Low-migratory ionic ferrocene-based burning rate catalysts with high combustion catalytic efficiency†
Xuelin
Liu
,
Weiqiang
Zhang
,
Guofang
Zhang
* and
Ziwei
Gao
*
Key Laboratory of Applied Surface and Colloid Chemistry, MOE/School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, China. E-mail: gfzhang@snnu.edu.cn; zwgao@snnu.edu.cn; Fax: +86 29 81530727; Tel: +86 29 81530830
First published on 1st October 2014
Abstract
Alkyl substituted ferrocenes can catalyze the burning of composited solid propellants efficiently. These non-polar and volatile ferrocenes, however, migrate to the surface of the propellants during prolonged storage, which would alter the designed burning parameters, and more seriously cause unexpected explosions. To tackle this problem, twelve ionic ferrocene derivatives bearing methylimidazolium and methyltriazolium substituents with nitrate and picrate anions as new ionic ferrocene-based burning rate catalysts were synthesized. The compounds were fully characterized by 1H NMR, 13C NMR and elemental analysis. Ten of them were characterized by single-crystal X-ray diffraction. Compounds 2 and 3 crystallize in the monoclinic space group P2(1)/n and P2(1)/c, respectively. Compounds 4·H2O, 6·1/2H2O, 8–10 and 12 crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Compounds 7 and 11 crystallize in the orthorhombic space group Pna21 and Pna2(1), respectively. Cyclic voltammetry investigations revealed that all the compounds exhibit quasi-reversible redox systems. Migration studies confirmed that the migration of these ionic ferrocenes is much slower than that of 2,2-bis(ethylferrocenyl)propane. The migration trend of the compounds is dependent on molecular structures of the ionic ferrocenes and that shorter alkyl chain lengths in the heterocyclic rings lead to slower migration rates. Their high thermal stability was determined by TG and DSC analyses (peak temperatures >172 °C). The thermal degradation of ammonium perchlorate (AP) catalyzed by the new burning-rate catalysts was evaluated by DSC methods. In the presence of nitrate (1–6) in 4 wt% or picrate (7–12) in 5 wt%, the peak decomposition temperature of AP shifts left significantly while the released heat increases dramatically. The catalytic activity of an ionic compound with a triazole ring in its cation is higher than its corresponding analog with an imidazole ring and the catalytic activity of a nitrate is generally higher than its picrate counterpart.
. Compounds 7 and 11 crystallize in the orthorhombic space group Pna21 and Pna2(1), respectively. Cyclic voltammetry investigations revealed that all the compounds exhibit quasi-reversible redox systems. Migration studies confirmed that the migration of these ionic ferrocenes is much slower than that of 2,2-bis(ethylferrocenyl)propane. The migration trend of the compounds is dependent on molecular structures of the ionic ferrocenes and that shorter alkyl chain lengths in the heterocyclic rings lead to slower migration rates. Their high thermal stability was determined by TG and DSC analyses (peak temperatures >172 °C). The thermal degradation of ammonium perchlorate (AP) catalyzed by the new burning-rate catalysts was evaluated by DSC methods. In the presence of nitrate (1–6) in 4 wt% or picrate (7–12) in 5 wt%, the peak decomposition temperature of AP shifts left significantly while the released heat increases dramatically. The catalytic activity of an ionic compound with a triazole ring in its cation is higher than its corresponding analog with an imidazole ring and the catalytic activity of a nitrate is generally higher than its picrate counterpart.
Introduction
Ferrocene and its numerous derivatives have found their application in almost all scientific fields such as catalysis,1 medicine,2 nanomaterials preparation3 and ion recognition.4 As burning rate (BR) catalysts, the ferrocene-based catalysts include tert-butylferrocene (TBF), n-butylferrocene (NBF), 2,2-bis(ethylferrocenyl)propane (Catocene), which significantly enhance BRs of the solid propellants that mainly consist of hydroxyl-terminated polybutadiene–ammonium perchlorate (HTPB/AP).5These ferrocene derivatives are, however, neutral and volatile, which might sublime during curing and processing, and migrate to the surface of the propellants after prolonged storage times. These shortcomings can destruct the steady combustion of the propellants and in some cases result in danger when mixing with ultrafine AP.5b Many efforts have been devoted to retard and even eliminate the migration tendency of ferrocene-based BR catalysts. One strategy is to incorporate ferrocenyl groups into the side-chain or backbone of polymers using ferrocene precursors with functional groups prone to polymerization.6 Another method is to design ferrocene compounds containing polar groups such as azolyl, hydroxyl, acyl groups or transition-metal complexes derived from ferrocene-based ligands.7 Although many efforts have been devoted to eliminate the migration of ferrocene-based BR catalysts, the preparation of the low-migratory ferrocenes are time-consuming and inefficient.5
In the last few decades, energetic ionic compounds, including ionic liquids and salts, have attracted great interest in the field of energetic materials due to their unique characteristics such as very lower vapor pressures and higher densities than their atomically similar non-ionic analogues.8 Moreover, the modular design principle of energetic ionic compounds will allow the prediction of ion combinations with the desired property sets for the custom design of energetic materials.8d Some of them may be as alternatives to common explosives.
Inspired by the unique characteristics of energetic ionic compounds, we introduced the concept of energetic ionic compounds and their modular synthetic strategy into the ferrocene-based BR catalysts, aiming to retard the migration rates of neutral BR catalysts and whilst enhance the energy level of the propellants. Therefore, we recently designed and synthesized a series of mono- and binuclear ferrocene-based ionic compounds with nitrate or picrate as the counter anions.9 They exhibited a low migration tendency and a highly catalytic effect on the thermal decomposition of AP.
As a continuous activity to design and synthesize energetic ionic ferrocene-based BR catalysts with higher catalytic activity, ferrocenylmethylalkylimidazolium(triazolium) iodides10 were selected as cation sources for the new energetic ionic ferrocene compounds described in this work. The selected cation sources contain nitrogen-rich heterocyclic rings, which will contribute to their thermal stability, low migration properties and the energetic level of the propellants, and more importantly, they can be easily prepared. Earlier, a few 1-ferrocenylmethylimidazolium (triazolium)-based ionic compounds with PF6− or NTf2− (NTf2− = bis(trifluoro-methanesulfonyl)amide) as counterions from their corresponding iodides were prepared and some of them are ionic liquids.11 In our work, new 1-ferrocenylmethyl-3(4)-alkylimidazolium (triazolium) (alkyl = CH3, C2H5, n-C3H7, i-C3H7 or n-C4H9) nitrates and picrates (Scheme 1) were designed, synthesized and most of them structurally characterized by single crystal X-ray diffraction. Their thermal stability, migration tendency and redox properties as well as their catalytic effect on the thermal decomposition of AP were evaluated.
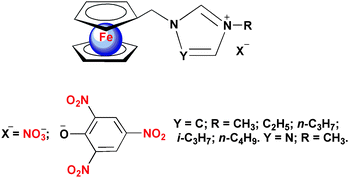 | ||
| Scheme 1 Molecular structures of 1-ferrocenylmethyl-3(4)-alkylimidazolium (triazolium) nitrates and picrates. | ||
Results and discussion
Synthesis
The desired products were prepared in high yields by an ion exchange reaction of their corresponding iodides with silver nitrate or sodium picrate. To purify the products, water and a minimum amount of methanol were used to dissolve the iodides. The melting points of all the new compounds except 5 are in the range of 78.5–164.1 °C. Compound 5 is oil. The two compounds with triazole rings in their cations show higher melting points, presumably due to the substitution of a carbon atom in imidazole ring with a nitrogen atom, which is prone to forming hydrogen bonds with hydrogen atoms. The 1H NMR spectra (ESI†) of the compounds exhibit a considerable chemical shift upwards for the hydrogen atom in the 2-position and downwards for the hydrogen atoms in the 3- and 4-positions of the imidazole rings, on comparison with their iodide precursors. The UV-Vis absorption spectra of all the picrates (1.0 × 10−4 mol L−1) and nitrate 5 (5.5 × 10−4 mol L−1) in methanol are presented in Fig. S1 (ESI†). Each compound displays a strong adsorption band around 229 and 245 nm, which are ascribed to the π–π* transition of the Cp groups and with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε in the range of 3.76–4.40 L mol−1 cm−1. The picrates show bands peaked in the range of 362–370 nm and the shoulders from 423 to 426 nm, which are assigned to the characteristic π–π* transition of the picrate anion.12
ε in the range of 3.76–4.40 L mol−1 cm−1. The picrates show bands peaked in the range of 362–370 nm and the shoulders from 423 to 426 nm, which are assigned to the characteristic π–π* transition of the picrate anion.12
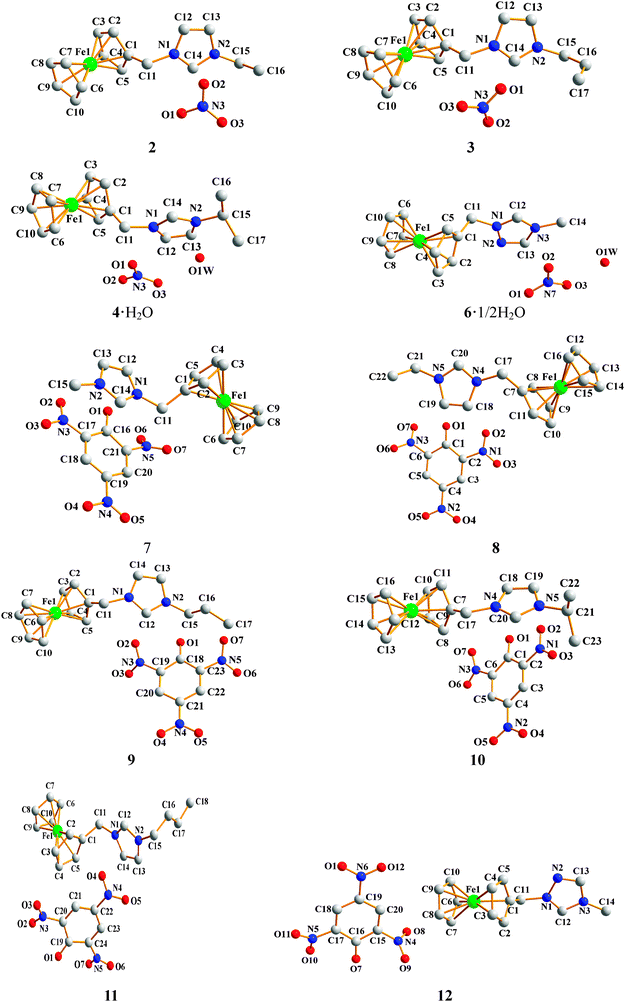
Crystal structure analysis
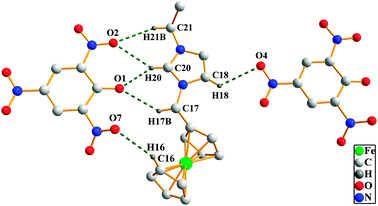
Single crystals of compounds 2–4·H2O and 6·1/2H2O–12 were grown from methanol–diethyl ether solution and characterized by single X-ray crystallography (Fig. 1). Selected data and parameters for the X-ray determination are given in Tables S1 and S2 (ESI†). 2 and 3 crystallize in the monoclinic space group P2(1)/n and P2(1)/c, respectively. 4·H2O, 6·1/2H2O, 8–10 and 12 crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . 7 and 11 crystallize in the orthorhombic space group Pna21 and Pna2(1), respectively. All the compounds, except 4·H2O and 6·1/2H2O contain lattice water molecules in their crystal structures and include no solvate molecules in their crystal lattices. The distances between Cp rings and Fe atoms are in the range of 1.6368 (1) Å to 1.6714 (7) Å. The five atoms of the imidazole and triazole ring are almost in the same plane, respectively. Their bond lengths are prone to be conjugated. The dihedral angles between the Cp rings (C7–C11 in 8 and 10, and C1–C5 in the other eight compounds) and the imidazole (triazole) rings are in the range of 61.4(2) to 79.0(1)° (61.4(2) to 65.7(9)°). The dihedral angles between the nitrate anions and the Cp rings in 2, 4·H2O and 6·1/2H2O are from 48.2° to 50.52°, but in 3 it is very large (72.2°). The dihedral angles of the benzene rings of the picrates and the Cp rings in 7–12 span 32.5(2) to 77.4(1)°. There are plenty of inter- and intramolecular hydrogen bonds in the spatial structures of the compounds (Table S3, ESI†). The hydrogen bonds in the crystal structure of 8, taken as an example, are discussed in detail. As shown in Fig. 2, O1, O2, O4 and O7 of the nitrate anion and the carbon atoms C16, C17, C18, C20 and C21 participate in medium to weak hydrogen bonding [C16–H16⋯O7 3.257(4), C17–H17⋯O1 3.351(3), C18–H18⋯O4 3.177(3), C20–H20⋯O1 2.994(3), C20–H20⋯O2 3.379(3) and C21–H21⋯O2 3.470(4) Å]. The existence of a large number of hydrogen bonds in the molecules leads to the stability of the compound.
. 7 and 11 crystallize in the orthorhombic space group Pna21 and Pna2(1), respectively. All the compounds, except 4·H2O and 6·1/2H2O contain lattice water molecules in their crystal structures and include no solvate molecules in their crystal lattices. The distances between Cp rings and Fe atoms are in the range of 1.6368 (1) Å to 1.6714 (7) Å. The five atoms of the imidazole and triazole ring are almost in the same plane, respectively. Their bond lengths are prone to be conjugated. The dihedral angles between the Cp rings (C7–C11 in 8 and 10, and C1–C5 in the other eight compounds) and the imidazole (triazole) rings are in the range of 61.4(2) to 79.0(1)° (61.4(2) to 65.7(9)°). The dihedral angles between the nitrate anions and the Cp rings in 2, 4·H2O and 6·1/2H2O are from 48.2° to 50.52°, but in 3 it is very large (72.2°). The dihedral angles of the benzene rings of the picrates and the Cp rings in 7–12 span 32.5(2) to 77.4(1)°. There are plenty of inter- and intramolecular hydrogen bonds in the spatial structures of the compounds (Table S3, ESI†). The hydrogen bonds in the crystal structure of 8, taken as an example, are discussed in detail. As shown in Fig. 2, O1, O2, O4 and O7 of the nitrate anion and the carbon atoms C16, C17, C18, C20 and C21 participate in medium to weak hydrogen bonding [C16–H16⋯O7 3.257(4), C17–H17⋯O1 3.351(3), C18–H18⋯O4 3.177(3), C20–H20⋯O1 2.994(3), C20–H20⋯O2 3.379(3) and C21–H21⋯O2 3.470(4) Å]. The existence of a large number of hydrogen bonds in the molecules leads to the stability of the compound.
Electrochemical behavior
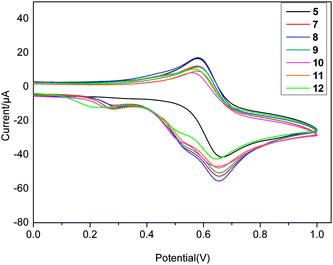
The redox properties of all the compounds in acetonitrile solution were studied by cyclic voltammetry (CV) to understand the electrochemical reaction mechanism of the ionic ferrocene compounds, which are related closely with the catalytic performance of the new compounds.6a The results of CV in the range of 0–1.0 V (Fig. 3) indicated that each compound exhibits a quasi-reversible ferrocene/ferrocenium wave in the range of 596–626 mV for E1/2p and 75–92 mV for ΔEp. Additionally, irreversible oxidation waves are displayed in the ranges of 0.270 mV to 0.295 mV (0.237 mV for 12), and 0.519 mV to 0.535 mV, respectively, ascribed tentatively to the oxidation of the picrate anion. Table S4 (ESI†) lists the electrochemical data for the ferrocenyl groups in all the new compounds, NBF, TBF and Catocene. From Table S4 (ESI†), it can be seen that the alkyl chain lengths in the imidazolium (triazolium) rings exert no significant influence on the redox potential. The oxidation potentials of the ionic compounds are ca. 200 mV larger than those of NBF, TBF and Catocene, indicating that the ionic compounds are more difficult to oxidized than the neutral ferrocene derivatives under similar conditions.Thermal stability studies
Thermal stability is an extremely important parameter for a burning rate catalyst since a higher thermal stability of a catalyst will be beneficial to its catalytic activity and to the combustion process of the solid propellants. Thermal behaviors of all the compounds were therefore investigated by TG/DSC methods. As shown in Fig. S2–S5 (ESI†), the nitrates exhibit a higher thermal stability than the picrates, with peaks in the range of 195.0–217.7 °C for nitrates 1–6·1/2H2O and 172.2–177.4 °C for picrates 7–12. The first sharp mass loss stages in the TG curves of the nitrates are assigned to the loss of the nitrate anions and the imidazole rings for 1–5, while the TG curves of the picrates display relatively lower weight loss rates. The two compounds bearing triazole rings show higher thermal stability than the others containing imidazole rings. NBF begins its weight loss from 64.2 °C and ceases at 171.5 °C, corresponding to an almost 100% weight loss, attributed mainly to the high volatilization of NBF. Therefore, the newly synthesized ionic ferrocene derivatives show much higher thermal stability than NBF.Migration studies
Significant migration of a burning rate catalyst will lead to an unsteady combustion process for the propellants and can even lead to an explosion during curing.5 The migration trend of the commercially available ferrocene-based BR catalysts is significant. A variety of strategies have been developed to decrease the migration trend, but in many cases other problems were introduced. For confirmation of our prospective that the ionic ferrocene compounds are low-migratory combustion modifiers, the migration rates of some selected compounds were determined and compared with Catocene (with the lowest migration rates of the three alkyl-substituted ferrocenes). The relative mobilities for nitrate 3 and three picrates 7, 8 and 9 as well as Catocene (Cat) against their migration distance and aging time are plotted in Fig. 4.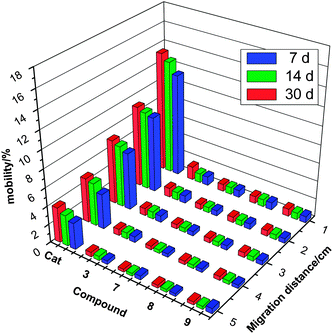 | ||
| Fig. 4 Dependence of migration percentage of 3, 7, 8, 9 and Catocene on migration distance and aging time at 70 °C. | ||
It is observed that the migration percentage of the ionic compounds were much lower than those of Catocene. For example, the total migration percentage of Catocene and ionic compounds 3 and 7 aged for 7 days are 32.86, 3.58 and 2.77%, respectively. It is also found that for 7, 8 and 9 the migration tendency increases with an increase in the alkyl chain length on the imidazole rings, as observed in other ionic ferrocene compounds reported earlier.9 A tentative explanation for this tendency is that the ionization degree of the ferrocene salts decreases with an increase of the alkyl chain length on the imidazole ring, thus leading to an increase of their volatility and migration trend. In addition, it is also notable that the migration rate of the picrate is lower than its nitrate analog, owing to the higher molecular weight of the picrate. The migration test results verify that the introduction of the concept of energetic ionic compounds into ferrocene-based BR catalysts effectively retards the migration rate of the ferrocene-based BR catalysts.
Catalytic performances for thermal degradation of AP
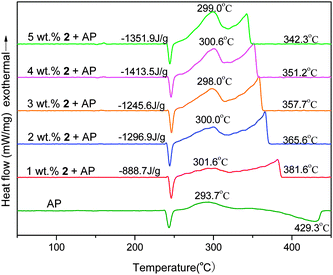
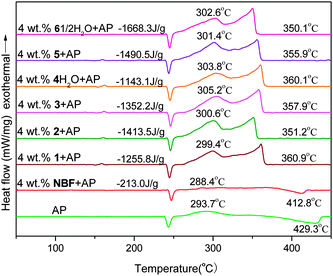
AP is a commonly used main component in HTPB–AP composite solid propellants. Its thermal decomposition has a close relationship with the combustion process of the propellants. Therefore, the combustion effect of a BR catalyst candidate on the combustion behavior of the HTPB–AP composite propellants is usually assessed by its effect on the thermal degradation of AP. The addition of a ferrocene-based BR catalyst as additive will give rise to a shift in the peak temperatures of AP left, and accelerating AP decomposition as well as increasing the released heat of AP.13 The catalytic performance of all the newly prepared ferrocene salts for thermal degradation of AP were evaluated by DSC methods. Fig. 5 and Fig. S6 (ESI†) show the catalytic effects of different addition contents of 2 and 8 on the thermal degradation of AP, respectively. It is observed that the phase transition endothermic process of AP peaked at 243.4 °C has almost no shift (max. ca. 3 °C) and exhibit a similar shape with 1–5 wt% of 2 or 8 as additive, indicating that the additives exert little effect on the crystallographic transition temperature of the AP. Both the low-temperature decomposition (LTD) stage (peak temperature at 293.7 °C) and the high-temperature decomposition (HTD) stage (peak temperature at 429.3 °C) of the AP are, however, significantly affected by the addition of 2 or 8.14 The wide decomposition temperature range of AP was considerably narrowed and the released heat of AP increased. 4 wt% of 2 was chosen as the optimum amount and each nitrate was then added in 4 wt% for the evaluation of their catalytic effects. Similarly, the optimum percentage of 8 in AP was 5 wt% and each picrate was also added in 5 wt% for the catalytic activity test.Both Fig. 6 and Fig. S7 (ESI†) present the DSC curves for the thermal degradation of AP and its mixtures with 4 wt% of 1–6·1/2H2O and 5 wt% of 7–12, respectively. It is noted that all the DSC curves, except that of pure AP, displayed a similar shape with two peaks in each DSC curve, suggesting that an identical decomposition mechanism of the mixture systems should be expected. For the two decomposition stages of pure AP, the HTD stage is changed dramatically on comparison with that of the LTD stage. The endothermic process of the AP peaked at 429.3 °C disappeared and a new exothermic process appeared, peaked in the region of 350.1–360.9 °C (a 68.4–79.2 °C downward-shift) for the addition of 1–6·1/2H2O and 361.0–365.9 °C (a 63.4–68.3 °C downward-shift) using 7–12 as additives, implying that the additives have a great catalytic effect on the HDT stage of AP and that the nitrates have more of an effect than the picrates in this case. Upon the addition of a new additive, AP narrowed its decomposition temperature range from ca. 170 °C to about 90–107 °C. The endothermic process predominated by sublimation of decomposition intermediates at the HTD stage of pure AP changed to an exothermic decomposition process with an ionic ferrocene additive, suggesting that a violent decomposition process had occurred. In contrast, NBF brought down the temperature peak of the HTD stage of AP by 16.5 °C and a sublimation phenomena can still be observed, suggesting that the endothermic process predominates the HTD stage of AP with the addition of NBF. NBF has therefore a relatively lower catalytic efficiency during the thermal degradation of AP.
The released heats (Fig. 6 and Fig. S7, ESI†) of the samples are between −1143.1 to −1668.3 J g−1 with 1–6·1/2H2O as additives and −1042.9 to −1464.0 J g−1 with 7–12 as additives. It can be seen that the AP releases the lowest heat with addition of 4·H2O in the nitrate family and 10 in the picrates, each containing a i-propyl group on the imidazole ring. AP gives the highest heat with incorporation of 6·1/2H2O (nitrates) and 12 (picrates), each containing a triazole ring in the cation. These results suggested that the substitution of the imidazole ring by a triazole ring exerts a positive effect on their catalytic activity, presumably due to the higher nitrogen content of the triazole ring, which will promote us to design higher nitrogen-content ferrocene derivatives later. The alkyl chain lengths in the azole rings also plays an important role in the catalytic performance of the compounds investigated. Additionally, compared to a picrate, a nitrate analog can significantly increase the heat released by AP decomposition, implying that a nitrate anion gives a greater influence on the catalytic performance of these compounds, verifying again the effect of a nitrate anion being more obvious than a picrate anion.9
Upon comparison with their structural analogs we reported earlier,9b3, 5, 9 and 11 have much more effect on thermal decomposition of AP, indicating again that a high nitrogen content in the molecules is beneficial to their combustion catalytic activity. However, compared to the binuclear ferrocenyl derivatives with similar alkyl(alkenyl) chain lengths,9a these mononuclear ionic ferrocenes present low catalytic efficiency in the thermal degradation of AP, demonstrating that both high iron and nitrogen contents in the ferrocenyl derivatives are necessary for their combustion catalytic performance.
Conclusions
Twelve new (ferrocenylmethyl)imidazole(triazole)-based nitrates and picrates were prepared in view of their potential lower migration in comparison to neutral alkyl-substituted ferrocenes. Single-crystal X-ray diffraction and other characterization techniques support the structure of the desired products. All the compounds are highly thermally stable and low-migratory ionic compounds. The catalytic evaluation results confirmed that they show high catalytic activity for the thermal decomposition of ammonium perchlorate and that the two compounds bearing triazole rings are more active than those with imidazole rings. However, their catalytic activities are lower than those of the binuclear ionic ferrocene compounds we reported earlier. This promotes us to design and synthesize high-nuclear ferrocene-containing ionic compounds with nitrogen-rich heterocyclic rings, such as triazole or tetrazole rings. They are currently being conducted in our laboratory.Experimental
General method
N,N′-Dimethylmethylferrocene was purchased from Meryer (Shanghai) Chemical Technology Co., Ltd and N-methylimidazole, N-ethylimidazole, N-propylimidazole, N-isopropylimidazole and N-butylimidazole from Alfa Aesar (Tianjin) Chemicals Co. Ltd. Additional reagents and chemicals used were of AR grade and used as received. All 1-ferrocenylmethy-3-alkylimidazolium iodides (alkyl = methyl; ethyl; n-propyl; i-propyl; n-butyl and 1-ferrocenylmethy-4-methyltriazolium iodide were synthesized as described by B. Bildstein).101H and 13C NMR spectra were performed on a Bruker Avance 400 MHz spectrometer unless indicated otherwise. Elemental analyses were carried out with a Vario EL III Elemental Analyzer, Germany. Low-temperature DSC data for the ionic liquids were obtained on a Q1000DSC + LNCS + FACS Q600SDT thermoanalyzer system from TA Company of USA. DSC and TG studies were undertaken on a HS-1 model from Beijing Henven Scientific Instrument Factory and Q50 model from TA company of USA, respectively, operating at 5 °C min−1 in an nitrogen atmosphere (50 mL min−1) with sample masses of ca. 3.0 mg. The mass spectrometry measurements were performed on a Bruker microflex MALDI-TOF mass spectrometer. The UV-Vis adsorption spectra were recorded on a UV-2450 spectrophotometer of Shimadzu Corporation. Cyclic voltammograms were recorded with an CHI660C analyzer. Redox potentials were measured at a scan rate of 100 mV s−1 in CH3CN containing 0.1 mol L−1n-Bu4PF6 as the supporting electrolyte. An Ag/Ag+ reference electrode and a platinum working electrode were used. Migration studies were performed according to our earlier works.9 Single-crystal X-ray structural measurements were carried out with a Bruker SMART APEX-II CCD detector using graphite monochromated MoKα radiation (λ = 0.71073). Structures were solved by applying a direct method using SHELXS-97.15 The full-matrix least-squares refinement on F2 included atomic coordinates and anisotropic thermal parameters for all non-H atoms. The H atoms were included using a riding model.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (21171112).Notes and references
- (a) M. Drusan and R. Šebesta, Tetrahedron, 2014, 70, 759–786 CrossRef CAS PubMed; (b) D. J. Young, S. W. Chien and T. S. A. Hor, Dalton Trans., 2012, 41, 12655–12665 RSC; (c) D. Schaarschmidt and H. Lang, Organometallics, 2013, 32, 5668–5704 CrossRef CAS.
- S. S. Braga and A. M. S. Silva, Organometallics, 2013, 32, 5626–5639 CrossRef CAS.
- (a) A. W. Orbaek, N. Aggarwal and A. R. Barron, J. Mater. Chem. A, 2013, 1, 14122–14132 RSC; (b) G. Huang; J. Weng, Curr. Org. Chem., 2011, 15, 3653–3666 CrossRef.
- (a) P. Molina, A. Tárraga and M. Alfonso, Dalton Trans., 2014, 43, 18–29 RSC; (b) M. Alfonso, A. Espinosa, A. Tárraga and P. Molina, Chem. Commun., 2012, 48, 6848–6850 RSC; (c) S. R. Beeren and J. K. M. Sanders, J. Am. Chem. Soc., 2011, 133, 3804–3807 CrossRef CAS PubMed.
- (a) R. Tong, Y. Zhao, L. Wang, H. Yu, F. Ren and M. Saleem, J. Organomet. Chem., 2014, 755, 16–32 CrossRef CAS PubMed; (b) J. Gao, L. Wang, H. Yu, A. Xiao and W. Ding, Propellants, Explos., Pyrotech., 2011, 36, 404–409 CrossRef CAS; (c) X. Zhang, Y. Xia, L. Jia, J. Chi, W. Chang and J. Wang, Chem. Propellants Polym. Mater., 2012, 10, 58–64 CAS; (d) X. Tang, J. Wuhan Inst. Technol., 2012, 34, 19–25 CAS; (e) K. Subramanian, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 4090–4099 CrossRef CAS.
- (a) F. J. Xiao, F. F. Feng, L. L. Li and D. Zhang, Propellants, Explos., Pyrotech., 2013, 38, 358–365 CrossRef CAS; (b) F. Xiao, X. Sun, X. Wu, J. Zhao and Y. Luo, J. Organomet. Chem., 2012, 713, 96–103 CrossRef CAS PubMed; (c) K. Gottlie, H. P. Hebekeuser, H. Jungbluth, H. P. MacKowiak and H. Neitsch, US Pat., 6313334, 2001 Search PubMed; (d) C. W. Huskins and A. J. Thomas, US Pat., 3781179, 1973 Search PubMed; (e) D. C. Sayles and H. Ala, US Pat., 3860463, 1975 Search PubMed; (f) W. E. Hill and H. Ala, US Pat, 3962297, 1976 Search PubMed.
- (a) D. Saravanakumar, N. Sengottuvelan, V. Narayanan, M. Kandaswamy and T. L. Varghese, J. Appl. Polym. Sci., 2011, 119, 2517–2524 CrossRef CAS; (b) C. Ke, H. Li, L. Xie and Y. Yuan, Chin. J. Energ. Mater., 2011, 19, 19–22 Search PubMed; (c) W. Liao, Y. Dou, J. Wang, L. Xie, F. Lin and Y. Yuan, Chem. Res. Chin. Univ., 2012, 33, 2244–2248 CAS; (d) J. Wang, L. Feng, F. Ma, F. Lin, L. Xie and Y. Yuan, Chin. J. Org. Chem., 2012, 32, 1479–1486 CrossRef CAS; (e) H. Zhao, L. Guo, S. Chen and Z. Bian, RSC Adv., 2013, 3, 19929–19932 RSC; (f) H. Zhao, M. Chen, X. Zhu, S. Chen and Z. Bian, Res. Chem. Intermed. DOI:10.1007/s11164-013-1503-7.
- (a) H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed; (b) G. Steinhauser and T. M. Klapoetke, Angew. Chem., Int. Ed., 2008, 47, 3330–3347 CrossRef CAS PubMed; (c) M. Smiglak, A. Metlen and R. D. Rogers, Acc. Chem. Res., 2007, 40, 1182–1192 CrossRef CAS PubMed; (d) N. Fischer, K. Huell, T. M. Klapoetke, J. Stierstorfer, G. Laus, M. Hummel, C. Froschauer, K. Wurst and H. Schottenberger, Dalton Trans., 2012, 41, 11201–11211 RSC; (e) S. Li, H. Gao and J. M. Shreeve, Angew. Chem., Int. Ed., 2014, 53, 2969–2972 CrossRef CAS PubMed; (f) C. He, J. Zhang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2013, 1, 2863–2868 RSC.
- (a) X. Liu, D. Zhao, F. Bi, X. Fan, F. Zhao, G. Zhang, W. Zhang and Z. Gao, J. Organomet. Chem., 2014, 762, 1–8 CrossRef CAS PubMed; (b) Z. Cheng, G. Zhang, X. Fan, F. Bi, F. Zhao, W. Zhang and Z. Gao, Inorg. Chim. Acta, 2014, 421, 191–199 CrossRef CAS PubMed.
- B. Bildstein, M. Malaun, H. Kopacka, K.-H. Ongania and K. Wurst, J. Organomet. Chem., 1998, 552, 55–61 CrossRef.
- Y. Gao, B. Twamley and J. M. Shreeve, Inorg. Chem., 2004, 43, 3406–3412 CrossRef CAS PubMed.
- (a) N. Camire, U. T. Mueller-Westerhoff and W. E. Geiger, J. Organomet. Chem., 2001, 637–639, 823–826 CrossRef CAS; (b) Y. Miura, F. Shimizu and T. Mochida, Inorg. Chem., 2010, 49, 10032–10040 CrossRef CAS PubMed.
- Q. Yang, S. Chen, G. Xie and S. Gao, J. Hazard. Mater., 2011, 197, 199–203 CrossRef CAS PubMed.
- (a) A. J. Han, J. J. Liao, M. Q. Ye, Y. Li and X. H. Peng, Chin. J. Chem. Eng., 2011, 19, 1047–1051 CrossRef CAS; (b) Z. R. Liu, C. M. Yin, Y. H. Kong, F. Q. Zhao, Y. Luo and H. Xiang, Chin. J. Energ. Mater., 2000, 8, 75–79 CAS; (c) A. J. Lang and S. Vyazovkin, Combust. Flame, 2006, 145, 779–790 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, Tables S1–S4 and Fig. S1–S7. Crystal data for compounds 2–4·H2O and 6·1/2H2O–12. CCDC 973759–973761, 992136, 992962, 997738, 997739, 999264, 1001648 and 1008574. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4nj01216j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |