruijl, Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct?, Photochem. Photobiol. Sci., 2010, 9, 11–17 CAS.
- A. R. Webb, B. R. DeCosta and M. F. Holick, Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation, J. Clin. Endocrinol. Metab., 1989, 68, 882–887 CrossRef CAS PubMed.
- A. R. Webb, R. Kift, J. L. Berry and L. E. Rhodes, The vitamin D debate: translating controlled experiments into reality for human sun exposure times, Photochem. Photobiol., 2011, 87, 741–745 CrossRef CAS PubMed.
- C. T. Sempos, H. Vesper, K. Phinney and L. Thienpont, P. Coates and Vitamin D Standardization Program, Vitamin D status as an international issue: National surveys and the problem of standardization, Scand. J. Clin. Lab. Invest., 2012, 72, 32–40 CAS.
- C. J. Rosen, S. A. Abrams, J. F. Aloia, P. M. Brannon, S. K. Clinton, R. A. Durazo-Arvizu, J. C. Gallagher, R. L. Gallo, G. Jones, C. S. Kovacs, J. E. Manson, S. T. Mayne, A. C. Ross, S. A. Shapses and C. L. Taylor, IOM committee members respond to Endocrine Society vitamin D guideline, J. Clin. Endocrinol. Metab., 2012, 97, 1146–1152 CrossRef CAS PubMed.
- B. W. Hollis, Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D, J. Nutr., 2005, 135, 317–322 CAS.
- Institute of Medicine, Dietary Reference Intakes for Calcium and Vitamin D, Institute of Medicine of the National Academies Report No., 2010. http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx.
- S. H. S. Pearce and T. D. Cheetham, Diagnosis and management of vitamin D deficiency, Br. Med. J., 2010, 340 Search PubMed.
- M. F. Holick, N. C. Binkley, H. A. Bischoff-Ferrari, C. M. Gordon, D. A. Hanley, R. P. Heaney, M. H. Murad and C. M. Weaver, Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline, J. Clin. Endocrinol. Metab., 2011, 96, 1911–1930 CrossRef CAS PubMed.
- C. Palacios and L. Gonzalez, Is vitamin D deficiency a major global public health problem?, J. Steriod Biochem. Mol. Biol., 2014, 144PA, 138–145 CrossRef PubMed.
- J. Hilger, A. Friedel, R. Herr, T. Rausch, F. Roos, D. A. Wahl, D. D. Pierroz, P. Weber and K. Hoffmann, A systematic review of vitamin D status in populations worldwide, Br. J. Nutr., 2014, 111, 23–45 CrossRef CAS PubMed.
- D. A. Wahl, C. Cooper, P. R. Ebeling, M. Eggersdorfer, J. Hilger, K. Hoffmann, R. Josse, J. A. Kanis, A. Mithal, D. D. Pierroz, J. Stenmark, E. Stocklin and B. Dawson-Hughes, A global representation of vitamin D status in healthy populations, Arch. Osteoporos., 2012, 7, 155–172 CrossRef CAS PubMed.
- R. M. Lucas, A. L. Ponsonby, K. Dear, P. C. Valery, B. Taylor, I. van der Mei, A. J. McMichael, M. P. Pender, C. Chapman, A. Coulthard, T. J. Kilpatrick, J. Stankovich, D. Williams and T. Dwyer, Vitamin D status: multifactorial contribution of environment, genes and other factors in healthy Australian adults across a latitude gradient, J. Steriod Biochem. Mol. Biol., 2013, 136, 300–308 CrossRef CAS PubMed.
- R. M. Lucas, P. Valery, I. van der Mei, T. Dwyer, M. P. Pender, B. Taylor, A. L. Ponsonby and G. Ausimmune Investigator, Sun exposure over a lifetime in Australian adults from latitudinally diverse regions, Photochem. Photobiol., 2013, 89, 737–744 CrossRef CAS PubMed.
- A. Bjork, A. Andersson, G. Johansson, K. Bjorkegren, A. Bardel and P. Kristiansson, Evaluation of sun holiday, diet habits, origin and other factors as determinants of vitamin D status in Swedish primary health care patients: a cross-sectional study with regression analysis of ethnic Swedish and immigrant women, BMC Fam. Pract., 2013, 14, 129 CrossRef PubMed.
- C. E. Powe, M. K. Evans, J. Wenger, A. B. Zonderman, A. H. Berg, M. Nalls, H. Tamez, D. Zhang, I. Bhan, S. A. Karumanchi, N. R. Powe and R. Thadhani, Vitamin D-binding protein and vitamin D status of black Americans and white Americans, N. Engl. J. Med., 2013, 369, 1991–2000 CrossRef CAS PubMed.
- A. M. Renzaho, J. A. Halliday and C. Nowson, Vitamin D, obesity, and obesity-related chronic disease among ethnic minorities: a systematic review, Nutrition, 2011, 27, 868–879 CrossRef CAS PubMed.
- R. Kift, J. L. Berry, A. Vail, M. T. Durkin, L. E. Rhodes and A. R. Webb, Lifestyle factors including less cutaneous sun exposure contribute to starkly lower vitamin D status in UK South Asians compared to the white Caucasian population, Br. J. Dermatol., 2013, 169(6), 1272–1278 CrossRef CAS PubMed.
- I. Bhan, C. E. Powe, A. H. Berg, E. Ankers, J. B. Wenger, S. A. Karumanchi and R. I. Thadhani, Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients, Kidney International, 2012, 82, 84–89 CrossRef CAS PubMed.
- C. E. Powe, C. Ricciardi, A. H. Berg, D. Erdenesanaa, G. Collerone, E. Ankers, J. Wenger, S. A. Karumanchi, R. Thadhani and I. Bhan, Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship, J. Bone Miner. Res., 2011, 26, 1609–1616 CrossRef CAS PubMed.
- F. Libon, E. Cavalier and A. F. Nikkels, Skin color is relevant to vitamin D synthesis, Dermatol., 2013, 227, 250–254 CrossRef CAS PubMed.
- P. Springbett, S. Buglass and A. R. Young, Photoprotection and vitamin D status, J. Photochem. Photobiol., B, 2010, 101, 160–168 CrossRef CAS PubMed.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation, J. Invest. Dermatol., 2010, 130, 546–553 CrossRef CAS PubMed.
- L. O. Bjorn, Vitamin D synthesis may be independent of skin pigmentation only with UV of short wavelength, J. Invest. Dermatol., 2010, 130, 2848–2850 CrossRef PubMed.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, Vitamin D production depends on ultraviolet-B dose but not on dose rate: a randomized controlled trial, Exp. Dermatol., 2011, 20, 14–18 CrossRef CAS PubMed.
- R. McKenzie, R. Scragg, B. Liley, P. Johnston, J. Wishart, A. Stewart and R. Prematunga, Serum 25-hydroxyvitamin-D responses to multiple UV exposures from solaria: inferences for exposure to sunlight, Photochem. Photobiol. Sci., 2012, 11, 1174–1185 CAS.
- L. E. Rhodes, A. R. Webb, H. I. Fraser, R. Kift, M. T. Durkin, D. Allan, S. J. O'Brien, A. Vail and J. L. Berry, Recommended summer sunlight exposure levels can produce sufficient (≥20 ng ml−1) but not the proposed optimal (≥32 ng ml−1) 25(OH)D levels at UK latitudes°, J. Invest. Dermatol., 2010, 130, 1411–1418 CrossRef CAS PubMed.
- A. C. Porojnicu, O. S. Bruland, L. Aksnes, W. B. Grant and J. Moan, Sun beds and cod liver oil as vitamin D sources, J. Photochem. Photobiol., B, 2008, 91, 125–131 CrossRef CAS PubMed.
- J. Moan, Z. Lagunova, E. Cicarma, L. Aksnes, A. Dahlback, W. B. Grant and A. C. Porojnicu, Sunbeds as vitamin D sources, Photochem. Photobiol., 2009, 85, 1474–1479 CrossRef CAS PubMed.
- M. D. Farrar, A. R. Webb, R. Kift, M. T. Durkin, D. Allan, A. Herbert, J. L. Berry and L. E. Rhodes, Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: implications for targeted guidance on sun exposure, Am. J. Clin. Nutr., 2013, 97, 1210–1216 CrossRef CAS PubMed.
- M. G. Kimlin, R. M. Lucas, S. L. Harrison, I. van der Mei, B. K. Armstrong, D. C. Whiteman, A. Kricker, M. Nowak, A. M. Brodie and J. Sun, The contributions of solar ultraviolet radiation exposure and other determinants to serum 25-hydroxyvitamin D concentrations in Australian adults: The AusD Study, Am. J. Epidemiol., 2014, 179(7), 864–874 CrossRef PubMed.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, Interdependence between body surface area and ultraviolet B dose in vitamin D production: a randomized controlled trial, Br. J. Dermatol., 2011, 164, 163–169 CrossRef CAS PubMed.
- B. Petersen, H. C. Wulf, M. Triguero-Mas, P. A. Philipsen, E. Thieden, P. Olsen, J. Heydenreich, P. Dadvand, X. Basagana, T. S. Liljendahl, G. I. Harrison, D. Segerback, A. W. Schmalwieser, A. R. Young and M. J. Nieuwenhuijsen, Sun and ski holidays improve vitamin D status, but are associated with high levels of DNA damage, J. Invest. Dermatol., 2014, 134(11), 2806–2813 CrossRef CAS PubMed.
- R. McKenzie, B. Liley, P. Johnston, R. Scragg, A. Stewart, A. I. Reeder and M. W. Allen, Small doses from artificial UV sources elucidate the photo-production of vitamin D, Photochem. Photobiol. Sci., 2013, 12, 1726–1737 CAS.
- A. R. Webb, R. Kift, M. T. Durkin, S. J. O'Brien, A. Vail, J. L. Berry and L. E. Rhodes, The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population, Br. J. Dermatol., 2010, 163, 1050–1055 CrossRef CAS PubMed.
- P. Autier, M. Boniol, C. Pizot and P. Mullie, Vitamin D status and ill health: a systematic review, Lancet Diab. Endocrinol., 2014, 2, 76–89 CrossRef CAS.
- E. Theodoratou, I. Tzoulaki, L. Zgaga and J. P. Ioannidis, Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials, Br. Med. J., 2014, 348, g2035 CrossRef PubMed.
- J. H. White, Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection, Arch. Biochem. Biophys., 2012, 523, 58–63 CrossRef CAS PubMed.
- M. Hewison, Vitamin D and immune function: An overview, Proc. Nutrit. Soc., 2012, 71, 50–61 CrossRef CAS PubMed.
- S. Christakos, M. Hewison, D. G. Gardner, C. L. Wagner, I. N. Sergeev, E. Rutten, A. G. Pittas, R. Boland, L. Ferrucci and D. D. Bikle, Vitamin D: beyond bone, Ann. N. Y. Acad. Sci., 2013, 1287, 45–58 CrossRef CAS PubMed.
- A. P. Ralph, R. M. Lucas and M. Norval, Vitamin D and solar ultraviolet radiation in the risk and treatment of tuberculosis, Lancet Infect. Dis., 2013, 13, 77–88 CrossRef CAS.
- C. Wejse, V. F. Gomes, P. Rabna, P. Gustafson, P. Aaby, I. M. Lisse, P. L. Andersen, H. Glerup and M. Sodemann, Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial, Am. J. Respir Crit. Care Med., 2009, 179, 843–850 CrossRef CAS PubMed.
- A. P. Ralph, G. Waramori, G. J. Pontororing, E. Kenangalem, A. Wiguna, E. Tjitra, Sandjaja, D. B. Lolong, T. W. Yeo, M. D. Chatfield, R. K. Soemanto, I. Bastian, R. Lumb, G. P. Maguire, J. Eisman, R. N. Price, P. S. Morris, P. M. Kelly and N. M. Anstey, L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial, PLoS One, 2013, 8, e70032 CAS.
- A. R. Martineau, P. M. Timms, G. H. Bothamley, Y. Hanifa, K. Islam, A. P. Claxton, G. E. Packe, J. C. Moore-Gillon, M. Darmalingam, R. N. Davidson, H. J. Milburn, L. V. Baker, R. D. Barker, N. J. Woodward, T. R. Venton, K. E. Barnes, C. J. Mullett, A. K. Coussens, C. M. Rutterford, C. A. Mein, G. R. Davies, R. J. Wilkinson, V. Nikolayevskyy, F. A. Drobniewski, S. M. Eldridge and C. J. Griffiths, High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial, Lancet, 2011, 377, 242–250 CrossRef CAS.
- D. Ganmaa, E. Giovannucci, B. R. Bloom, W. Fawzi, W. Burr, D. Batbaatar, N. Sumberzul, M. F. Holick and W. C. Willett, Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial, Am. J. Clin. Nutr., 2012, 96, 391–396 CrossRef CAS PubMed.
- K. Bloom-Feshbach, W. J. Alonso, V. Charu, J. Tamerius, L. Simonsen, M. A. Miller and C. Viboud, Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review, PLoS One, 2013, 8, e54445 CAS.
- M. Science, J. L. Maguire, M. L. Russell, M. Smieja, S. D. Walter and M. Loeb, Low serum 25-hydroxyvitamin D level and risk of upper respiratory tract infection in children and adolescents, Clin. Infect. Dis., 2013, 57, 392–397 CrossRef CAS PubMed.
- A. A. Ginde, J. M. Mansbach and C. A. Camargo, Jr., Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey, Arch. Intern. Med., 2009, 169, 384–390 CrossRef CAS PubMed.
- D. J. Berry, K. Hesketh, C. Power and E. Hypponen, Vitamin D status has a linear association with seasonal infections and lung function in British adults, Br. J. Nutr., 2011, 106, 1433–1440 CrossRef CAS PubMed.
- V. Hirani, Associations between vitamin D and self-reported respiratory disease in older people from a nationally representative population survey, J. Am. Geriat. Soc., 2013, 61, 969–973 CrossRef PubMed.
- K. J. Bryson, A. A. Nash and M. Norval, Does vitamin D protect against respiratory viral infections?, Epidemiol. Infect., 2014, 142, 1789–1801 CrossRef CAS PubMed.
- M. Urashima, T. Segawa, M. Okazaki, M. Kurihara, Y. Wada and H. Ida, Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren, Am. J. Clin. Nutr., 2010, 91, 1255–1260 CrossRef CAS PubMed.
- C. A. Camargo, Jr., D. Ganmaa, A. L. Frazier, F. F. Kirchberg, J. J. Stuart, K. Kleinman, N. Sumberzul and J. W. Rich-Edwards, Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia, Pediatrics, 2012, 130, e561–e567 CrossRef PubMed.
- J. F. Aloia and M. Li-Ng, Re: epidemic influenza and vitamin D, Epidemiol. Infect., 2007, 135, 1095–1096 Search PubMed.
- I. Laaksi, J. P. Ruohola, V. Mattila, A. Auvinen, T. Ylikomi and H. Pihlajamaki, Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men, J. Infect. Dis., 2010, 202, 809–814 CrossRef CAS PubMed.
- B. Tran, B. K. Armstrong, P. R. Ebeling, D. R. English, M. G. Kimlin, J. C. van der Pols, A. Venn, V. Gebski, D. C. Whiteman, P. M. Webb and R. E. Neale, Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial, Am. J. Clin. Nutr., 2014, 99, 156–161 CrossRef CAS PubMed.
- M. Li-Ng, J. F. Aloia, S. Pollack, B. A. Cunha, M. Mikhail, J. Yeh and N. Berbari, A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections, Epidemiol. Infect., 2009, 137, 1396–1404 CrossRef CAS PubMed.
- J. R. Rees, K. Hendricks, E. L. Barry, J. L. Peacock, L. A. Mott, R. S. Sandler, R. S. Bresalier, M. Goodman, R. M. Bostick and J. A. Baron, Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial, Clin. Infect. Dis., 2013, 57, 1384–1392 CrossRef CAS PubMed.
- D. R. Murdoch, S. Slow, S. T. Chambers, L. C. Jennings, A. W. Stewart, P. C. Priest, C. M. Florkowski, J. H. Livesey, C. A. Camargo and R. Scragg, Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial, J. Am. Med. Assoc., 2012, 308, 1333–1339 CrossRef CAS PubMed.
- A. Ascherio, K. L. Munger, R. White, K. Kochert, K. C. Simon, C. H. Polman, M. S. Freedman, H. P. Hartung, D. H. Miller, X. Montalban, G. Edan, F. Barkhof, D. Pleimes, E. W. Radu, R. Sandbrink, L. Kappos and C. Pohl, Vitamin D as an Early Predictor of Multiple Sclerosis Activity and Progression, J. Am. Med. Assoc. Neurol., 2014, 71(3), 306–314 Search PubMed.
- D. Reid, B. J. Toole, S. Knox, D. Talwar, J. Harten, D. S. O'Reilly, S. Blackwell, J. Kinsella, D. C. McMillan and A. M. Wallace, The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty, Am. J. Clin. Nutr., 2011, 93, 1006–1011 CrossRef CAS PubMed.
- R. Lucas and B. Taylor, Challenges in exposure and outcome definition in neuroepidemiology: the case of vitamin D and multiple sclerosis, Australas. Epidemiol., 2013, 20, 4–8 Search PubMed.
- G. Bjelakovic, L. L. Gluud, D. Nikolova, K. Whitfield, G. Krstic, J. Wetterslev and C. Gluud, Vitamin D supplementation for prevention of cancer in adults, Cochrane Database Syst. Rev., 2014, 6, CD007469 Search PubMed.
- C. Gordon-Thomson, R. Gupta, W. Tongkao-on, A. Ryan, G. M. Halliday and R. S. Mason, 1alpha,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin, Photochem. Photobiol. Sci., 2012, 11, 1837–1847 CAS.
- D. L. Damian, Y. J. Kim, K. M. Dixon, G. M. Halliday, A. Javeri and R. S. Mason, Topical calcitriol protects from UV-induced genetic damage but suppresses cutaneous immunity in humans, Exp. Dermatol., 2010, 19, e23–e30 CrossRef PubMed.
- J. Y. Tang, N. Parimi, A. Wu, W. J. Boscardin, J. M. Shikany, M. M. Chren, S. R. Cummings, E. H. Epstein, Jr., D. C. Bauer and G. Osteoporotic Fractures in Men Study, Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men, Cancer Causes Control, 2010, 21, 387–391 CrossRef PubMed.
- J. C. van der Pols, A. Russell, U. Bauer, R. E. Neale, M. G. Kimlin and A. C. Green, Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community, J. Invest. Dermatol., 2013, 133, 637–641 CrossRef CAS PubMed.
- S. Afzal, B. G. Nordestgaard and S. E. Bojesen, Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study, J. Invest. Dermatol., 2013, 133, 629–636 CrossRef CAS PubMed.
- J. M. Major, C. Kiruthu, S. J. Weinstein, R. L. Horst, K. Snyder, J. Virtamo and D. Albanes, Pre-diagnostic circulating vitamin D and risk of melanoma in men, PLoS One, 2012, 7, e35112 CAS.
- J. Y. Tang, T. Fu, E. Leblanc, J. E. Manson, D. Feldman, E. Linos, M. Z. Vitolins, N. C. Zeitouni, J. Larson and M. L. Stefanick, Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women's health initiative randomized controlled trial, J. Clin. Oncol., 2011, 29, 3078–3084 CrossRef CAS PubMed.
- M. Peterlik, Vitamin D insufficiency and chronic diseases: hype and reality, Food Funct., 2012, 3, 784–794 CAS.
- T. D. Shanafelt, M. T. Drake, M. J. Maurer, C. Allmer, K. G. Rabe, S. L. Slager, G. J. Weiner, T. G. Call, B. K. Link, C. S. Zent, N. E. Kay, C. A. Hanson, T. E. Witzig and J. R. Cerhan, Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia, Blood, 2011, 117, 1492–1498 CrossRef CAS PubMed.
- K. Zhu, A. J. Whitehouse, P. Hart, M. Kusel, J. Mountain, S. Lye, C. Pennell and J. P. Walsh, Maternal Vitamin D Status During Pregnancy and Bone Mass in Offspring at 20 Years of Age: A Prospective Cohort Study, J. Bone Miner. Res., 2013, 29(5), 1088–1095 CrossRef PubMed.
- A. J. Whitehouse, B. J. Holt, M. Serralha, P. G. Holt, M. M. Kusel and P. H. Hart, Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development, Pediatrics, 2012, 129, 485–493 CrossRef PubMed.
- K. L. Allen, S. M. Byrne, M. M. Kusel, P. H. Hart and A. J. Whitehouse, Maternal vitamin D levels during pregnancy and offspring eating disorder risk in adolescence, Int. J. Eat. Disord., 2013, 46, 669–676 CrossRef PubMed.
- J. J. McGrath, D. W. Eyles, C. B. Pedersen, C. Anderson, P. Ko, T. H. Burne, B. Norgaard-Pedersen, D. M. Hougaard and P. B. Mortensen, Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study, Arch. Gen. Psychiatry, 2010, 67, 889–894 CrossRef CAS PubMed.
- D. Durup, H. L. Jorgensen, J. Christensen, P. Schwarz, A. M. Heegaard and B. Lind, A Reverse J-Shaped Association of All-Cause Mortality with Serum 25-Hydroxyvitamin D in General Practice, the CopD Study, J. Clin. Endocrinol. Metab., 2012, 97(8), 2644–2652 CrossRef CAS PubMed.
- M. L. Melamed, E. D. Michos, W. Post and B. Astor, 25-hydroxyvitamin D levels and the risk of mortality in the general population, Arch. Intern. Med., 2008, 168, 1629–1637 CrossRef PubMed.
- C. T. Sempos, R. A. Durazo-Arvizu, B. Dawson-Hughes, E. A. Yetley, A. C. Looker, R. L. Schleicher, G. Cao, V. Burt, H. Kramer, R. L. Bailey, J. T. Dwyer, X. Zhang, J. Gahche, P. R. Thomas, P. M. Coates and M. F. Picciano, Is there a Reverse J-shaped Association between 25-Hydroxyvitamin D and All-Cause Mortality? Results from the US Nationally Representative NHANES, J. Clin. Endocrinol. Metab., 2013, 98, 3001–3009 CrossRef CAS PubMed.
- Y. Dror, S. M. Giveon, M. Hoshen, I. Feldhamer, R. D. Balicer and B. S. Feldman, Vitamin D levels for preventing acute coronary syndrome and mortality: evidence of a nonlinear association, J. Clin. Endocrinol. Metab., 2013, 98, 2160–2167 CrossRef CAS PubMed.
- A. Zittermann, J. Kuhn, J. Dreier, C. Knabbe, J. F. Gummert and J. Borgermann, Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery, Eur. Heart J., 2013, 34, 1358–1364 CrossRef CAS PubMed.
- D. M. Freedman, A. C. Looker, C. C. Abnet, M. S. Linet and B. I. Graubard, Serum 25-hydroxyvitamin D and cancer mortality in the NHANES IIIstudy (1988–2006), Cancer Res., 2010, 70, 8587–8597 CrossRef CAS PubMed.
- K. Michaelsson, J. A. Baron, G. Snellman, R. Gedeborg, L. Byberg, J. Sundstrom, L. Berglund, J. Arnlov, P. Hellman, R. Blomhoff, A. Wolk, H. Garmo, L. Holmberg and H. Melhus, Plasma vitamin D and mortality in older men: a community-based prospective cohort study, Am. J. Clin. Nutr., 2010, 92, 841–848 CrossRef CAS PubMed.
- P. Tuohimaa, L. Tenkanen, M. Ahonen, S. Lumme, E. Jellum, G. Hallmans, P. Stattin, S. Harvei, T. Hakulinen, T. Luostarinen, J. Dillner, M. Lehtinen and M. Hakama, Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries, Int. J. Cancer, 2004, 108, 104–108 CrossRef CAS PubMed.
- J. Brandstedt, M. Almquist, J. Manjer and J. Malm, Vitamin D, PTH, and calcium and the risk of prostate cancer: a prospective nested case-control study, Cancer Causes Control, 2012, 23, 1377–1385 CrossRef PubMed.
- R. Z. Stolzenberg-Solomon, E. J. Jacobs, A. A. Arslan, D. Qi, A. V. Patel, K. J. Helzlsouer, S. J. Weinstein, M. L. McCullough, M. P. Purdue, X. O. Shu, K. Snyder, J. Virtamo, L. R. Wilkins, K. Yu, A. Zeleniuch-Jacquotte, W. Zheng, D. Albanes, Q. Cai, C. Harvey, R. Hayes, S. Clipp, R. L. Horst, L. Irish, K. Koenig, L. Le Marchand and L. N. Kolonel, Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, Am. J. Epidemiol., 2010, 172, 81–93 CrossRef PubMed.
- J. Rothers, A. L. Wright, D. A. Stern, M. Halonen and C. A. Camargo, Jr., Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona, J. Allergy. Clin. Immunol., 2011, 128, 1093–1099.e5 CrossRef CAS PubMed.
- N. O. Nielsen, T. Skifte, M. Andersson, J. Wohlfahrt, B. Soborg, A. Koch, M. Melbye and K. Ladefoged, Both high and low serum vitamin D concentrations are associated with tuberculosis: a case-control study in Greenland, Br. J. Nutr., 2010, 104, 1487–1491 CrossRef CAS PubMed.
- L. M. Bodnar, J. M. Catov, J. M. Zmuda, M. E. Cooper, M. S. Parrott, J. M. Roberts, M. L. Marazita and H. N. Simhan, Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women, J. Nutr., 2010, 140, 999–1006 CrossRef CAS PubMed.
- K. E. Ensrud, S. K. Ewing, L. Fredman, M. C. Hochberg, J. A. Cauley, T. A. Hillier, S. R. Cummings, K. Yaffe and P. M. Cawthon, Circulating 25-hydroxyvitamin D levels and frailty status in older women, J. Clin. Endocrinol. Metab., 2010, 95, 5266–5273 CrossRef CAS PubMed.
- A. Bearden, C. Abad, R. Gangnon, J. M. Sosman, N. Binkley and N. Safdar, Cross-sectional study of vitamin D levels, immunologic and virologic outcomes in HIV-infected adults, J. Clin. Endocrinol. Metab., 2013, 98, 1726–1733 CrossRef CAS PubMed.
- Vitamin D Council, Johnson Avenue, San Luis Obispo, California, United States, 93401, http://www.vitamindcouncil.org, accessed 18 October 2011.
- H. Bak, S. P. Hong, S. K. Jeong, E. H. Choi, S. E. Lee, S. H. Lee and S. K. Ahn, Altered epidermal lipid layers induced by long-term exposure to suberythemal-dose ultraviolet, Int. J. Dermatol., 2011, 50, 832–837 CrossRef PubMed.
- E. J. Kim, Y. K. Kim, J. E. Kim, S. Kim, M. K. Kim, C. H. Park and J. H. Chung, UV modulation of subcutaneous fat metabolism, J. Invest. Dermatol., 2011, 131, 1720–1726 CrossRef CAS PubMed.
- T. Michikawa, Y. Nishiwaki, K. Asakura, G. Hillebrand, K. Miyamoto, M. Ono, Y. Kinjo, S. Akiba and T. Takebayashi, Sunlight Exposure May Be a Risk Factor of Hearing Impairment: A Community-Based Study in Japanese Older Men and Women, J. Gerontol. A Biol. Sci. Med. Sci., 2013, 68(1), 96–103 CrossRef PubMed.
- R. A. Miller, D. Dolan, M. Han, W. Kohler and J. Schacht, Resistance of skin fibroblasts to peroxide and UV damage predicts hearing loss in aging mice, Aging Cell, 2011, 10, 362–363 CrossRef CAS PubMed.
- A. Szilagyi, H. Leighton, B. Burstein and I. Shrier, Significant positive correlation between sunshine and lactase nonpersistence in Europe may implicate both in similarly altering risks for some diseases, Nutr. Cancer, 2011, 63, 991–999 CrossRef PubMed.
- V. Nair-Shalliker, D. P. Smith, S. Egger, A. M. Hughes, J. M. Kaldor, M. Clements, A. Kricker and B. K. Armstrong, Sun exposure may increase risk of prostate cancer in the high UV environment of New South Wales, Australia: a case-control study, Int. J. Cancer, 2012, 131, E726–E732 CrossRef CAS PubMed.
- B. Q. Wang, Z. Y. Shen, Y. Fei, H. Li, J. H. Liu, H. Xu, Z. Zhang, X. H. Yu and X. D. Chen, A population-based study of acquired bilateral nevus-of-Ota-like macules in Shanghai, China, J. Invest. Dermatol., 2011, 131, 358–362 CrossRef CAS PubMed.
- B. B. Koo, Restless legs syndrome: relationship between prevalence and latitude, Sleep Breath., 2012, 16(4), 1237–1245 CrossRef PubMed.
- Y. W. Wirohadidjojo, S. Radiono, A. Budiyanto and H. Soebono, Cellular viability, collagen deposition, and transforming growth factor beta1 production among ultraviolet B-irradiated keloid fibroblasts, Aesthetic. Plast. Surg., 2011, 35, 1050–1055 CrossRef PubMed.
- D. Liu, B. O. Fernandez, A. Hamilton, N. N. Lang, J. M. Gallagher, D. E. Newby, M. Feelisch and R. B. Weller, UVA Irradiation of Human Skin Vasodilates Arterial Vasculature and Lowers Blood Pressure Independently of Nitric Oxide Synthase, J. Invest. Dermatol., 2014, 134(7), 1839–1846 CrossRef CAS PubMed.
- L. Yang, M. B. Veierod, M. Lof, S. Sandin, H. O. Adami and E. Weiderpass, Prospective study of UV exposure and cancer incidence among Swedish women, Cancer Epidemiol. Biomarkers Prev., 2011, 20, 1358–1367 CrossRef CAS PubMed.
- P. Brondum-Jacobsen, B. G. Nordestgaard, S. F. Nielsen and M. Benn, Skin cancer as a marker of sun exposure associates with myocardial infarction, hip fracture and death from any cause, Int. J. Epidemiol., 2013, 42, 1486–1496 CrossRef PubMed.
- R. Hoyos-Bachiloglu, P. S. Morales, J. Cerda, E. Talesnik, G. Gonzalez, C. A. Camargo, Jr. and A. Borzutzky, Higher latitude and lower solar radiation influence on anaphylaxis in Chilean children, Pediatr. Allergy Immunol., 2014, 25, 338–343 CrossRef PubMed.
- S. J. Balk, Ultraviolet radiation: a hazard to children and adolescents, Pediatrics, 2011, 127, e791–e817 CrossRef PubMed.
- I. Galan, A. Rodriguez-Laso, L. Diez-Ganan and E. Camara, Prevalence and correlates of skin cancer risk behaviors in Madrid (Spain), Gac. Sanit., 2011, 25, 44–49 CrossRef PubMed.
- D. B. Buller, V. Cokkinides, H. I. Hall, A. M. Hartman, M. Saraiya, E. Miller, L. Paddock and K. Glanz, Prevalence of sunburn, sun protection, and indoor tanning behaviors among Americans: review from national surveys and case studies of 3 states, J. Am. Acad. Dermatol., 2011, 65, S114–S123 CrossRef PubMed.
- D. Reinau, C. Meier, N. Gerber, G. F. Hofbauer and C. Surber, Sun protective behaviour of primary and secondary school students in North-Western Switzerland, Swiss Med. Wkly., 2012, 142, w13520 Search PubMed.
- S. W. Dusza, A. C. Halpern, J. M. Satagopan, S. A. Oliveria, M. A. Weinstock, A. Scope, M. Berwick and A. C. Geller, Prospective study of sunburn and sun behavior patterns during adolescence, Pediatrics, 2012, 129, 309–317 CrossRef PubMed.
- S. Dobbinson, M. Wakefield, D. Hill, A. Girgis, J. F. Aitken, K. Beckmann, A. I. Reeder, N. Herd, M. J. Spittal, A. Fairthorne and K. A. Bowles, Children's sun exposure and sun protection: Prevalence in Australia and related parental factors, J. Am. Acad. Dermatol, 2012, 66, 938–947 CrossRef PubMed.
- C. Battie, M. Gohara, M. Verschoore and W. Roberts, Skin cancer in skin of color: an update on current facts, trends, and misconceptions, J. Drugs Dermatol., 2013, 12, 194–198 Search PubMed.
- S. Ergul and E. Ozeren, Sun protection behavior and individual risk factors of Turkish Primary School Students associated with skin cancer: a questionnaire-based study, Asian Pac. J. Cancer Prev., 2011, 12, 765–770 Search PubMed.
- F. Mousavi, B. Golestan, M. Vaseie, L. Vaseie and R. Khajeh-Kazemi, Knowledge, attitude, and practice of adults to the protective actions against sun in northwest Tehran, Iran, Arch. Iran. Med., 2011, 14, 126–131 Search PubMed.
- J. Li, W. Uter, A. Pfahlberg and O. Gefeller, A comparison of patterns of sun protection during beach holidays and everyday outdoor activities in a population sample of young German children, Br. J. Dermatol., 2012, 166, 803–810 CrossRef CAS PubMed.
- B. Koster, C. Thorgaard, A. Philip and I. H. Clemmensen, Vacations to sunny destinations, sunburn, and intention to tan: a cross-sectional study in Denmark, 2007–2009, Scand. J. Public Health, 2011, 39, 64–69 CrossRef PubMed.
- T. Tempark, S. Chatproedprai and S. Wanankul, Attitudes, knowledge, and behaviours of secondary school adolescents regarding protection from sun exposure: a survey in Bangkok, Thailand, Photodermatol. Photoimmunol. Photomed., 2012, 28, 200–206 Search PubMed.
- C. J. Heckman and J. Cohen-Filipic, Brief Report: Ultraviolet Radiation Exposure, Considering Acculturation Among Hispanics (Project URECAH), J. Cancer Educ., 2012, 27, 342–346 CrossRef PubMed.
- S. Potente, K. Coppa, A. Williams and R. Engels, Legally brown: using ethnographic methods to understand sun protection attitudes and behaviours among young Australians ‘I didn't mean to get burnt–it just happened!’, Health Educ. Res., 2011, 26, 39–52 CrossRef PubMed.
- Cancer Council Victoria, SunSmart, http://www.sunsmart.com.au, accessed 31 July, 2014.
- Australian Radiation and Nuclear Safety Agency, Australian UV levels for mobile phones, http://www.arpansa.gov.au/uvindex/realtime/cellrt.htm, accessed 31 July, 2014.
- K. Dunstone and C. Conway, There is an app for that! Communicating UV via the SunSmart app, in UV radiation and its effects: an update 2014, National Institute for Water and Atmosphere, Auckland, NZ, 2014.
- G. R. Casale, A. M. Siani and A. Colosimo, A UV Index Sundial on compact disk, Household Person. Care Today, 2014, 9, 45–47 Search PubMed.
- J. Morris, T. Laing-Morton, P. Marno and A. Curnow, An investigation into the awareness and understanding of the ultraviolet index forecasts in the South West of England, Photochem. Photobiol. Sci., 2011, 10, 103–108 CAS.
- B. Rajiv and R. Gray, Sun Protection Alert - a simple tool for a complex issue, in UV radiation and its effects: an update 2014, Auckland, NZ, 2014 Search PubMed.
- EPA, UV Alert, United States Environmental Protection Agency, http://www2.epa.gov/sunwise/uv-alert, accessed 11 May, 2014.
- P. A. Andersen, D. B. Buller, B. J. Walkosz, M. D. Scott, J. A. Maloy, G. R. Cutter and M. D. Dignan, Environmental cues to UV radiation and personal sun protection in outdoor winter recreation, Arch. Dermatol., 2010, 146, 1241–1247 Search PubMed.
- D. Vernez, A. Milon, L. Vuilleumier and J. L. Bulliard, Anatomical exposure patterns of skin to sunlight: relative contributions of direct, diffuse and reflected ultraviolet radiation, Br. J. Dermatol., 2012, 167(2), 383–390 CrossRef CAS PubMed.
- A. I. Kudish, M. Harari and E. G. Evseev, The solar ultraviolet B radiation protection provided by shading devices with regard to its diffuse component, Photodermatol. Photoimmunol. Photomed., 2011, 27, 236–244 CrossRef PubMed.
- J. C. van der Pols, G. M. Williams, N. Pandeya, V. Logan and A. C. Green, Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use, Cancer Epidemiol. Biomarkers Prev., 2006, 15, 2546–2548 Search PubMed.
- A. C. Green, G. M. Williams, V. Logan and G. M. Strutton, Reduced melanoma after regular sunscreen use: randomized trial follow-up, J. Clin. Oncol., 2011, 29, 257–263 CrossRef CAS PubMed.
- M. C. Hughes, G. M. Williams, P. Baker and A. C. Green, Sunscreen and prevention of skin aging: a randomized trial, Ann. Intern. Med., 2013, 158, 781–790 CrossRef PubMed.
- A. Rodrigues, F. F. Sniehotta and V. Araujo-Soares, Are interventions to promote sun-protective behaviors in recreational and tourist settings effective? A systematic review with meta-analysis and moderator analysis, Ann. Behav. Med., 2013, 45, 224–238 CrossRef PubMed.
- R. Jansen, U. Osterwalder, S. Q. Wang, M. Burnett and H. W. Lim, Photoprotection: Part II. Sunscreen: Development, efficacy, and controversies, J. Am. Acad. Dermatol, 2013, 69, 867 e861–867 e814 CrossRef PubMed.
- A. Fourtanier, D. Moyal and S. Seite, UVA filters in sun-protection products: regulatory and biological aspects, Photochem. Photobiol. Sci., 2012, 11, 81–89 CAS.
- B. L. Diffey and M. W. Brown, The ideal spectral profile of topical sunscreens, Photochem. Photobiol., 2012, 88, 744–747 CrossRef CAS PubMed.
- A. L. Andrady and H. H. Redhwi, AyakoTorikai, K. K. Pandey and P. Gies, Consequences of stratospheric ozone depletion and climate change on the use of materials, Photochem. Photobiol. Sci., 2015, 14 Search PubMed , this issue.
- C. Couteau, C. Chauvet, E. Paparis and L. Coiffard, UV filters, ingredients with a recognized anti-inflammatory effect, PLoS One, 2012, 7, e46187 CAS.
- R. M. Sayre, J. C. Dowdy and E. W. Rosenberg, Sun-protection factor confounded by anti-inflammatory activity of sunscreen agents?, J. Am. Acad. Dermatol, 2013, 69, 481 CrossRef CAS PubMed.
- B. Petersen, P. Datta, P. A. Philipsen and H. C. Wulf, Sunscreen use and failures–on site observations on a sun-holiday, Photochem. Photobiol. Sci., 2013, 12, 190–196 CAS.
- A. Diaz, R. E. Neale, M. G. Kimlin, L. Jones and M. Janda, The children and sunscreen study: a crossover trial investigating children's sunscreen application thickness and the influence of age and dispenser type, Arch. Dermatol., 2012, 148, 606–612 Search PubMed.
- M. Pissavini and B. Diffey, The likelihood of sunburn in sunscreen users is disproportionate to the SPF, Photodermatol. Photoimmunol. Photomed., 2013, 29, 111–115 CrossRef PubMed.
- T. Teramura, M. Mizuno, H. Asano, N. Naito, K. Arakane and Y. Miyachi, Relationship between sun-protection factor and application thickness in high-performance sunscreen: double application of sunscreen is recommended, Clin. Exp. Dermatol., 2012, 37, 904–908 CrossRef CAS PubMed.
- A. Faurschou, D. M. Beyer, A. Schmedes, M. K. Bogh, P. A. Philipsen and H. C. Wulf, The relation between sunscreen layer thickness and vitamin D production after ultraviolet B exposure: a randomized clinical trial, Br. J. Dermatol., 2012, 167, 391–395 CrossRef CAS PubMed.
- E. Linos, E. Keiser, M. Kanzler, K. L. Sainani, W. Lee, E. Vittinghoff, M. M. Chren and J. Y. Tang, Sun protective behaviors and vitamin D levels in the US population: NHANES 2003–2006, Cancer Causes Control, 2012, 23, 133–140 CrossRef PubMed.
- R. Jansen, S. Q. Wang, M. Burnett, U. Osterwalder and H. W. Lim, Photoprotection: Part I. Photoprotection by naturally occurring, physical, and systemic agents, J. Am. Acad. Dermatol, 2013, 69, 853 e851–853 e812 CrossRef PubMed.
- A. K. Haylett, Z. Nie, M. Brownrigg, R. Taylor and L. E. Rhodes, Systemic photoprotection in solar urticaria with alpha-melanocyte-stimulating hormone analogue [Nle4-D-Phe7]-alpha-MSH, Br. J. Dermatol., 2011, 164, 407–414 CrossRef CAS PubMed.
- E. A. Langan, Z. Nie and L. E. Rhodes, Melanotropic peptides: more than just ‘Barbie drugs’ and ‘sun-tan jabs’?, Br. J. Dermatol., 2010, 163, 451–455 CrossRef CAS PubMed.
- L. Chen, J. Y. Hu and S. Q. Wang, The role of antioxidants in photoprotection: A critical review, J. Am. Acad. Dermatol, 2012, 67(5), 1013–1024 CrossRef CAS PubMed.
- A. C. Chen and D. L. Damian, Nicotinamide and the skin, Australas. J. Dermatol., 2014, 55(3), 169–175 CrossRef PubMed.
- Y. Wu, L. L. Jia, Y. N. Zheng, X. G. Xu, Y. J. Luo, B. Wang, J. Z. Chen, X. H. Gao, H. D. Chen, M. Matsui and Y. H. Li, Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation, J. Eur. Acad. Dermatol. Venereol., 2013, 27(3), 345–350 CrossRef CAS PubMed.
- U. Wolfle, P. R. Esser, B. Simon-Haarhaus, S. F. Martin, J. Lademann and C. M. Schempp, UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo, Free Radicals Biol. Med., 2011, 50, 1081–1093 CrossRef PubMed.
- M. Rizwan, I. Rodriguez-Blanco, A. Harbottle, M. A. Birch-Machin, R. E. Watson and L. E. Rhodes, Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial, Br. J. Dermatol., 2011, 164, 154–162 CrossRef CAS PubMed.
- U. Heinrich, C. E. Moore, S. De Spirt, H. Tronnier and W. Stahl, Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women, J. Nutr., 2011, 141, 1202–1208 CrossRef CAS PubMed.
- L. E. Rhodes, G. Darby, K. A. Massey, K. A. Clarke, T. P. Dew, M. D. Farrar, S. Bennett, R. E. Watson, G. Williamson and A. Nicolaou, Oral green tea catechin metabolites are incorporated into human skin and protect against UV radiation-induced cutaneous inflammation in association with reduced production of pro-inflammatory eicosanoid 12-hydroxyeicosatetraenoic acid, Br. J. Nutr., 2013, 110, 891–900 CrossRef CAS PubMed.
- Y. R. Lou, Q. Y. Peng, T. Li, C. M. Medvecky, Y. Lin, W. J. Shih, A. H. Conney, S. Shapses, G. C. Wagner and Y. P. Lu, Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on UVB-induced skin carcinogenesis in SKH-1 mice, Carcinogenesis, 2011, 32, 1078–1084 CrossRef CAS PubMed.
- J. C. van der Pols, C. Xu, G. M. Boyle, M. C. Hughes, S. J. Carr, P. G. Parsons and A. C. Green, Serum omega-3 and omega-6 fatty acids and cutaneous p53 expression in an Australian population, Cancer Epidemiol. Biomarkers Prev., 2011, 20, 530–536 CrossRef CAS PubMed.
- T. DeBoyes, D. Kouba, D. Ozog, E. Fincher, L. Moy, K. Iwata and R. Moy, Reduced number of actinic keratoses with topical application of DNA repair enzyme creams, J. Drugs Dermatol., 2010, 9, 1519–1521 Search PubMed.
- A. Hofer, F. J. Legat, A. Gruber-Wackernagel, F. Quehenberger and P. Wolf, Topical liposomal DNA-repair enzymes in polymorphic light eruption, Photochem. Photobiol. Sci., 2011, 10, 1118–1128 CAS.
- E. Berardesca, M. Bertona, K. Altabas, V. Altabas and E. Emanuele, Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: clues to skin cancer prevention, Mol. Med. Rep., 2012, 5, 570–574 CAS.
- R. C. Ojah and J. M. Welch, Vitamin D and musculoskeletal status in Nova Scotian women who wear concealing clothing, Nutrients, 2012, 4, 399–412 CrossRef CAS PubMed.
- S. Hatun, O. Islam, F. Cizmecioglu, B. Kara, K. Babaoglu, F. Berk and A. S. Gokalp, Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing, J. Nutr., 2005, 135, 218–222 CAS.
- D. Bassil, M. Rahme, M. Hoteit and H. Fuleihan Gel, Hypovitaminosis D in the Middle East and North Africa: Prevalence, risk factors and impact on outcomes, Dermato-endocrinol., 2013, 5, 274–298 CrossRef PubMed.
- P. S. Sobolewski, J. W. Krzyscin, J. Jaroslawski, J. Wink, A. Lesiak and J. Narbutt, Controlling adverse and beneficial effects of solar UV radiation by wearing suitable clothes - Spectral transmission of different kinds of fabrics, J. Photochem. Photobiol., B, 2014, 140C, 105–110 CrossRef PubMed.
- E. Linos, E. Keiser, T. Fu, G. Colditz, S. Chen and J. Y. Tang, Hat, shade, long sleeves, or sunscreen? Rethinking US sun protection messages based on their relative effectiveness, Cancer Causes Control, 2011, 22, 1067–1071 CrossRef PubMed.
- A. V. Parisi, R. Eley and N. Downs, Determination of the usage of shade structures via a dosimetry technique, Photochem. Photobiol., 2012, 88, 1012–1015 CrossRef CAS PubMed.
- R. L. McKenzie, J. B. Liley and L. O. Bjorn, UV radiation: balancing risks and benefits, Photochem. Photobiol., 2009, 85, 88–98 CrossRef CAS PubMed.
- A. R. Webb and O. Engelsen, Ultraviolet exposure scenarios: risks of erythema from recommendations on cutaneous vitamin D synthesis, Adv. Exp. Med. Biol., 2008, 624, 72–85 CrossRef CAS.
- F. S. Rosenthal, C. Phoon, A. E. Bakalian and H. R. Taylor, The ocular dose of ultraviolet radiation to outdoor workers, Invest. Ophthalmol. Visual Sci., 1988, 29, 649–656 CAS.
- D. H. Sliney, Intraocular and crystalline lens protection from ultraviolet damage, Eye Contact Lens, 2011, 37, 250–258 Search PubMed.
- U. L. Osuagwu, K. C. Ogbuehi and T. M. Almubrad, Changes in ultraviolet transmittance of hydrogel and silicone-hydrogel contact lenses induced by wear, Eye Contact Lens, 2014, 40, 28–36 Search PubMed.
- N. Gao, L. W. Hu, Q. Gao, T. T. Ge, F. Wang, C. Chu, H. Yang and Y. Liu, Diurnal variation of ocular exposure to solar ultraviolet radiation based on data from a manikin head, Photochem. Photobiol., 2012, 88, 736–743 CrossRef CAS PubMed.
- F. Wang, Q. Gao, L. Hu, N. Gao, T. Ge, J. Yu and Y. Liu, Risk of eye damage from the wavelength-dependent biologically effective UVB spectrum irradiances, PLoS One, 2012, 7, e52259 CAS.
- L. Hu, Q. Gao, N. Gao, G. Liu, Y. Wang, H. Gong and Y. Liu, Solar UV exposure at eye is different from environmental UV: diurnal monitoring at different rotation angles using a manikin, J. Occup. Environ. Hyg., 2013, 10, 17–25 CrossRef PubMed.
- J. Turner and A. V. Parisi, Influence of reflected UV irradiance on occupational exposure from combinations of reflective wall surfaces, Photochem. Photobiol. Sci., 2013, 12, 1589–1595 CAS.
- H. Chandler, Ultraviolet absorption by contact lenses and the significance on the ocular anterior segment, Eye Contact Lens, 2011, 37, 259–266 Search PubMed.
- W. Lan, A. Petznick, S. Heryati, M. Rifada and L. Tong, Nuclear Factor-kappaB: central regulator in ocular surface inflammation and diseases, Ocul. Surf., 2012, 10, 137–148 CrossRef PubMed.
- D. Ali, A. Verma, F. Mujtaba, A. Dwivedi, R. K. Hans and R. S. Ray, UVB-induced apoptosis and DNA damaging potential of chrysene via reactive oxygen species in human keratinocytes, Toxicol. Lett., 2011, 204, 199–207 CrossRef CAS PubMed.
- M. A. Beketov, A. Speranza and M. Liess, Ultraviolet radiation increases sensitivity to pesticides: synergistic effects on populationgrowth rate of Daphnia magna at low concentrations, Bull. Environ. Contam. Toxicol., 2011, 87, 231–237 CrossRef CAS PubMed.
- B. Epe, DNA damage spectra induced by photosensitization, Photochem. Photobiol. Sci., 2012, 11, 98–106 CAS.
- G. Miolo, S. Caffieri, D. Dalzoppo, F. Gallocchio, E. Fasani and G. M. Beyersbergen van Henegouwen, Photoactivation of corticosteroids in UVB-exposed skin, J. Photochem. Photobiol., B, 2011, 103, 35–41 CrossRef CAS PubMed.
- B. Gulson, M. McCall, M. Korsch, L. Gomez, P. Casey, Y. Oytam, A. Taylor, M. McCulloch, J. Trotter, L. Kinsley and G. Greenoak, Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin, Toxicol. Sci., 2010, 118, 140–149 CrossRef CAS PubMed.
- T. Kunisue, Z. Chen, G. M. Buck Louis, R. Sundaram, M. L. Hediger, L. Sun and K. Kannan, Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis, Environ. Sci. Technol., 2012, 46, 4624–4632 CrossRef CAS PubMed.
- Therapeutic Goods Administration, Literature review on the safety of titanium dioxide and zinc oxide nanoparticles in sunscreens. Scientific review report, Department of Health and Ageing, Canberra, 2013, pp. 0–19 Search PubMed.
- S. Jatana and L. A. Delouise, Understanding engineered nanomaterial skin interactions and the modulatory effects of ultraviolet radiation skin exposure, Wiley Interdiscip. Rev.: Nanomed. and Nanobiotechnol., 2014, 6, 61–79 CrossRef CAS PubMed.
- D.-P. Häder, C. E. Williamson, S.-Å. Wängberg, M. Rautio, K. C. Rose, K. Gao, E. W. Helbling, R. P. Sinha and R. Worrest, Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors, Photochem. Photobiol. Sci., 2015, 14 Search PubMed , this issue.
- K. G. McGuigan, R. M. Conroy, H. J. Mosler, M. du Preez, E. Ubomba-Jaswa and P. Fernandez-Ibanez, Solar water disinfection (SODIS): a review from bench-top to roof-top, J. Hazard. Mater., 2012, 235–236, 29–46 CrossRef CAS PubMed.
- H. Slaper, G. J. Velders, J. S. Daniel, F. R. de Gruijl and J. C. van der Leun, Estimates of ozone depletion and skin cancer incidence to examine the Vienna Convention achievements, Nature, 1996, 384, 256–258 CrossRef CAS PubMed.
- A. van Dijk, H. Slaper, P. N. den Outer, O. Morgenstern, P. Braesicke, J. A. Pyle, H. Garny, A. Stenke, M. Dameris, A. Kazantzidis, K. Tourpali and A. F. Bais, Skin cancer risks avoided by the Montreal Protocol–worldwide modeling integrating coupled climate-chemistry models with a risk model for UV, Photochem. Photobiol., 2013, 89, 234–246 CrossRef CAS PubMed.
- A. F. Bais, K. Tourpali, A. Kazantzidis, H. Akiyoshi, S. Bekki, P. Braesicke, M. P. Chipperfield, M. Dameris, V. Eyring, H. Garny, D. Iachetti, P. Jockel, A. Kubin, U. Langematz, E. Mancini, M. Michou, O. Morgenstern, T. Nakamura, P. A. Newman, G. Pitari, D. A. Plummer, E. Rozanov, T. G. Shepherd, K. Shibata, W. Tian and Y. Yamashita, Projections of UV radiation changes in the 21st century: impact of ozone recovery and cloud effects, Atmos. Chem. Phys., 2011, 11, 7533–7545 CrossRef CAS.
- P. Correa Mde, S. Godin-Beekmann, M. Haeffelin, S. Bekki, P. Saiag, J. Badosa, F. Jegou, A. Pazmino and E. Mahe, Projected changes in clear-sky erythemal and vitamin D effective UV doses for Europe over the period 2006 to 2100, Photochem. Photobiol. Sci., 2013, 12, 1053–1064 Search PubMed.
- B. Petersen, E. Thieden, P. A. Philipsen, J. Heydenreich, H. C. Wulf and A. R. Young, Determinants of personal ultraviolet-radiation exposure doses on a sun holiday, Br. J. Dermatol., 2013, 168, 1073–1079 CrossRef CAS PubMed.
- L. Lemus-Deschamps and J. K. Makin, Fifty years of changes in UV Index and implications for skin cancer in Australia, Int. J. Biometeorol., 2012, 56, 727–735 CrossRef PubMed.
- J. Makin, Implications of climate change for skin cancer prevention in Australia, Health Promo. J. Aust., 2011, 22(Spec No), S39–S41 Search PubMed.
- J. C. van der Leun, R. D. Piacentini and F. R. de Gruijl, Climate change and human skin cancer, Photochem. Photobiol. Sci., 2008, 7, 730–733 CAS.
- W. G. Tsiaras and M. A. Weinstock, Factors influencing vitamin D status, Acta Derm. Venereol., 2011, 91, 115–124 Search PubMed.
- P. Thomas, A. Swaminathan and R. M. Lucas, Climate change and health with an emphasis on interactions with ultraviolet radiation: a review, Global. Change Biol., 2012, 18, 2392–2405 CrossRef PubMed.
- J. F. Bornman, P. W. Barnes, S. A. Robinson, C. L. Ballaré, S. D. Flinte and M. M. Caldwell, Solar ultraviolet radiation and its interaction with other ozone depletion and associated climate effects on terrestrial ecosystems, Photochem. Photobiol. Sci., 2015, 14 Search PubMed , this issue.
- D. J. Erickson, III, B. Sulzberger, R. Zepp, A. T. Austin and N. Paul, Effects of solar UV radiation and climate change on biogeochemical cycling: Interactions and feedbacks, Photochem. Photobiol. Sci., 2015, 14 Search PubMed , this issue.
- N. Principi, P. Marchisio, L. Terranova, A. Zampiero, E. Baggi, C. Daleno, S. Tirelli, C. Pelucchi and S. Esposito, Impact of vitamin D administration on immunogenicity of trivalent inactivated influenza vaccine in previously unvaccinated children, Hum. Vaccines Immunother., 2013, 9, 969–974 CrossRef CAS PubMed.
- S. Esposito, E. Baggi, S. Bianchini, P. Marchisio and N. Principi, Role of vitamin D in children with respiratory tract infection, Int. J. Immunopathol. Pharmacol., 2013, 26, 1–13 CAS.
- S. N. Byrne, How much sunlight is enough?, Photochem. Photobiol. Sci., 2014, 13, 840–852 CAS.
- S. Seite, A. Fourtanier, D. Moyal and A. R. Young, Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review, Br. J. Dermatol., 2010, 163, 903–914 CrossRef CAS PubMed.
- K. D. Cashman, M. Kiely, M. Kinsella, R. A. Durazo-Arvizu, L. Tian, Y. Zhang, A. Lucey, A. Flynn, M. J. Gibney, H. W. Vesper, K. W. Phinney, P. M. Coates, M. F. Picciano and C. T. Sempos, Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program's potential for national nutrition and health surveys, Am. J. Clin. Nutr., 2013, 97, 1235–1242 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2015 |