Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereoselective synthesis of glycosides using (salen)Co catalysts as promoters†
Sandra
Medina
,
Alexander S.
Henderson
,
John F.
Bower
and
M. Carmen
Galan
*
School of Chemistry, University of Bristol, Cantock's Close, Bristol BS8 1TS, UK. E-mail: m.c.galan@bris.ac.uk
First published on 23rd April 2015
Abstract
The use of (salen)Co catalysts as a new class of bench-stable stereoselective glycosylation promoters of trichloroacetimidate glycosyl donors at room temperature is described. The conditions are practical and do not require the use of molecular sieves with products being isolated in good to high yields.
Access to structurally defined oligosaccharides in sufficient amounts to be used as probes for biological research, still represents a significant challenge due to the structural complexity of carbohydrates. The stereoselective coupling of glycosides is often considered the main bottleneck towards this goal and despite significant efforts devoted to the development of efficient glycosylation strategies, there is still a need for robust and practical methods to achieve this crucial step.1
Transition metal catalysis applied to oligosaccharide synthesis offers great promise at achieving high yields and anomeric selectivities by the careful choice of the metal and ligands attached to the metal centre.2
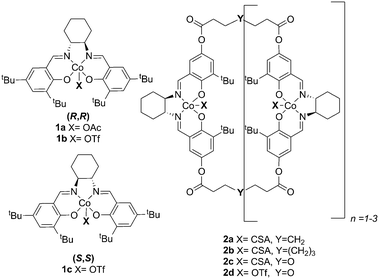
As part of our ongoing interest in developing catalytic methods for the stereoselective synthesis of glycosides,3 we decided to focus our attention on the use of transition metal complexes in glycosylation reactions. Chiral (salen)Co(III) complexes have been developed by the Jacobsen team for the elegant preparation of both enantiopure terminal epoxides and highly enantioenriched 1,2-diols.4 The (salen)Co catalysed asymmetric epoxide ring-opening reactions proceed via cooperative bimetallic mechanisms, whereby both the epoxide electrophile and nucleophile are activated by separate (salen)Co complexes in the rate-limiting ring opening step.5 We envisioned that these chiral Co complexes would be applicable to glycosylation reactions where an electrophile glycosyl donor reacts with the corresponding nucleophile glycoside acceptor and could provide a more efficient glycosylation methodology than currently available processes.
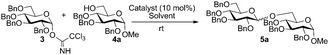
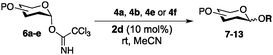
Anomeric trichloroacetimidates are among the most effective and commonly used glycosyl donors in the chemical synthesis of oligosaccharides.6 These type of glycosides are activated by Lewis acids such as TMSOTf,7 boron trifluoride etherate8 or lithium triflate,9 ionic liquids,3a,b,10 transition metals11 and organocatalysts12 among others. However, many of the strategies developed thus far require the use of strict anhydrous conditions/molecular sieves and low temperatures, and suffer from additional problems associated with the stability and reactivity of the promoters.6a Herein we report the application of chiral oligomeric (salen)Co complexes as bench-stable stereoselective glycosylation promoters of trichloroacetimidate glycoside donors.
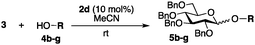
In our initial studies, perbenzylated glucose trichloroacetimidate 3 was employed as the model glycoside donor in reactions with glycoside acceptor 4a (Fig. 1 and Table 1). No reaction was observed when monomeric (salen)Co complex 1a (10 mol%) was used as the catalyst (Table 1, entry 1), however when triflate (−OTf, 1b) was used as the counter ion, the reaction proceeded to give the desired glycosylation product 5a, albeit slowly (22 h), in 89% yield with no stereocontrol (entry 2). Changing the catalyst to the opposite enantiomer 1c did not yield any significant changes in yield or stereo-outcome of the reaction. Based on these results, we decided to move onto the cyclic, oligomeric linked C2 symmetric (salen)Co catalysts, which have been shown to be more reactive than their monomeric counterparts and able to provide better stereocontrol in some cases.4c,d,f Encouragingly, reactions in the presence of 10 mol% of 1st generation oligomeric (salen)Co catalyst 2a4c in dichloromethane, afforded the product in 64% yield and with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 α
2.6 α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β anomeric ratio after 24 h (entry 4). Extending the linker chain by two carbon units as in azelate-linked 2b4d resulted in an inactive catalyst (entry 5). However, introduction of an oxygen atom in the linker while maintaining the linker length (2cvs.2a) yielded 5 in 70% isolated yield and in a 1
β anomeric ratio after 24 h (entry 4). Extending the linker chain by two carbon units as in azelate-linked 2b4d resulted in an inactive catalyst (entry 5). However, introduction of an oxygen atom in the linker while maintaining the linker length (2cvs.2a) yielded 5 in 70% isolated yield and in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 α
2.6 α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β ratio (Table 1, entry 6). It has been shown that small changes in the oligomeric catalyst structure can have a noticeable effect on the range of reactive conformations available4d and thus we attribute the lack of reactivity of 2b to the added flexibility that this catalyst possesses in comparison to the other oligomeric ones tested. Remarkably, when oligomeric Co catalyst 2d, which bears a triflate (−OTf) as the counter ion instead of (1S)-(+)-10-camphorsulfonate (CSA), was used, the reaction proceeded faster (under 30 min.) and afforded the product in 87% yield and with similar levels of anomeric stereocontrol as observed with the other active oligomeric catalysts (Table 1, entry 7).13 Furthermore, lowering the catalyst (2d) loading to 5 or 2 mol% was detrimental to the reaction yield and stereoselectivity (Table 1, entries 8 and 9). It is noteworthy that molecular sieves, which are typically used in glycosylation reactions to remove traces of water, were not needed. Next, we decided to explore solvent effects. Reactions using either THF or toluene using 10 mol% of 2d afforded low yields and anomeric mixtures of glycosylated products (Table 1, entries 10 and 11). Stereoselectivity was improved towards the β-anomer when acetonitrile was used as the solvent, and 5a was isolated in 92% yield as a 1
β ratio (Table 1, entry 6). It has been shown that small changes in the oligomeric catalyst structure can have a noticeable effect on the range of reactive conformations available4d and thus we attribute the lack of reactivity of 2b to the added flexibility that this catalyst possesses in comparison to the other oligomeric ones tested. Remarkably, when oligomeric Co catalyst 2d, which bears a triflate (−OTf) as the counter ion instead of (1S)-(+)-10-camphorsulfonate (CSA), was used, the reaction proceeded faster (under 30 min.) and afforded the product in 87% yield and with similar levels of anomeric stereocontrol as observed with the other active oligomeric catalysts (Table 1, entry 7).13 Furthermore, lowering the catalyst (2d) loading to 5 or 2 mol% was detrimental to the reaction yield and stereoselectivity (Table 1, entries 8 and 9). It is noteworthy that molecular sieves, which are typically used in glycosylation reactions to remove traces of water, were not needed. Next, we decided to explore solvent effects. Reactions using either THF or toluene using 10 mol% of 2d afforded low yields and anomeric mixtures of glycosylated products (Table 1, entries 10 and 11). Stereoselectivity was improved towards the β-anomer when acetonitrile was used as the solvent, and 5a was isolated in 92% yield as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 α
4 α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β ratio. On the other hand, when diethyl ether was employed, the α-product was favoured and the desired disaccharide was isolated in 96% as a 2.6
β ratio. On the other hand, when diethyl ether was employed, the α-product was favoured and the desired disaccharide was isolated in 96% as a 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 α
1 α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β mixture (Table 1, entries 12 and 13).
β mixture (Table 1, entries 12 and 13).
| Entry | Catalyst | Solvent | T (h) | Yield (%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βa βa |
|---|---|---|---|---|---|
| a Measured by 1H NMR unless stated otherwise. b 2 mol% catalyst loading. c 5 mol% catalyst loading. d Starting material recovered. e Product isolated mixed with hydrolyzed starting materials. n/o = not observed. | |||||
| 1 | 1a | CH2Cl2 | 24 | n/o | n/o |
| 2 | 1b | CH2Cl2 | 22 | 89 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
| 3 | 1c | CH2Cl2 | 0.5 | 74 | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 2a | CH2Cl2 | 24 | 64 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6 |
| 5 | 2b | CH2Cl2 | 24 | n/o | n/o |
| 6 | 2c | CH2Cl2 | 22 | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6 |
| 7 | 2d | CH2Cl2 | 0.5 | 89 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6 |
| 8 | 2d | CH2Cl2 | 1 | n/o | n/o |
| 9 | 2d | CH2Cl2 | 1 | 70d | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 2d | THF | 1 | <50e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 2d | Toluene | 1 | <50e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 2d | MeCN | 1 | 92 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 13 | 2d | Diethyl ether | 1 | 96 | 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
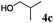
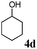
Having established optimal reaction conditions, we turned our attention to exploring the acceptor scope of the reaction. To that end, glycosyl donor 3 was reacted with a range of OH nucleophiles (Table 2). In all cases, reactions proceeded smoothly within 1 h. Glycosylations with aliphatic alcohols (4b–d) led to the expected products in high yields (78–94%) and anomeric ratios ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9 to 1
4.9 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.3 α
5.3 α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
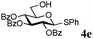
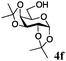
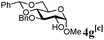
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β (Table 2, entries 1–3). Glycosyl acceptors with a primary or secondary alcohol and bearing either benzyl, benzoyl or acetal protecting groups, and either methoxy or thiophenyl as the anomeric substituent (4e–g) were also screened and gave isolated yields of 74–85%, with anomeric ratios that range from 1
β (Table 2, entries 1–3). Glycosyl acceptors with a primary or secondary alcohol and bearing either benzyl, benzoyl or acetal protecting groups, and either methoxy or thiophenyl as the anomeric substituent (4e–g) were also screened and gave isolated yields of 74–85%, with anomeric ratios that range from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.3 to complete α-selectivity (Table 2, entries 4–6). These results highlight that oligomeric salen(Co) catalyst 2d is tolerant of most commonly used alcohol functional groups and that the reactivity is independent of the position of the free hydroxyl on the acceptor. Furthermore, the reaction conditions are semi-orthogonal to thioglycoside chemistry (Table 2, entry 4), making this reagent suitable for chemoselective glycosylations where a trichloroacetimidate glycosyl donor can be selectively activated in the presence of a thioglycoside building block.
4.3 to complete α-selectivity (Table 2, entries 4–6). These results highlight that oligomeric salen(Co) catalyst 2d is tolerant of most commonly used alcohol functional groups and that the reactivity is independent of the position of the free hydroxyl on the acceptor. Furthermore, the reaction conditions are semi-orthogonal to thioglycoside chemistry (Table 2, entry 4), making this reagent suitable for chemoselective glycosylations where a trichloroacetimidate glycosyl donor can be selectively activated in the presence of a thioglycoside building block.
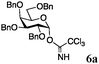
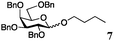
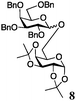
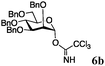
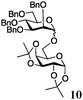
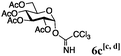
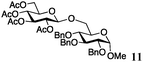
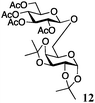
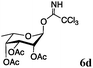
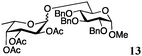
Encouraged by the acceptor scope of the catalyst, glycosylations with other common glycoside donors were also investigated. Reactions of perbenzylated galactose 6a with either 4b or 4f led to the desired products 7 and 8 in 82% and 90% yields with good to moderate anomeric β-selectivities (Table 3, entries 1 and 2). When activated mannose 6b was reacted with 4e or 4f as the nucleophiles, the corresponding disaccharides 9 and 10 were isolated in 60% and 62% yields with reasonable to moderate α-selectivity, respectively (Table 3, entries 3 and 4). Reactions involving “disarmed” donor 6c with 4a or 4f were less efficient and proceeded in modest yields, albeit with complete β-stereocontrol as expected from less reactive glycosyl donors bearing a participating group at C-214 (Table 3, entries 5 and 6). 6-Deoxyglycosides are also an important class of compounds often found as conjugates of natural products. Moreover, the stereoselective synthesis of this type of glycans is further complicated by the lack of oxygen substituents at C-6.15 Coupling between fucosyl trichloroacetimidate 6d with 4a proceeded in good yields and the corresponding disaccharide 13 was isolated in 83% yield with a preference for the β-product (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9 α
4.9 α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) (Table 3, entry 7).
β) (Table 3, entry 7).
In conclusion, we have described the application of oligomeric salen(Co) catalysts as a new class of stereoselective promoters in glycosylation reactions involving trichloroacetimidate glycosyl donors. The bench-stable catalyst can be easily prepared in multigram quantities from inexpensive and commercially available starting materials. The reactions proceed cleanly and in good to excellent yields at room temperature and without the need for molecular sieves. Furthermore, the conditions are practical, mild and applicable to different types of glycosyl donors and acceptors, tolerant of most common hydroxyl protecting groups (e.g. acetates, benzoates, alkyl and benzyl ethers and acetals) and semi-orthogonal to thioglycoside chemistry. The robustness of the catalyst and ease of use in often troublesome coupling reactions, makes this class of catalyst a promising synthetic tool for diastereoslective acetal chemistry.
MCG thanks the EPSRC EP/L001926/1 CAF and EP/J002542/1 (MCG, SM) for funding. ASH thanks EPSRC Bristol Chemical Synthesis CDT EP/G036764/1 and JFB thanks the Royal Society for a University Research Fellowship.
Notes and references
- (a) M. C. Galan, D. Benito-Alifonso and G. M. Watt, Org. Biomol. Chem., 2011, 9, 3598 RSC; (b) T. Nokami, J. Synth. Org. Chem., Jpn., 2014, 72, 797 CrossRef CAS.
- M. J. McKay and H. M. Nguyen, ACS Catal., 2012, 2, 1563 CrossRef CAS PubMed.
- (a) M. C. Galan, C. Brunet and M. Fuensanta, Tetrahedron Lett., 2009, 50, 442 CrossRef CAS PubMed; (b) M. C. Galan, A. T. Tran and S. Whitaker, Chem. Commun., 2010, 46, 2106 RSC; (c) E. I. Balmond, D. M. Coe, M. C. Galan and E. M. McGarrigle, Angew. Chem., Int. Ed., 2012, 51, 9152 CrossRef CAS PubMed; (d) E. I. Balmond, M. C. Galan and E. M. McGarrigle, Synlett, 2013, 2335 CAS; (e) E. I. Balmond, D. Benito-Alifonso, D. M. Coe, R. W. Alder, E. M. McGarrigle and M. C. Galan, Angew. Chem., Int. Ed., 2014, 53, 8190 CrossRef CAS PubMed.
- (a) E. N. Jacobsen, Acc. Chem. Res., 2000, 33, 421 CrossRef CAS PubMed; (b) R. G. Konsler, J. Karl and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 10780 CrossRef CAS; (c) J. M. Ready and E. N. Jacobsen, J. Am. Chem. Soc., 2001, 123, 2687 CrossRef CAS; (d) J. M. Ready and E. N. Jacobsen, Angew. Chem., Int. Ed., 2002, 41, 1374 CrossRef CAS; (e) S. E. Schaus, B. D. Brandes, J. F. Larrow, M. Tokunaga, K. B. Hansen, A. E. Gould, M. E. Furrow and E. N. Jacobsen, J. Am. Chem. Soc., 2002, 124, 1307 CrossRef CAS PubMed; (f) D. E. White, P. M. Tadross, Z. Lu and E. N. Jacobsen, Tetrahedron, 2014, 70, 4165 CrossRef CAS PubMed.
- D. D. Ford, L. P. C. Nielsen, S. J. Zuend, C. B. Musgrave and E. N. Jacobsen, J. Am. Chem. Soc., 2013, 135, 15595 CrossRef CAS PubMed.
- (a) X. M. Zhu and R. R. Schmidt, Angew. Chem., Int. Ed., 2009, 48, 1900 CrossRef CAS PubMed; (b) S. C. Ranade and A. V. Demchenko, J. Carbohydr. Chem., 2013, 32, 1 CrossRef CAS.
- R. R. Schmidt, Angew. Chem., Int. Ed., 1986, 25, 212 CrossRef PubMed.
- R. R. Schmidt and J. Michel, Angew. Chem., Int. Ed., 1980, 19, 731 CrossRef PubMed.
- A. Lubineau and B. Drouillat, J. Carbohydr. Chem., 1997, 16, 1179 CrossRef CAS.
- (a) A. Rencurosi, L. Lay, G. Russo, E. Caneva and L. Poletti, J. Org. Chem., 2005, 70, 7765 CrossRef CAS PubMed; (b) M. C. Galan, A. T. Tran, J. Boisson, D. Benito, C. Butts, J. Eastoe and P. Brown, J. Carbohydr. Chem., 2011, 30, 486 CrossRef CAS; (c) M. C. Galan, R. A. Jones and A. T. Tran, Carbohydr. Res., 2013, 375, 35 CrossRef CAS PubMed.
- (a) J. M. Yang, C. Cooper-Vanosdell, E. A. Mensah and H. M. Nguyen, J. Org. Chem., 2008, 73, 794 CrossRef CAS PubMed; (b) E. A. Mensah, J. A. Azzarelli and H. M. Nguyen, J. Org. Chem., 2009, 74, 1650 CrossRef CAS PubMed; (c) M. J. Mckay, B. D. Naab, G. J. Mercer and H. M. Nguyen, J. Org. Chem., 2009, 74, 4705 CrossRef CAS PubMed; (d) E. A. Mensah and H. M. Nguyen, J. Am. Chem. Soc., 2009, 131, 8778 CrossRef CAS PubMed; (e) E. A. Mensah, F. Yu and H. M. Nguyen, J. Am. Chem. Soc., 2010, 132, 14288 CrossRef CAS PubMed.
- Y. Q. Geng, A. Kumar, H. M. Faidallah, H. A. Albar, I. A. Mhkalid and R. R. Schmidt, Angew. Chem., Int. Ed., 2013, 52, 10089 CrossRef CAS PubMed.
- All starting material was consumed within 1 h when the reaction was carried out in the presence of 2,6-di-tert-butylpyridine as an acid scavenger, suggesting that TfOH is not the source of catalysis. However, the addition of the base was detrimental to product yield and diastereocontrol.
- B. Fraser-Reid and J. C. Lopez, Armed-Disarmed Effects in Carbohydrate Chemistry: History, Synthetic and Mechanistic Studies, Springer Berlin Heidelberg, 2011, vol. 301 Search PubMed.
- D. Hou and T. L. Lowary, J. Org. Chem., 2009, 74, 2278 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, data and NMR spectra. See DOI: 10.1039/c5cc02552d |
| This journal is © The Royal Society of Chemistry 2015 |