The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling's role in its reduction†
J. B.
Dunn
*a,
L.
Gaines
a,
J. C.
Kelly
a,
C.
James
b and
K. G.
Gallagher
c
aEnergy Systems Division, Argonne National Laboratory, 9700 South Cass Avenue, Argonne, IL 60439, USA. E-mail: jdunn@anl.gov; Tel: +1-630-252-4667
bDepartment of Chemical Engineering and Materials Science, Michigan State University, 2527 Engineering Building, East Lansing, MI 48824, USA
cChemical Sciences and Engineering Division, Argonne National Laboratory, 9700 South Cass Avenue, Argonne, IL 60439, USA
First published on 11th November 2014
Abstract
Three key questions have driven recent discussions of the energy and environmental impacts of automotive lithium-ion batteries. We address each of them, beginning with whether the energy intensity of producing all materials used in batteries or that of battery assembly is greater. Notably, battery assembly energy intensity depends on assembly facility throughput because energy consumption of equipment, especially the dry room, is mainly throughput-independent. Low-throughput facilities therefore will have higher energy intensities than near-capacity facilities. In our analysis, adopting an assembly energy intensity reflective of a low-throughput plant caused the assembly stage to dominate cradle-to-gate battery energy and environmental impact results. Results generated with an at-capacity assembly plant energy intensity, however, indicated cathode material production and aluminium use as a structural material were the drivers. Estimates of cradle-to-gate battery energy and environmental impacts must therefore be interpreted in light of assumptions made about assembly facility throughput. The second key question is whether battery recycling is worthwhile if battery assembly dominates battery cradle-to-gate impacts. In this case, even if recycled cathode materials are less energy and emissions intensive than virgin cathode materials, little energy and environmental benefit is obtained from their use because the energy consumed in assembly is so high. We reviewed the local impacts of metals recovery for cathode materials and concluded that avoiding or reducing these impacts, including SOx emissions and water contamination, is a key motivator of battery recycling regardless of the energy intensity of assembly. Finally, we address whether electric vehicles (EV) offer improved energy and environmental performance compared to internal combustion-engine vehicles (ICV). This analysis illustrated that, even if a battery assembly energy reflective of a low-throughput facility is adopted, EVs consume less petroleum and emit fewer greenhouse gases (GHG) than an ICV on a life-cycle basis. The only scenario in which an EV emitted more GHGs than an ICV was when it used solely coal-derived electricity as a fuel source. SOx emissions, however, were up to four times greater for EVs than ICVs. These emissions could be reduced through battery recycling.
Broader contextIn this paper, we address three key questions in automotive lithium-ion battery energy and environmental analysis: whether materials production or battery assembly drive these batteries' energy and environmental impacts; what motivates battery recycling if it is the assembly step that is the major energy consumer; and how the energy and environmental performance of electric vehicles (EV) and internal combustion-engine vehicles (ICV) compare. Our analysis indicates that, even if battery assembly drives battery energy and environmental impacts, EVs offer lower petroleum consumption and GHG emissions on a life-cycle basis compared to ICVs unless the EVs use electricity produced exclusively from coal-fired power plants. Further, if the energy intensity of battery assembly were very high, batteries incorporating recycled components (e.g., cathode) may not offer significant energy savings compared to batteries containing only virgin material. Even in that case, battery recycling is still critical to avoid the local impacts of metals production for use in battery cathode materials, among other reasons. Finally, although uncertainty still hampers estimates of battery assembly energy intensity, plants operating at or near capacity have low energy intensities reflective of a mature industry. In this case, materials production, not battery assembly, drives the energy and environmental impacts of automotive lithium-ion batteries. |
Introduction
Electric vehicles (EV) are touted as one of a suite of technologies that can drive down fossil fuel consumption and GHG emissions from the transportation sector, which contributes about one-third of U.S. total GHG emissions each year. There are several types of EVs, but all rely on batteries. One type is a plug-in hybrid electric vehicle (PHEV) that uses both a battery and an engine to power the vehicle. Extended range electric vehicles (EREV) are a type of PHEV that may have a larger battery to extend vehicle range. PHEVs operating in charge-depleting (CD) mode pull energy from the battery whereas charge-sustaining mode operation relies on the engine combusting a liquid fuel. Battery electric vehicles (BEVs) rely solely on a battery as an energy source. Eberle and von Helmolt1 review the technology, advantages, and drawbacks of these types of EVs, as well as fuel cell electric vehicles, which use hydrogen fuel.Without a comprehensive picture of the battery's contribution to EV life-cycle impacts, it is difficult to compare GHG emissions of EVs and conventional internal combustion engine vehicles (ICV) and assess whether EVs offer GHG emissions reductions and other energy and environmental benefits. Recently, a suite of analyses2–6 has improved understanding of the energy and environmental impacts of automotive lithium-ion batteries, although several key uncertainties remain. The battery contribution to life-cycle EV energy consumption and environmental impacts must be based on sound data and analyses to reduce uncertainty comparing EVs and ICVs.
Lithium-ion battery cradle-to-gate energy consumption results in the literature, however, complicate this comparison because they diverge, ranging from about 100 MJ kg−1 to 200 MJ kg−1.2–4,6 An examination of the literature reveals that the key point of disagreement among these studies is the energy intensity of the assembly of the battery, not the materials production stage. The definition of these two stages can be confusing and can vary among studies. We define these terms as follows. Battery assembly constitutes steps that put together a battery from its component parts including the electrodes, cells, battery management system, and packaging. This step could occur all in one building as sketched in Fig. S1.† Ellingsen et al.6 report that battery manufacturer Miljøbil Grenland manufactures cells at one facility and these cells and other components are assembled into battery packs at a separate facility. On the other hand, we define materials production as all the steps that come before the final assembly of the battery. Fig. S2† is a diagram of the steps involved in making a lithium-ion battery with a LiMn2O4 (LMO) cathode material and graphite anode with the assembly step clearly separated from the material production steps that precede it. Different literature accounts report the assembly step, as we define it here, as ranging from about 1% to over 60% of the total cradle-to-gate energy of producing an automotive lithium-ion battery.2–4,6
This discrepancy in the literature raises the first of three key issues that we will address in this paper with the aim of clarifying outstanding issues in the energy and environmental analysis of automotive lithium-ion batteries. The first issue is whether it is more energy intensive to put a battery together than to produce all of its component parts. The second issue is whether recycling of automotive lithium-ion batteries is of environmental value if battery assembly is as energy intensive as some reports indicate. Battery recycling consumes energy presumably with the aim of recovering materials (especially the cathode) that can be reassembled into batteries.4 If, however, the assembly step is already the greatest contributor to battery cradle-to-gate energy intensity, it may not be possible to achieve an energy reduction in cradle-to-gate energy consumption through recycling. Finally, there has been some question, especially in the popular press, as to the relative benefits of EVs as compared to ICVs if producing batteries is as energy intensive as some suggest.7
In addition to addressing these three important issues, we report new cradle-to-gate energy consumption, GHG emissions, and air pollutant emissions results for lithium-ion batteries. Existing literature studies cover lithium ion batteries with LMO, LiFePO4 (LFP), and LiNi0.4Co0.2Mn0.4O2 (NMC) cathode materials paired with graphite anodes. Li et al. pair NMC cathode materials with silicon nanowires as the anode.8 Herein we report results for these chemistries and LiCoO2 (LCO) paired with graphite anodes in addition to an advanced cathode material under development at Argonne National Laboratory, 0.5Li2MnO3·0.5LiNi0.44Co0.25Mn0.31O2 (LMR-NMC) paired with either graphite or a graphite–silicon blend. For LCO and LFP, we consider both hydrothermal (HT) and solid state (SS) preparation techniques to get a sense of how the energy intensity of these two preparation routes could differ. The former technique uses a solvent and low reaction temperatures, whereas the latter involves dry reactions generally at high temperatures. It is not always clear which route would be less energy intensive. Cradle-to-gate impacts of producing automotive lithium-ion batteries with these chemistries were calculated on a consistent basis with Argonne's Greenhouse gases, Regulated Emissions, and Energy use in Transportation (GREET™) model. Comparing these different battery types on a consistent basis allows insight into the factors that drive their energy and environmental impacts. Furthermore, we used previously-developed estimates of the energy intensity of battery recycling4 to estimate the possible energy and emissions savings associated with recycling each of these battery types by pyrometallurgical or physical recycling processes.
Taken together, this comprehensive reporting of cradle-to-gate energy consumption and GHG emissions results for a range of lithium-ion battery chemistries and the discussion of the three overarching issues in battery energy and environmental analysis provides insight into the key question of whether EVs offer improved energy and environmental performance compared to ICVs.
Methodology
We developed material and energy flows for the cradle-to-gate production and recycling of lithium-ion batteries with LMO, LFP, NMC, LCO, and LMR-NMC cathodes and report our methodology and assumptions in two peer-reviewed reports.8,9 We combined these data for each of these cathode materials along with battery compositions (dependent on chemistry and battery type) generated with Argonne's Battery Performance and Cost (BatPaC) model9 into GREET. Table S1† contains inputs used for each of the cathode materials and Table S2† summarizes their properties. Tables S3–S6† summarize properties and composition of the PHEV and BEV batteries we considered in this analysis.Results and discussion
In this section, we first present results for the energy and emissions intensity of preparing different cathode chemistries. This discussion informs the subsequent investigation of the three key issues in battery energy and environmental analysis: the relative energy intensities of material production versus battery assembly, the benefits of battery recycling if battery assembly is more energy intensive than material production, and the relative performance of EVs as compared to conventional vehicles.Energy and emissions intensity of cathode preparation
Previous analyses2–4 indicate cathode materials contribute significantly to total battery energy and environmental impacts, which led us to characterize the impacts of producing batteries with different cathode materials. Incorporating the cathodes' material and energy flow data in GREET yields results for the total energy consumed in production of each cathode material. Fig. 1 shows the contribution of major inputs in the cathode material supply chain to the total cradle-to-gate energy consumption. Fig. 1 combines several cathode material inputs; Table S7† reports the contribution to cathode cradle-to-gate energy intensity separately. The topmost bar for each cathode represents the energy consumed in the preparation step, which is either HT or SS. We developed estimates of the material and energy intensity of both HT and SS preparation routes for two cathode materials: LCO and LFP.10 In both cases, HT preparation is more energy intensive than the SS method. Despite our consideration of some heat recovery, the energy consumed in solvent heating drove results for HT preparation technique energy consumption. It is important to note that the energy and materials consumed in the preparation steps are based on best available public information in the literature. The rates of material consumption are likely suboptimal and potential for recycling of some of the inputs could exist. The values in Fig. 1 therefore serve as estimates that indicate the relative energy intensity of the different cathode preparation routes and identify drivers of energy consumption results. In the case of LMO and LFP prepared by the HT route, the preparation step is the key contributor to energy consumption. When LFP is prepared by the SS route, production of diammonium phosphate, an input to the preparation step, is the key contributor to cathode material energy consumption.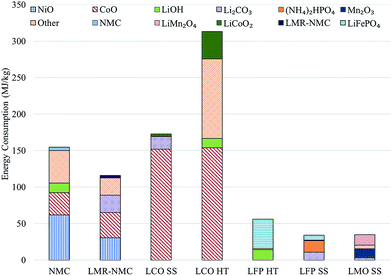 | ||
| Fig. 1 Cradle-to-gate energy consumption in the production of different cathode materials (HT: hydrothermal; SS: solid state). | ||
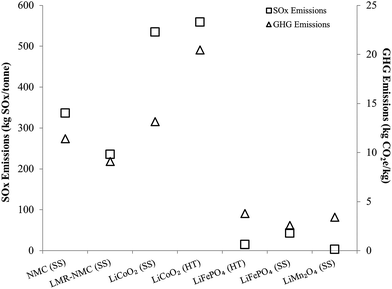
Another important result in Fig. 1 is that cathode materials with cobalt and nickel have higher cradle-to-gate energy intensities than cathode materials that do not contain these metals (LMO and LFP). The processes to recover cobalt and nickel from ores drive results in Fig. 1 for cobalt- and nickel-containing cathode materials. These processes use energy-intensive and high-emitting steps like smelting. On the other hand, recovery of lithium from brines consumes little energy. It is this basic difference at the start of the cathode material supply chain that drives differences in cradle-to-gate energy consumption of cathode materials. The smelting step in cobalt and nickel recovery strongly influences another key environmental impact of cathode material production, SOx emissions, as Fig. 2 illustrates. Cradle-to-gate SOx emissions are significantly higher for LCO, NMC, and LMR-NMC compared to cathodes that do not contain cobalt or nickel. The highest cradle-to-gate SOx emissions are for HT production of LCO, 560 kg SOx per tonne LCO. Cradle-to-gate GHG emissions (Fig. 2) for the different cathode materials range from 3 kg CO2e kg−1 to 20 kg CO2e kg−1. The drivers behind GHG emissions in cathode materials supply chains mirrors those behind energy consumption (Fig. 1). Emissions of other air pollutants in the cathode materials' supply chain (NOx, VOC, PM10, and PM2.5) are also higher for cobalt- and nickel-containing cathodes but are roughly an order of magnitude below SOx emissions (Fig. S3†).
Overall, as new cathode materials are developed, researchers can use tools, such as the GREET battery module, to guide material selection towards lower-impact inputs and to assess the relative impacts of different preparation techniques. While cathode materials that do not contain cobalt or nickel have lower cradle-to-gate energy consumption, they commonly have lower specific energies than nickel- and cobalt-containing cathodes, which can mean greater masses of these cathodes need to be included in a battery to achieve the same performance. For example, the 28 kWh BEV (approximately 110 km range) batteries we modeled with BatPaC contain either 71 kg of LMO (405 Wh kg−1vs. Li metal, 100 mA h g−1) or 48 kg of LCO (610 Wh kg−1vs. Li metal, 150 mA h g−1) (see Table S2† for a summary of cathode material properties). It is therefore important to consider whole-battery environmental impacts of the different cathode materials we examined.
To construct a picture of whole-battery impacts, two key elements are necessary. The first element is a material inventory for the battery. In our work, we use the BatPaC model to define a materials inventory based on material types (e.g., cathode material, plastics, aluminum, steel). We take this approach to facilitate analysis in GREET, which assigns material, energy, and emissions intensity based on battery component type (e.g., polyethylene, aluminum, graphite). Using BatPaC allows us to construct and analyze on a consistent basis batteries that use different cathode and anode materials but have the same performance. Other researchers take an approach that may use the breakdown of a battery3 or a battery's bill of materials.6 We compared the breakdown of components of a lithium-ion battery with NMC as a cathode between BatPaC and Ellingsen et al.6 and found very similar mass contributions of the different elements. Key differences were a higher (by 11%) mass contribution from packaging and a lower (by 5%) positive electrode paste contribution in Ellingsen et al.'s battery. The second essential element for whole-battery analysis is the battery assembly energy, which is the topic of the next subsection.
Energy intensity of battery assembly
As mentioned previously, there is a good deal of uncertainty in the literature concerning the energy intensity of battery assembly. Before describing literature estimates of this parameter, we will first review the major stages of battery assembly.11,12In the first stage of battery assembly, electrodes are produced. In this stage, the cathode active material is mixed with a binder in a solvent to achieve a slurry with homogeneous distribution of cathode components. Separately, the anode material is mixed. Next, a coating process applies the cathode and anode slurries to the current collectors (aluminium and copper foils, respectively) and dries the composite thus forming the electrodes. A calendering process presses the composite to the desired, reduced porosity. The large coils of coated foil are then slit and cut down to sheets of electrodes of appropriate size. At this point, the second stage of battery assembly, cell assembly, begins. In this step, a separator is placed between electrodes, which are connected to poles and other cell components as necessary. In a dry room, the electrolyte is added and the cells are sealed (some or all of the previously described steps may also occur in a dry room, depending on the facility design). Outside of the dry room, the sealed cells subsequently undergo charging and discharging cycles to age them. The next stage of battery assembly involves mounting the battery module and begins with setting the cells in a prepared module base. The cell conductors are connected before the battery management system is attached. Next, the cooling system is integrated and the module tested. The final stage of battery assembly is the assembly of the battery pack itself in which the modules are attached to the pack base and the cooling and managements systems are installed. Final assembly and testing yields a completed pack. Cell assembly and final pack assembly can occur at a single facility11 or be distributed among multiple facilities.6 It is common today for automotive companies to design and assemble their own battery packs from cells that have been purchased from a supplier.
Three different approaches have been taken to estimating the energy required in battery assembly. Some studies use a bottom-up approach, in which the energy of individual steps in battery manufacturing are estimated and summed.2,4,8 These studies generally assume assembly facilities operate at capacity. Only one of these4 takes into account the energy consumed by the dry room. Nonetheless, these studies estimate battery assembly to be between about 1 and 5 MJ kg−1 battery. Other studies use the second, top-down approach that seeks to apportion either a fraction of total corporate energy consumption13 or a literature estimate for total primary fuel consumption for lithium-ion battery production from cradle-to-gate3,14 to battery assembly. Top-down estimates of the energy intensity of battery assembly place it between 74 and 80 MJ kg−1 battery. The third approach, using real-world energy consumption data, was taken by Ellingsen et al., who were able to obtain energy consumption data for a battery cell manufacturer and a battery pack assembler. The latter plant, which employed mostly manual labor, had a very small energy consumption below 0.01 MJ kg−1 battery. The cell assembly plant, however exhibited a wide range of energy consumption between 100 and 400 MJ kg−1 battery. Ellingsen et al. note that given the swings in energy consumption, it is very likely that energy conservation opportunities exist at the cell assembly plant that could drive down energy consumption and state that the lower end of the range is most characteristic of large-scale production (using the high end of the range would place total battery energy consumption at about 500 MJ kg−1 battery). Secondly, the facility from which they gathered data was operating only at about one-third of its capacity.15 This latter point is critical because if the plant were operating at full capacity, the energy consumption would range from about 35 MJ kg−1 to 130 MJ kg−1.
At present, as lithium-ion battery production expands, cell and battery assembly facilities may, at first, operate well below capacity.16 An important point is that certain equipment, especially the dry room, likely consume the same amount of energy regardless of throughput, rendering the energy intensity of battery production high when throughput is low. A question in battery energy and environmental analysis then is whether it is appropriate to use the energy intensity of “pioneer plants” that are operating at low capacity or “nth plants” that have benefitted from increased demand and the maturing of battery assembly equipment and processes as representative. It is perhaps best to track both, recognizing that the energy and emissions burdens of lithium ion battery assembly will decline over time. When comparing BEVs to conventional vehicles that have benefited from decades of development, however, it may be most appropriate to use the “nth plant” approach.
Clearly facility capacity is a key factor that influences energy intensity of battery assembly. As the battery industry matures, it is likely that facilities will operate at or near the capacity for which they were designed, minimizing energy intensity and optimizing profits.
In Fig. 3, we provide the energy intensity of battery production from cradle-to-gate using both (a) low (nth plant, high throughput)4 and (b) high (pioneer plant, low throughput)6 assembly step energy intensities (Table S8† breaks out the contribution of all battery components to cradle-to-gate energy intensity). Fig. 3 includes batteries made with the cathodes in Fig. 1. Total energy consumption to produce a battery from cradle-to-gate with the higher assembly energy, which we take to be an upper bound that is not indicative of typical operation, can be up to ten times greater than total energy consumption estimates that use the lower assembly energy.
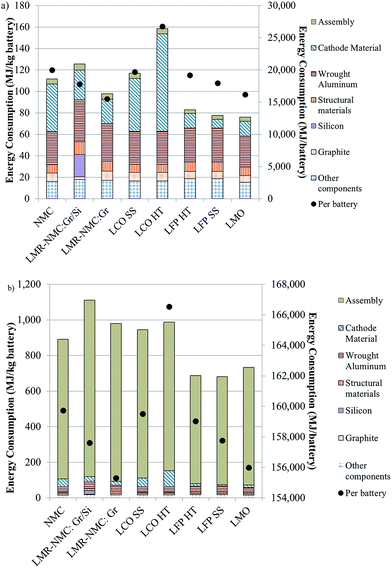 | ||
| Fig. 3 Energy intensity of BEV production from cradle-to-gate with different cathode materials using assembly step energy intensity from Dunn et al.4 (a) and Ellingsen et al.6 (b). | ||
If the high, “pioneer plant” energy intensity reflected the state of the industry with no expectation for improvement, battery recycling, from an energy standpoint, would not be advantageous. That is, even if it were possible to produce recycled cathode materials at a lower energy intensity than producing virgin cathode material, on a whole-battery level, the energy saved would be diminutive in comparison to the energy consumed in assembling the battery, which ranges from 88–90%, depending on the cathode chemistry. Other benefits of battery recycling merit consideration, including reduction of air emissions associated with mining of metals for cathodes, reducing waste sent to landfills and stewardship of key metals like cobalt and copper. If, however, we expect battery assembly facilities to operate at or near capacity, the assembly step contributes less than 10% of total battery energy, and recycling offers considerable energy benefits.
In this latter case, it is either the energy intensity of the cathode or of wrought aluminium used as a structural material that drives cradle-to-gate energy consumption. Of the cathode materials, LCO is the most significant contributor (42–57%) to a given battery's energy intensity. Fig. 1 indicates that the bulk of this contribution is from the recovery and purification of CoO. On the other hand, LMO and LFP contribute relatively little (11–18%) to cradle-to-gate battery energy intensity. For batteries with these cathode materials, it is aluminium that drives overall battery energy intensity (∼40%). In the case of the battery with an NMC cathode, the cathode material is the main contributor (40%) to battery energy intensity. Another key result from this analysis is that silicon, when added to the anode, increases the energy intensity of a battery with an LMR-NMC cathode by about 30%. The energy intensity of silicon production is driven by the deposition process that converts metallurgical grade silicon to technical grade silicon.17,18 On a per battery, cradle-to-gate basis, the batteries that use graphite as the anode with LMO or LMR-NMC cathodes consume the least energy. In the case of the LMO-containing battery, this result is driven by the low energy intensity of producing LMO. LMR-NMC is about three times as energy intensive to produce as LMO (Fig. 1) but about 41% less of it is needed in the battery (when both batteries use graphite as the anode material) because its capacity is 250 mA h g−1, 2.5 times greater than that of LMO (Table S2†).10 Consequently, the cathode material contribution to total battery energy consumption when LMR-NMC is the cathode is just under double the cathode's contribution to total energy consumption of a battery. Energy consumed in LCO production with an HT preparation step causes the cradle-to-gate energy consumption to be nearly 7000 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MJ per battery (34–72%) higher than the other battery chemistries we considered.
000 MJ per battery (34–72%) higher than the other battery chemistries we considered.
The role of battery recycling
Production of cathodes, especially those with cobalt and nickel, is a key driver of lithium-ion battery cradle-to-gate impacts (energy, GHG emissions, SOx emissions) when the assembly energy reflects at-capacity assembly. If battery recycling can recover cathodes at a lower emissions and energy intensity than producing virgin cathode materials, it is important to pursue battery recycling. Other considerations in favour of battery recycling include solid waste reduction and material scarcity concerns. Lithium supplies are sufficient even under a high-demand scenario with large-scale EV deployment world-wide.19 Current trends in cathode chemistries are moving away from cobalt and nickel because they are expensive. If this trend continues, existing cobalt and nickel supplies could be sufficient.19 (In addition to being recycled, batteries could also be used in alternative second-life applications such as grid-level storage,20,21 but analysis of this latter scenario is outside the scope of this paper).We have previously examined the role of battery recycling in reducing impacts of overall battery production (with an LMO cathode), including the use of aluminium and copper recovered through battery recycling in a closed-loop scenario.4 We estimated that whole-battery GHG emissions could be up to 50% less when batteries used recycled cathode, aluminium, and copper as compared to batteries using entirely virgin materials. Although it is still true that little information about battery recycling is in the public domain and that recycling of automotive lithium-ion batteries is still in its infancy, we have extended this analysis beyond batteries with LMO cathodes to investigate potential GHG and SOx benefits of recovering cathode materials from batteries with all the cathodes in Fig. 1. In this case, we quantified potential reductions in these emissions from recycling of the cathode material only, not from recovery of any other battery constituents to be conservative.
The recycling processes we considered in this analysis include a pyrometallurgical process modelled after that of Umicore, an intermediate process modelled after the one used by Retriev Technologies (formerly Toxco), and a direct recycling process analogous to that of a process under development by Onto. We describe these processes and our development of their respective material and energy flows in an earlier report.22 Briefly, in the pyrometallurgical process, batteries are fed to a furnace where they are smelted. This process recovers an alloy of Co, Cu, Ni, and Fe. Lithium, however, exits the furnace in a slag, along with aluminum. Lithium's recovery is not economically viable. The four-metal alloy proceeds through a series of leaching, solvent extraction, and purification stages to yield Cu, Fe, and Ni(OH)2. LCO can be reproduced after CoO undergoes oxidation and firing. It is only for this cathode that we quantified the potential benefits of battery recycling with the pyrometallurgical process because cobalt recovery drives process economics in this case.
In the intermediate process, batteries are shredded, pass through a hammermill, and undergo a series of physical steps to separate battery components including mixed metals and, after some purification, Li2CO3. Finally, the direct process, which is still at the bench scale, aims to recover cathode material, electrolyte and metals. The process targets recovery of LCO after limited relithiation, but can also be used to recover other cathode materials or precursors.
To estimate the potential benefits of recovering the metals in cathode materials through recycling, we calculated the energy and material intensity of recovering cobalt as LCO from the pyrometallurgical process. We assumed that Co is recovered as a salt from the intermediate process, which then re-enters the supply chain for NCM or LMR-NMC. We also assumed the intermediate process could recover Li2CO3 that could replace virgin Li2CO3 in the supply chain of LMO and LMR-NMC. Finally, we assumed that each cathode material was recoverable from the direct recycling process with limited additional processing. It is important to note that our estimates are based on basic engineering calculations and that recycled cathode materials may require additional processing to achieve identical performance to virgin material-derived cathode materials. Nonetheless, these estimates inform the question of whether it could be possible to derive energy and environmental benefits from battery recycling.
Fig. 4 displays the GHG and SOx reductions for different cathode materials produced from recycled compounds as described above as compared to producing them from virgin materials (Fig. 1). In the case of the commercial pyrometallurgical process that we analyze only for the case of LCO-containing batteries, GHG reductions for producing the cathode material could be between 60–75%. If recycled LCO were incorporated into automotive batteries that would have used virgin LCO prepared hydrothermally, the cathode material contribution to overall battery GHG intensity would decline from 57% to 25% and overall battery GHG intensity would decline by 43%. Importantly, the SOx intensity of recycled LCO is nearly 100% lower than from production of virgin LCO. This significant SOx reduction holds true even for the energy-intensive pyrometallurgical process because the SOx-intensive smelting step that occurs during recovery of virgin Co is completely avoided.
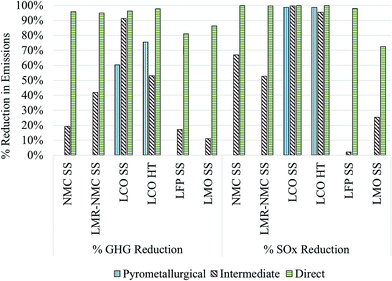 | ||
| Fig. 4 GHG and SOx reductions for different cathode materials recovered from pyrometallurgical, indirect physical, and direct physical as compared to producing them from virgin materials. | ||
GHG emissions from production of different cathode materials drop by between 11% and 91% when Co and Li2CO3 are recovered with the intermediate recycling process and integrated into cathode material supply chains. Li2CO3 is used in the supply chains of LMR-NMC, LFP (SS), and LCO (SS). Using these recycled components in production of LCO has the potential to significantly decrease SOx emissions. Overall, SOx emissions are low for the production of LMO and LFP (SS) cathode materials. Production of LMO consumes more Li2CO3 than the production of LFP10,22 and therefore the drop in the Li2CO3 SOx intensity is more noticeable for LMO than for LFP. Finally, the direct recycling process has the potential to reduce GHG and SOx emissions from cathode material production by 81–98% and 72% to nearly 100%, respectively. To reiterate, these estimates of the GHG and SOx benefits of battery recycling should be regarded as indicators of recycling's potential to decrease automotive lithium ion batteries' environmental burdens rather than as absolute results. They indicate that, especially for cobalt- and nickel-containing cathode materials, recycled cathode materials are likely less GHG- and SOx-intensive than cathode materials produced from scratch. In the case of the least energy- and GHG-intensive cathode material to produce, LMO, using recycled cathode material from the intermediate and direct processes could reduce overall battery GHG emissions by 2% and 16%, respectively. Emissions reductions for overall battery production can increase when Al and Cu are recovered and re-used.4 It is also important to keep in mind that SOx benefits of recycling cobalt-containing cathode materials are likely to increase as nickel–cobalt production shifts from sulphite ores to laterite ores16 as discussed in the next subsection along with the local effects of metals mining, which could be reduced through battery recycling.
Local impacts of metals production
In this section, we provide an overview of the local impacts of mining three metals that are key to cathode material production: nickel, cobalt, and lithium. Nuss and Eckelman23 provide a detailed analysis of the cradle-to-gate environmental burdens of producing 63 metals, including these three.As Mudd24 details for Canadian sulfide ore Ni production, while environmental impacts have improved over time, Ni mining has produced major historical local impacts: acid rain from SO2 emissions, heavy metal soil contamination, wetland acidification, biodiversity loss (especially in fish populations), vegetation die back, and soil erosion. Similar detrimental effects are reported for Russian sulfide ore mining areas.24 In Canada, modernization and process improvements have led to reduced lake damage, reduced SO2 emissions (hence, reduced acid rain), and improved fish stocks.
Despite the relative ease of recovering Ni from sulfide ores as compared to laterite ores, laterite ores are increasingly mined for Ni because sulfide ore mines are becoming less productive.24 Ni production from laterite ores is more complex, with greater variance in mining and purification techniques depending upon the laterite ore type. An open cut process is typically used because most laterite ores are at shallow depths. Then a beneficiation step is generally required before drying or calcining to reduce the high moisture content of the ores. Following that step, ores are typically treated with a rotary kiln electric furnace, a Caron ammonia leach process, or high pressure acid leaching (HPAL) depending on the laterite ore type.24 The most significant impact of the Caron process is the energy consumption during the drying stage; the ammonia used in this process is generally recovered. The sulfuric acid used in the HPAL process is neutralized after it is used to leach Ni and is typically not regenerated. Laterite-produced Ni is roughly 2.5–5.7 times more energy intensive, and approximately 2.5–4.6 times more GHG intensive than its sulfide-derived counterpart.24 SO2 emissions do not follow the same ore-based trend because several mines of different types have effectively implemented pollution controls, and many others show a year-over-year reduction in emissions per tonne Ni.
Laterite ore Ni mining has historically taken place in New Caledonia, an island in the South Pacific Ocean. Garcin et al.25 suggest that this mining is responsible for the creation of bare soil, influencing coastline changes over the past 50 years. Research indicates that mining efforts have caused large-scale contamination of anguilliform fish (moray eels and congers) and coral reefs within this biodiversity hotspot.26,27 Newer laterite ore Ni mines could pose similar environmental risks. As more Ni is sourced from laterite ores in places such as Australia, Colombia, Cuba, and Indonesia, energy consumption and GHG emissions associated with Ni production will increase.
The downward pressure on sulfide ore grades over time24 has caused them to be less economically viable than they have historically been. Laterite ore grades are also declining. This reduction in Ni ore grades necessitates larger investments of energy (and subsequent SO2 and GHG emissions) unless nickel mines adopt more efficient, lower emitting technologies. Process efficiency improvements in both laterite and sulfide ore mining operations have substantially reduced pollutant emissions and energy use, but reduced ore grades could drive up the per-tonne-Ni intensity for both processes. The broader transition from sulfide to laterite ores will likely increase energy use and GHG emissions associated with nickel mining, while possibly reducing SO2 emissions. Downward trends in energy and SO2 intensity of Ni mining suggest the potential to reduce some local impacts of Ni production.24
The previously mentioned local impacts of Ni apply to Co.24,29 It is also important, however, to consider the local impacts of Cu–Co mines, which can have up to 10 times the annual Co production of Ni laterite mines. Not all Cu mining operations yield Co; only the local impacts of exclusively Cu–Co mines are examined here. Cu–Co mines are economically dominated by those in the Katangan Cu-belt of central Africa, which stretches across Zambia and the Democratic Republic of Congo and have produced 25–50% of the world's Co since the 1970s.29,33 The techniques for these mining operations typically include open cut or underground methods, with subsequent crushing, grinding, and flotation. Smelting and refining follow.29
Mining activity in the Katangan Cu-belt is less well documented than mining activity in other areas of the world, but Nriagu34 states broadly that African “[mining] operations rely on pollution prone technologies and the controls on the discharge of pollutants from African mines and smelters are lax or non-existent. The net result is that the air, water, soils and vegetation near the mining centers of Africa tend to be severely contaminated with toxic metals.” This statement seems to be supported by more recent biomonitoring studies from Banza, et al.28 More data are needed to evaluate the full nature and scope of the local impacts of Co.
Relative performance of EVs and conventional vehicles
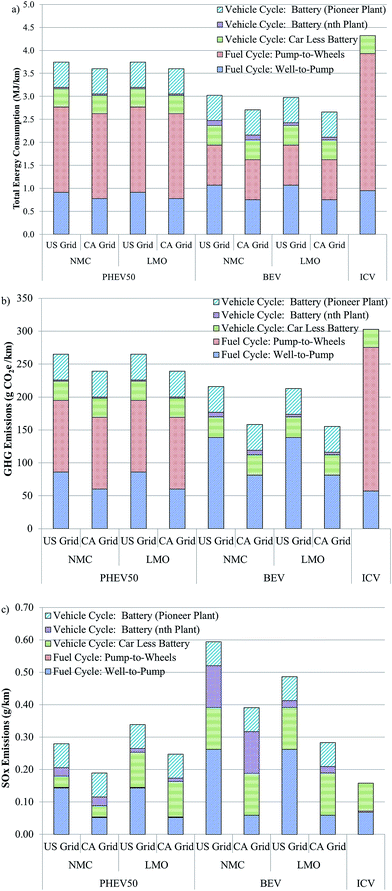
The energy and emissions intensity and local impacts of battery production from cradle-to-gate influence not only the weighing of the merits of battery recycling, but the performance of EVs as compared to ICVs. As mentioned earlier, several recent reports have questioned whether the production and operation of EVs is less energy and emissions intensive that the production and operation of ICVs. The GREET model can be used to address this question when vehicle cycle (manufacturing the vehicle and battery) and fuel cycle (producing and using electricity and/or gasoline) are combined as in Fig. 5.Fig. 5a compares the total energy consumption of plug-in hybrid vehicles (PHEV) with an approximately 50 km range with either an NMC or LMO cathode, of BEV with NMC or LMO cathode, and of an ICV. The PHEV50 could be considered similar to a Toyota Prius whereas the BEV could be considered akin to an electric Ford Focus. Battery parameters are reported in Tables S3–S6.† We selected a BEV battery with an NMC cathode material to represent a worst case scenario for BEVs. It is more likely to be used in a BEV than LCO (the most energy-intensive cathode material we examined) and its production is more energy intensive than cathodes that don't contain nickel or cobalt (Fig. 1). BEVs and PHEVs with LMO battery chemistry were also included as a best case scenario because LMO is the least energy-intensive cathode to produce of those we considered. We chose NMC as the second PHEV battery cathode material for this analysis because it is unlikely that LCO, the most energy intensive cathode material to produce, would be used in high-power applications.
Fig. 5 breaks down total energy consumption into four contributors. The first of these is the fuel cycle from well-to-pump (WTP). In the case of BEVs, this step includes generation of electricity reaching back in the supply chain to coal mining, natural gas extraction, and so forth to represent the full fuel cycle energy of providing electricity. In the case of the ICV, this step represents the full fuel cycle of gasoline going back to crude oil extraction. The PHEV WTP stage includes both electricity and gasoline production to the point of use. The PHEV50 is modelled as being in charge-depleting (CD) and charge-sustaining (CS) modes during 47.5% and 52.5% of operation, respectfully. The second category, pump-to-wheels (PTW), is the energy expended during vehicle use. The PHEV50 is assumed to have a fuel economy of 3.2 and 8.5 gasoline equivalent L per 100 km in CD and CS modes, respectively. The BEV range is assumed to be about 110 km. The BEV is assumed to have a fuel economy of 2.9 gasoline equivalent L per 100 km while the ICV operates at 1 L per 100 km. The liquid fuel used by the ICV and the PHEV50 during CS mode is conventional gasoline, 4% of which derives from oil sands recovered via in situ production.
Fig. 5 breaks the vehicle cycle into two categories. The first is the energy consumed in producing all of the vehicle but the battery. The battery category is subdivided so that it is possible to see the range of battery contribution to the total result, which depends on the choice of assembly energy in the analysis. From this figure it is possible to see that the total energy associated with ICV production and operation exceeds each EV scenario considered. If the higher battery assembly energy is adopted, production of the vehicle, including the battery, is about 2.5 times as energy intensive than producing the ICV. If, on the other hand, the battery assembly plants are assumed to operate at capacity, EV production is approximately 10–40% more energy intensive than producing ICVs. Operating an EV, however, consumes about 1.6–3.4 times less energy than an ICV on a per km basis.
A similar pattern is seen with the results for GHG emissions (Fig. 5b). A key feature of these results is that the BEV does not emit any GHGs during the PTW stage. Additionally, when a cleaner grid, such as the California grid (see Table S9† for contribution of different energy types to each grid mix considered) is used to charge the BEV, WTP emissions are low, reducing overall GHG emissions. The GHG emissions “debt” incurred during the vehicle production stage is paid back within the first 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km driven using the national average grid to charge the vehicle.
000 km driven using the national average grid to charge the vehicle.
A different picture emerges when SOx emissions are compared among these three vehicle types (Fig. 5c). First, WTP SOx emissions are higher for EVs that rely entirely or in part on electricity as a fuel as compared to ICVs. Second, PTW SOx emissions are low for both ICVs and PHEVs. They are zero for BEVs. Whereas vehicle cycle SOx emissions are somewhat comparable if the battery's contribution is ignored, its addition adds significant SOx emissions for PHEV and BEV alike, especially in the case of the higher assembly energy. It should be noted that the dry room energy consumption we estimated based on a vendor quote22 had about even contributions from natural gas and electricity consumption (natural gas is used to dry the desiccant wheel in this particular dry room design). It is this assembly energy that is used for the nth plant. The facility that Ellingsen et al. analysed, however, relied solely on electricity, which is more SOx-intensive than natural gas when we assume the U.S. grid supplies the electricity. Using GREET to incorporate Ellingsen et al.'s data for the pioneer plant, we assumed the assembly facility uses the U.S. grid, which is about 46% coal-fired. Therefore, using Ellingsen et al.'s energy intensity, which reflects the low throughput of the facility that provided the data these authors used, notably increases the SOx emissions in the battery assembly step. Compared to EVs with batteries produced in a pioneer plant, the SOx intensity of EVs with batteries produced in a high-throughput nth plant is about 10–60% lower on a g km−1 basis.
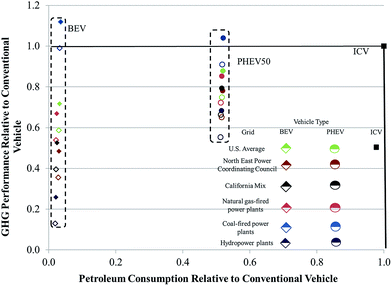
The source of electricity used to power EVs can significantly influence life-cycle GHG emissions and petroleum consumption of these vehicles.42,43 In Fig. 6, we examine the influence of electricity source on relative petroleum consumption and GHG emissions of a BEV with an NMC cathode and a PHEV50 with an LMO cathode as compared to an ICV. We include results that incorporated both assembly energies. If the relative energy consumed and GHGs emitted are less than one, they will fall inside the box bounded by unity on the abscissa and ordinate in the figure. Any result inside this box offers improved energy and GHG performance as compared to an ICV. The different power sources we incorporated were the US grid, the California grid, the Northeastern US grid, exclusively natural gas-fired power plants, exclusively coal-fired power plants, and hydropower. As might be expected, EVs charged with hydropower-sourced electricity performed the best. EVs reliant on electricity from coal-fired power plants performed the worst, with the only two results that illustrated worse GHG emissions than an ICV being a BEV and PHEV50 powered with electricity from a coal-fired power plant and manufactured in a pioneer plant. In general, however, BEVs offer higher reductions in petroleum usage as compared to PHEVs. BEVs operating on electricity from the CA grid, the NE US grid, and hydropower have lower GHG emissions than PHEVs. Undoubtedly, the electricity mix in the United States and internationally will continue to evolve with a corresponding influence on the energy and environmental impacts of EVs.44
A key message from this analysis is that electric vehicles, unless they power up with exclusively coal-derived electricity, have improved energy and GHG performance as compared to ICVs, regardless of whether the energy consumed during battery assembly is closer to our estimate or that of Ellingsen et al.
Conclusions
To reiterate, this analysis strove to address three key questions in automotive lithium-ion battery energy and environmental analysis: first, whether materials production or battery assembly drive these batteries' energy and environmental impacts; next, what motivates battery recycling if it is the assembly step that is the major energy consumer; finally, how the energy and environmental performance of EVs and ICVs compare.A key point in the assessment of the first question is the throughput of the battery assembly facility. Low-throughput facilities, which could be called pioneer plants, will have high energy intensities because some equipment, notably the energy-intensive dry room, likely consume the same amount of energy regardless of the number of batteries the plant is producing. As production increases with demand, energy intensity will decline. The proposed Tesla Gigafactory presumably will take advantage of lower energy intensity of battery assembly with its plans for a high-throughput facility in the United States.45 In this high-throughput, nth plant scenario, it is the materials production stage that drives battery impacts.
An important consideration in examining the second question is the local impacts of the recovery of metals in cathode materials' supply chains, including high SOx emissions. Battery recycling minimizes these impacts and can reduce the overall energy and emissions intensity of battery production, especially when assembly facilities operate at high capacity.
Finally, given today's knowledge about energy and emissions burdens associated with producing batteries, EVs offer petroleum consumption and GHG emissions savings as compared to ICVs. This result holds true even when assembly facilities operate at low capacity. The exceptions are when BEVs or PHEV50s power up with exclusively coal-derived electricity. SOx and other air emissions are of concern but can be reduced through battery recycling, using cleaner power sources in the battery supply chain and at the assembly plant, and controlling emissions from mines producing cathode metals.
It is important to continue to examine these critical issues in lithium-ion battery energy and environmental analysis and obtain improved data both for materials production and battery assembly stages.
Acknowledgements
The authors are very grateful to Michael Wang, Amgad Elgowainy, and Jeongwoo Han for helpful communication and discussions. This work was supported by the Vehicle Technologies Office of the Office of Energy Efficiency and Renewable Energy of the United States Department of Energy, under contract DE-AC02-06CH11357. We acknowledge Dave Howell of this office for support and guidance.References
- D. U. Eberle and D. R. von Helmolt, Energy Environ. Sci., 2010, 3, 689–699 Search PubMed.
- D. A. Notter, M. Gauch, R. Widmer, P. Wäger, A. Stamp, R. Zah and H.-J. Althaus, Environ. Sci. Technol., 2010, 44, 6550–6556 CrossRef CAS PubMed.
- G. Majeau-Bettez, T. R. Hawkins and A. H. Strømman, Environ. Sci. Technol., 2011, 45, 4548–4554 CrossRef CAS PubMed.
- J. B. Dunn, L. Gaines, J. Sullivan and M. Q. Wang, Environ. Sci. Technol., 2012, 46, 12704–12710 CrossRef CAS PubMed.
- T. R. Hawkins, B. Singh, G. Majeau-Bettez and A. H. Strømman, J. Ind. Ecol., 2013, 17, 53–64 CrossRef CAS PubMed.
- L. A. Ellingsen, G. Majeau-Bettez, B. Singh, A. K. Srivastava, L. O. Valøen and A. H. Strømman, J. Ind. Ecol., 2014, 18, 113–124 CrossRef CAS PubMed.
- B. Lomborg, Wall St. J., March 11, 2013, Section A, p. 15 Search PubMed.
- B. Li, X. Gao, J. Li and C. Yuan, Environ. Sci. Technol., 2014, 48, 3047–3055 CrossRef CAS PubMed.
- Argonne National Laboratory, BatPaC Model, 2011, http://www.cse.anl.gov/batpac.
- J. B. Dunn, C. James, L. Gaines and K. Gallagher, Material and Energy Flows in the Production of Cathode and Anode Materials for Lithium Ion Batteries, ANL/ESD/14/10, Argonne National Laboratory, 2014 Search PubMed.
- P. A. Nelson, K. G. Gallagher, I. Bloom and D. W. Dees, Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles, ANL-11/32, Argonne National Laboratory, 2011 Search PubMed.
- J. Jackson, presented at the 31st International Battery Seminar and Exhibit, Broward County, FL, 2014 Search PubMed.
- M. Zackrisson, L. Avellán and J. Orlenius, J. Cleaner Prod., 2010, 18, 1519–1529 CrossRef CAS PubMed.
- C. J. Rydh and B. A. Sandén, Energy Convers. Manage., 2005, 46, 1980–2000 CrossRef CAS PubMed.
- L. Ellingsen, personal communication, 2014.
- M. Anderman, presented at the National Academy of Sciences Committee on Overcoming Barriers to Electric Vehicle Deployment, Irvine, CA, 2014 Search PubMed.
- I. Boustead and G. F. Hancock, Handbook of industrial energy analysis, E. Horwood; Halsted Press, Chichester, Eng., New York, 1979 Search PubMed.
- M. J. deWilde-Scholten and E. A. Alsema, presented at the Materials Research Society Fall Meeting, Boston, MA, 2005 Search PubMed.
- L. Gaines and P. Nelson, presented at The Materials Society Annual Meeting and Exhibition, Seattle, WA, 2010 Search PubMed.
- J. Neubauer and A. Pesaran, J. Power Sources, 2011, 196, 10351–10358 CrossRef CAS PubMed.
- D. Strickland, L. Chittock, D. A. Stone, M. P. Foster and B. Price, IEEE Trans. Sustain. Energy, 2014, 5, 795–803 CrossRef.
- J. B. Dunn, L. Gaines, M. Barnes, J. Sullivan and M. Q. Wang, Material and Energy Flows in Materials Production, Assembly, and End-of-Life Stages of the Life Cycle of Lithium-Ion Batteries, ANL/ESD/12–3 Rev., Argonne National Laboratory, 2014 Search PubMed.
- P. Nuss and M. J. Eckelman, PLoS One, 2014, 9, e101298 Search PubMed.
- G. M. Mudd, Ore Geol. Rev., 2010, 38, 9–26 CrossRef PubMed.
- M. Garcin, A. Bails, G. Le Cozannet, T. Bulteau, A. Auboin and J. Sauter, J. Coastal Res., 2013, 65, 494–499 Search PubMed.
- X. Bonnet, M. J. Briand, F. Brischoux, Y. Letourneur, T. Fauvel and P. Bustamante, Sci. Total Environ., 2014, 470, 876–882 CrossRef PubMed.
- N. Myers, R. A. Mittermeier, C. G. Mittermeier, G. A. B. da Fonseca and J. Kent, Nature, 2000, 403, 853–858 CrossRef CAS PubMed.
- C. L. N. Banza, T. S. Nawrot, V. Haufroid, S. Decrée, T. De Putter, E. Smolders, B. I. Kabyla, O. N. Luboya, A. N. Ilunga, A. M. Mutombo and B. Nemery, Environ. Res., 2009, 109, 745–752 CrossRef CAS PubMed.
- G. M. Mudd, Z. Weng, S. M. Jowitt, I. D. Turnbull and T. E. Graedel, Ore Geol. Rev., 2013, 55, 87–98 CrossRef PubMed.
- A. H. Ahmed, S. Arai and M. Ikenne, Econ. Geol., 2009, 104, 249–266 CrossRef CAS.
- J. F. Slack, Econ. Geol., 2012, 107, 1089–1113 CrossRef CAS.
- E. M. Harper, G. Kavlak and T. E. Graedel, Environ. Sci. Technol., 2011, 46, 1079–1086 CrossRef PubMed.
- C. G. Smith, Trans. Inst. Min. Metall., Sect. B, 2001, 110, 75–80 Search PubMed.
- J. O. Nriagu, Sci. Total Environ., 1992, 121, 1–37 CrossRef CAS.
- B. W. Jaskula, 2012 Minerals Yearbook-Lithium, U.S. Department of the Interior, U.S. Geological Survey, Reston, Virginia, 2013 Search PubMed.
- A. Stamp, D. J. Lang and P. A. Wäger, J. Cleaner Prod., 2012, 23, 104–112 CrossRef CAS PubMed.
- D. Kushnir and B. A. Sandén, Resour. Policy, 2012, 37, 93–103 CrossRef PubMed.
- A. Ebensperger, P. Maxwell and C. Moscoso, Resour. Policy, 2005, 30, 218–231 CrossRef PubMed.
- R. A. Boelens and H. de Vos, Cult. Surviv. Q, 2006, 29, 18–21 Search PubMed.
- R. Boelens and M. Zwarteveen, Dev. Change, 2005, 36, 735–758 CrossRef PubMed.
- T. C. Wanger, Conserv. Lett., 2011, 4, 202–206 CrossRef PubMed.
- R. Edwards, J. Larive, D. Rickeard and W. Weindorf, Well-to-Tank Report Version 4.a JRC Well-to-Wheels Analysis, Ispra, Italy, 2014 Search PubMed.
- A. Elgowainy, J. Han, L. Poch, M. Wang, A. Vyas, M. Mahalik and A. Rousseau, Well-to-Wheels Analysis of Energy Use and Greenhouse Gas Emissions of Plug-In Hybrid Electric Vehicles, ANL/ESD/10–1, Argonne National Laboratory, 2010 Search PubMed.
- N. Armaroli and V. Balzani, Energy Environ. Sci., 2011, 4, 3193–3222 Search PubMed.
- M. Maynard, Forbes, July 31, 2014 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ee03029j |
| This journal is © The Royal Society of Chemistry 2015 |