Advances in f-element cyanide chemistry
Abstract
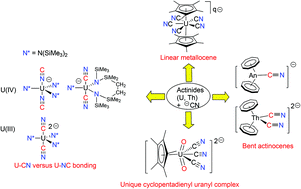
This Dalton perspective gives an overview of the development of cyanide chemistry of 4f- and 5f-elements, a field which was poorly explored in contrast to the attention paid to the cyanide complexes of the d transition metals. The use of the cyanide ligand led to the discovery of mono- and polycyanide complexes which exhibit unprecedented and unexpected coordination geometries. A new type of linear metallocenes including [U(Cp*)2(CN)5]3− (Cp* = C5Me5) and the first bent actinocenes [An(Cot)2(CN)]− (An = Th, U; Cot = C8H8) were isolated. Thorocene was found to be much more reactive than uranocene since a series of sterically crowded cyanide complexes have been obtained only from [Th(Cot)2]. A series of cyanido-bridged dinuclear compounds and mononuclear mono-, bis- and tris(cyanide) complexes were prepared by addition of cyanide salts to [MN*3] (M = Ce, U) and [UN*3]+ [N* = N(SiMe3)2]. The CeIII, UIII and UIV ions were clearly differentiated in these reactions by cyanide linkage isomerism, as shown for example by the structures of the cyanide complex [UIIIN*3(CN)2]2− and of the isocyanide derivatives [CeIIIN*3(NC)2]2− and [UIVN*3(NC)]−. While the U–CN/NC coordination preference towards the UIII/UIV pair is related to the subtle balance between steric, covalent and ionic factors, DFT computations and in particular the calculated total bonding energies between the metal and the cyanide ligand allowed the observed coordination mode to be predicted. The ability of the cyanide ligand to stabilize the high oxidation states was assessed with the synthesis of UV and UVI complexes in the inorganic and organometallic series.

 Please wait while we load your content...
Please wait while we load your content...