Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Indirect electroanalytical detection of phenols†
Athanasios V.
Kolliopoulos
,
Dimitrios K.
Kampouris
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Chemistry and the Environment, Division of Chemistry and Environmental Science, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, UK. E-mail: c.banks@mmu.ac.uk; Web: http://www.craigbanksresearch.com Fax: +44 (0)1612476831; Tel: +44 (0)1612471196
First published on 16th March 2015
Abstract
A novel indirect electrochemical protocol for the electroanalytical detection of phenols is presented for the first time. This methodology is demonstrated with the indirect determination of the target analytes phenol, 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol through an electrochemically adapted optical protocol. This electrochemical adaptation allows the determination of the above mentioned phenols without the use of any oxidising agents, as is the case in the optical method, where pyrazoline compounds (mediators) chemically react with the target phenols forming a quinoneimine product which is electrochemically active providing an indirect analytical signal to measure the target phenol(s). A range of commercially available pyrazoline substitution products, namely 4-dimethylaminoantipyrine, antipyrine, 3-methyl-1-(2-phenylethyl)-2-pyrazolin-5-one, 3-amino-1-(1-naphthylmethyl)-2-Pyrazolin-5-one, 4-amino-1,2-dimethyl-3-pentadecyl-3-pyrazolin-5-one hydrochloride, 3-amino-1-(2-amino-4-methylsulfonylphenyl)-2-pyrazolin-5-one hydrochloride and 4-aminoantipyrine are evaluated as mediators for the indirect detection of phenols. The indirect electrochemical detection of phenol, 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol through the use of 4-aminoantipyrine as a mediator are successfully determined in drinking water samples at analytically useful levels. Finally, the comparison of the direct (no mediator) and the proposed indirect determination (with 4-aminoantipyrine) towards the analytical detection of the target phenols in drinking water is presented. The limitation of the proposed electroanalytical protocol is quantified for all the four target phenols.
Introduction
Chlorinated phenols represent a group of commercially produced, substituted phenols and cresols referred to as chlorophenols and chlorocresols. Chlorinated phenols are used as intermediates in the synthesis of dyes, pigments, phenolic resins and pesticides.1 Certain chlorophenols are also used in flea repellents, fungicides, wood preservatives2 mould inhibitors, antiseptics, disinfectants and anti-gumming agents for gasoline.3 The chlorination of tap water may also produce chlorinated phenols;4 their analytical monitoring is without question.Electrochemical methods for the detection of phenolic compounds have been developed recently by using novel working electrodes such as pencil graphite based electrodes,5 boron-doped nanocrystalline diamond electrodes,6p-aminophenol-modified carbon nanotube paste electrode,7 platinum–polytyramine composite electrodes8 and ultrafine silica-PVA-tyrosinase fibers.9
An established optical analytical protocol involves the reaction of phenolic compounds with 4-aminoantipyrine, a pyrazoline substitution product, in the presence of potassium ferricyanide at a pH of 10 forms a stable reddish-brown coloured antipyrine dye.10 The colour intensity produced is a function of the concentration of the phenolic material;11,12 these dyes are measured spectrophotometrically at 510 nm and this protocol is the official analytical method for the determination of phenolic compounds in many countries13 and can be used over the linear range of 0.1 to 5 mg L−1. The proposed reaction is shown in Scheme 1 (step 1).10
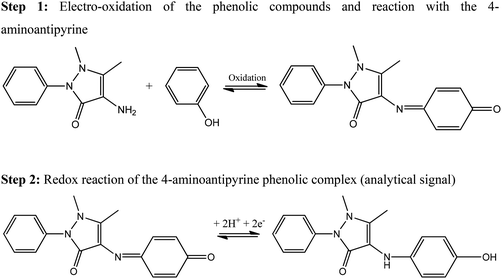 | ||
| Scheme 1 Possible reaction of 4-aminoantipyrine with the target phenol to produce the quinoneimine dye.25 In the colorimetric method, the dye is measured spectrophotometrically where the colour intensity is a function of concentration and the oxidising agent potassium ferricyanide is required; this chemically oxidises the target phenol to phenoxy radical which reacts with the 4-aminoantipyrine. In the electrochemically adapted optical protocol the phenol is oxidised electrochemically (step 1) and the dye is electrochemically active (step 2) which provides an indirect electroanalytical protocol for the electroanalytical sensing of phenols. | ||
Elsenstaed first proposed this optical method and studied the reactive limits of 69 phenols and 17 naphthols making the following conclusions:10
● There must be at least one free phenolic hydroxyl group in the molecule for a positive test; substituent's in the para position to the hydroxyl group prevent the reaction except as follows: halogen, carboxyl, sulfonic acid, hydroxyl and methoxyl. These groups are probably expelled;
● A nitro group in the ortho position prevents the reaction and a nitro group in meta position inhibits the colorimetric test, but not completely;
● Coupling of 4-aminoantipyrine with the phenol takes place in the para position rather than in the ortho position;
● When the para position is blocked by an alkyl, aryl, ester, nitro, benzoyl, nitroso, or aldehyde groups, no reaction takes place even if the ortho positions are un-substituted.
● Phenols in sewage determined by the 4-aminoantipyrine optical method are phenolic compounds in which there is no substituent in the para position (of the previous conclusion) to the hydroxyl group except for halogen, COOH, SO3H, OH, OCH3 groups.
This paper presents an electrochemical adaptation of the 4-aminoantipyrine optical methodology14,15 which avoids the use of an oxidising agent that is commonly included in the optical protocol; Scheme 1 displays the associated mechanism. This new proposed indirect electrochemical method is exemplified towards the electroanalytical determination of phenol, 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol. A range of pyrazoline substituted compounds are evaluated towards the detection of the target phenols. Finally, a direct comparison between the proposed indirect protocol with that of the direct electrochemical detection of the target phenols is presented to critically evaluate the two approaches.
Experimental
All chemicals used were obtained by Sigma Aldrich. Deionised water of resistivity 18.2 MΩ cm was used for the preparation of all solutions. All solutions were thoroughly degassed using high purity nitrogen gas prior to analysis. Voltammetric measurements were carried out using μ AUTOLAB Type III potentiostat by Metrohm Autolab B.V. Measurements were conducted utilising a three-electrode arrangement comprising a Boron-Doped Diamond Electrode (BDDE; 3 mm diameter, BAS, USA) with a platinum wire counter and a Saturated Calomel Electrode (SCE) as the reference electrode completing the circuit. All reference potential are reported relative to the SCE. The BDDE was thoroughly cleaned and polished with 1 micron and 0.25 micron diamond sprays before use. All the electrochemical measurements were obtained straight after the addition of the reagents.Note that in this work the concentration of the 4-aminoantipyrine (400 mg L−1) in this work was based on the optical method 9065 of United States Environmental Protection Agency (EPA). Throughout the paper we demonstrate proof-of-concept that the optical approach can be electrochemically adapted and use a constant amount of 4-aminoantipyrine (400 mg L−1). In fact as long as there is an excess of 4-aminoantipyrine in comparison to the levels of the target analyte, the amount of 4-Aminoantipyrine can be reduced as long as the observed current is substantially about that of the capacitive currents.
Drinking water was obtained from a drinking water tap (Manchester City Centre, UK) which was run for a minute before a sample being obtained. The sample was then stored at room temperature and used within a day of sampling. Prior to electroanalytical measurements the drinking water samples were simply modified to pH 10 with the addition of sodium hydroxide and spiked to obtain a concentration of 0.1 M potassium chloride serving as the electrolyte.
Results and discussion
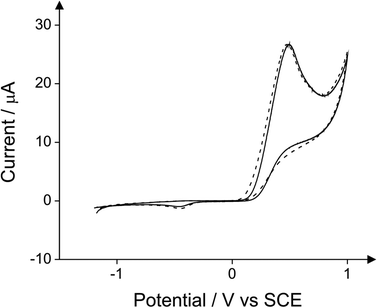
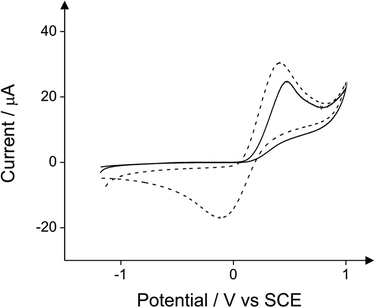
First, the cyclic voltammetry responses of seven different commercially available pyrazoline substituted compounds were explored towards the detection of 10 mg L−1 phenol in a pH 10 carbonate buffer solution using a boron-doped diamond electrode (BDDE). Pyrazolines are the five membered heterocyclic chemical compounds which have two adjacent nitrogen atoms within the ring and one endocyclic double bond.16 Fig. S1–S7† depict the responses of 4-dimethylaminoantipyrine, antipyrine, 3-methyl-1-(2-phenylethyl)-2-pyrazolin-5-one, 3-amino-1-(1-naphthylmethyl)-2-pyrazolin-5-one, 4-amino-1,2-dimethyl-3-pentadecyl-3-pyrazolin-5-one hydrochloride, 3-amino-1-(2-amino-4-methylsulfonylphenyl)-2-pyrazolin-5-one hydrochloride and 4-aminoantipyrine in the presence and absence of 10 mg L−1 phenol. It is evident that only 4-aminoantipyrine produces a new voltammetric peak which is a result of its chemical reaction with the target phenol producing an electrochemically active product producing the reduction peak observed at −0.5 V, as shown in Fig. S7;† this new voltammetric feature can provide the basis for the indirect electrochemical signal. For all the other compounds, no new voltammetric peaks are evident. Note the new peak which arises in Fig. S2† at +0.1 V is because of the direct reduction of phenol17 (see later in Fig. 8) and not of the product as a result of the reaction with the antipyrine. Antipyrine is the only pyrazoline of the seven studied here which does not have an amine group bonded with the pyrazoline ring. For this reason, it was not expected to react with the oxidised form of phenol producing the Schiff base in accordance with Scheme 1 (step 1).The analytical signal is due to the reduction of the quinoneimine dye produced by the coupling of 4-aminoantipyrine with the oxidised form of phenol. The reaction of the 4-aminoantipyrine with the target phenol requires first the oxidation of phenol to occur. For this purpose potassium ferricyanide is used in the standard optical method. Fig. 1 demonstrates that the use of ferricyanide ions at a concentration of 0.16 mg mL−1 does not optimise the analytical method. Additionally the potassium ferricyanide is electrochemically active and as it can be seen in Fig. 2, its reduction to ferrocyanide at high concentration (1.6 mg mL−1) induces a peak that overlaps with the analytical signal. In the proposed new methodology the oxidation of phenol occurs electrochemically alleviating the need of an oxidising agent. The electro-oxidation of phenol to phenoxy radical is shown in eqn (1):17–19
| C6H5OH → C6H5O˙ + H+ + e− | (1) |
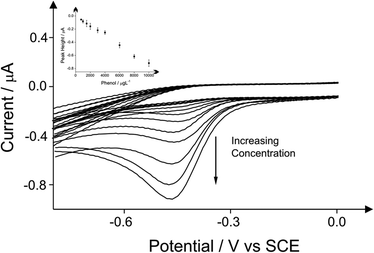
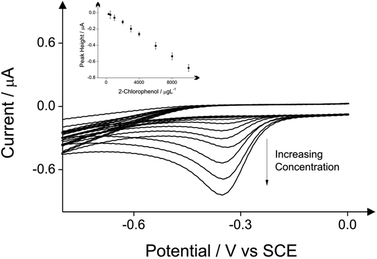
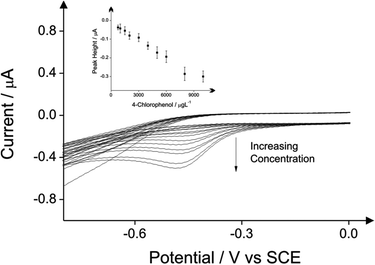
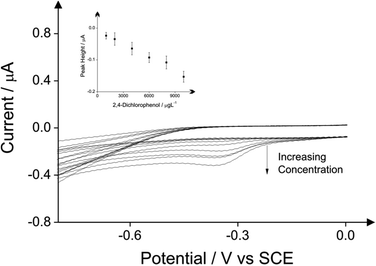
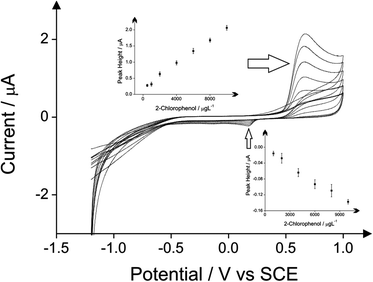
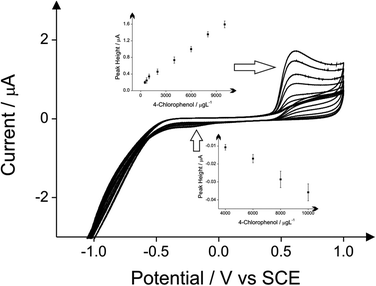
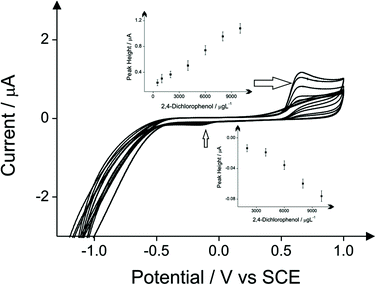
The detection of phenol and three chlorophenols, namely 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol in drinking water is the main subject of this investigation; consequently investigations focused on drinking water (tap) sample adjusted to pH 10 as this is the optimum pH for the electroanalytical protocol (see above). Additions of these target phenols into the drinking water samples was explored with corresponding voltammetric responses and calibration plots shown in Fig. 4–7. The limits of detection (LOD) for these four compounds with the proposed method using cyclic voltammetry on BDDE are 500 μg L−1, 300 μg L−1, 750 μg L−1 and 1000 μg L−1 respectively into drinking water (tap) sample adjusted to pH 10.
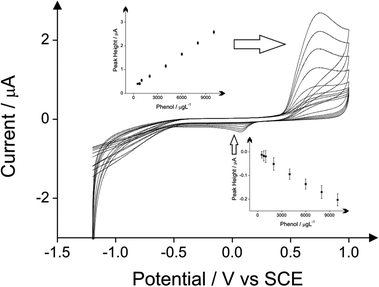
The relative high detection limits of the proposed electrochemical method with the 4-aminoantipyrine leads this work to consider the direct oxidation of phenol and chlorophenols, which is known to be attainable from inspection of the literature.18,20–23 Consequently voltammetric responses of the BDDE using the same experimental parameters without the addition of 4-aminoantipyrine were conducted for phenol, 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol. Analysis of the voltammetric response from this direct electroanalytical approach in the form of calibration plots for both the oxidation and reduction peaks of these four phenols are shown in Fig. 8–11.
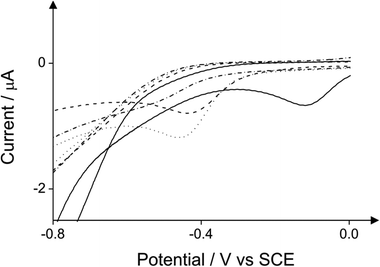
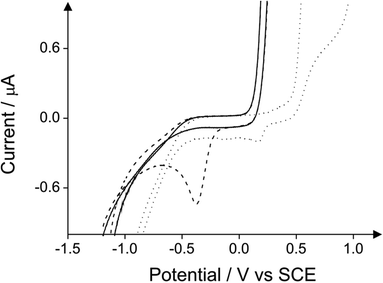
Fig. 12 depicts a comparison of the two reduction peaks in the case of 2-chlorophenol with and without 4-aminoantipyrine. In the presence of 4-aminoantipyrine the reduction peak prevails over the reduction peak arising from the direct reduction of 2-chlorophenol in the absence of 4-aminoantipyrine.
The comparison of the three analytical peaks for the electrochemical detection of phenols is presented in Tables 1–4 for each of the four phenols studied. The three analytical peaks refer to the oxidation peak of phenols without the addition of 4-aminoantipyrine, the reduction peak of the oxidised phenols without the addition of 4-aminoantipyrine and the reduction of the oxidised product of the reaction between the phenols and the 4-aminoantipyrine. All the peaks are responses obtained using a BDDE in drinking water adjusted pH 10, which was proved as the optimum electrode for this method based on literature reports due to its wide potential window, low background current and avoidance of passivation. These comparisons are based upon the lowest detection limits and the coefficient of determination that are possible. The potential where the voltammetric peaks appears is displayed as well in these tables. The direct reduction peaks of chlorophenols appear at more electronegative potentials than the direct reduction peak of phenol. This is probably due to the significant resonance stabilization of the chlorophenoxy radicals.24
| Limit of detection (μg L−1) | Peak position (V) of 10 mg L−1 phenol | R-Squared value | |
|---|---|---|---|
| Direct oxidation of phenol | 500 | 0.757 | 0.9973 (8 points) |
| Direct reduction of phenol | 500 | 0.082 | 0.9934 (8 points) |
| Indirect protocol | 500 | −0.465 | 0.9936 (9 points) |
| Limit of detection (μg L−1) | Peak position (V) of 10 mg L−1 2-chlorophenol | R-Squared value | |
|---|---|---|---|
| Direct oxidation of 2-chlorophenol | 500 | 0.669 | 0.9988 (8 points) |
| Direct reduction of 2-chlorophenol | 1000 | 0.172 | 0.9893 (6 points) |
| Indirect protocol | 300 | −0.352 | 0.9940 (9 points) |
| Limit of detection (μg L−1) | Peak position (V) of 10 mg L−1 4-chlorophenol | R-Squared value | |
|---|---|---|---|
| Direct oxidation of 4-chlorophenol | 500 | 0.589 | 0.9973 (8 points) |
| Direct reduction of 4-chlorophenol | 4000 | −0.179 | 0.9869 (4 points) |
| Indirect protocol | 750 | −0.474 | 0.9831 (10 points) |
| Limit of detection (μg L−1) | Peak position (V) of 10 mg L−1 2,4-dichlorophenol | R-Squared value | |
|---|---|---|---|
| Direct oxidation of 2,4-dichlorophenol | 500 | 0.654 | 0.9924 (7 points) |
| Direct reduction of 2,4-dichlorophenol | 2000 | −0.152 | 0.9650 (5 points) |
| Indirect protocol | 1000 | −0.358 | 0.9860 (6 points) |
In the case of phenol, the limit of detection of 500 μg L−1 is reached using all the three analytical peaks. The coefficient of the direct oxidation of phenol is relatively higher (see Table 1). In case of 2-chlorophenol the reduction peak of its product after its reaction with the 4-aminoantipyrine displays the lowest limit of detection which is 300 μg L−1 (see Table 2). In case of 4-chlorophenol the lowest detection limit of 500 μg L−1 can be reached when the direct oxidation peak is used as analytical peak (see Table 3). Finally the detection of 2,4-dichlorophenol by utilising again the direct oxidation peak as the analytical signal exhibits the lowest detection limit of 500 μg L−1 (see Table 4). Note that inspection of these tables and the potentials at which the indirect approach produces the new signal indicates that if all four phenols were present in solution, this indirect approach would unlikely allow their simultaneous detection due to overlapping peaks/signals and the whole phenolic concentration would not be able to be deduced; that said, the same is true for the optical approach.
Conclusions
The indirect determination of phenol, 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol by electrochemically adapting the 4-aminoantipyrine optical method is examined for the first time in drinking water. This electrochemical adaptation allows the determination of the phenol, 2-chlorophenol, 4-chlorophenol and 2,4-dichlorophenol with limits of detection of 500 μg L−1, 300 μg L−1, 750 μg L−1 and 1000 μg L−1 respectively without the use of an oxidant agent. Furthermore, other pyrazoline substitution products have been evaluated for the detection of phenol.Finally the comparison of the direct (without mediator) and the proposed indirect determination (with 4-aminoantipyrine) of phenol and the three chlorophenols is presented. The detection limits achieved in drinking water by the proposed electrochemical indirect method are lower in case of 2-chlorophenol but higher in case of phenol, 4-chlorophenol and 2,4-dichlorophenol in comparison to the data obtained when their direct oxidation peak is analysed. Such work then questions the need for the indirect electrochemical sensing protocol even with its advantage of alleviating the need for the oxidising agent. That said, as researchers extend this indirect methodology, there may well be cases where the indirect approach is advantageous over that of the direct electroanalytical sensing of phenols.
References
- D. Li, J. Park and J.-R. Oh, Anal. Chem., 2001, 73, 3089–3095 CrossRef CAS
.
- P. de Morais, T. Stoichev, M. C. P. Basto and M. T. S. D. Vasconcelos, Talanta, 2012, 89, 1–11 CrossRef CAS PubMed
.
- U.S.E.P.A. (EPA), 1980.
- M. Jin and Y. Yang, Anal. Chim. Acta, 2006, 566, 193–199 CrossRef CAS PubMed
.
- N. Vishnu and A. S. Kumar, Anal. Methods, 2015 10.1039/c4ay02798a
.
- A. F. Azevedo, F. A. Souza, J. T. Matsushima, M. R. Baldan and N. G. Ferreira, J. Electroanal. Chem., 2011, 658, 38–45 CrossRef CAS PubMed
.
- A. A. Ensafi, E. Heydari-Bafrooei and B. Rezaei, Chin. J. Catal., 2013, 34, 1768–1775 CrossRef CAS
.
- T. Spătaru and N. Spătaru, J. Hazard. Mater., 2010, 180, 777–780 CrossRef PubMed
.
- D. A. Oriero, I. O. Gyan, B. W. Bolshaw, I. F. Cheng and D. E. Aston, Microchem. J., 2015, 118, 166–175 CrossRef CAS PubMed
.
- Y. Cun-guang, J. Environ. Sci., 1998, 10, 76–86 Search PubMed
.
- The American Public Health Association, 16th edition, 1985 part 510.
- W. Frenzel, J. Oleksy-Frenzel and J. Mörlen, Anal. Chim. Acta, 1992, 261, 253–259 CrossRef CAS
.
- H.-q. Ni, J. Dong, J.-j. Shi and W. Wang, J. Sep. Sci., 2010, 33, 1356–1359 CAS
.
- R. J. Lacoste, S. H. Venable and J. C. Stone, Anal. Chem., 1959, 31, 1246–1249 CrossRef CAS
.
- M. Ettinger, C. Ruchhoft and R. Lishka, Anal. Chem., 1951, 23, 1783–1788 CrossRef CAS
.
- R. Azizur and A. A. Siddiqui, Int. J. Pharm. Sci. Drug Res., 2010, 2, 165–175 Search PubMed
.
- J. Mathiyarasu, J. Joseph, K. L. N. Phani and V. Yegnaraman, Indian J. Chem. Technol., 2004, 11 Search PubMed
.
- T. A. Enache and A. M. Oliveira-Brett, J. Electroanal. Chem., 2011, 655, 9–16 CrossRef CAS PubMed
.
- H. Sharifian and D. W. Kirk, J. Electrochem. Soc., 1986, 133, 921–924 CrossRef CAS PubMed
.
- G. V. Kornienko, N. V. Chaenko, N. G. Maksimov, V. L. Kornienko and V. P. Varnin, Russ. J. Electrochem., 2011, 47, 225–229 CrossRef CAS
.
- J. Iniesta, P. A. Michaud, M. Panizza, G. Cerisola, A. Aldaz and C. Comninellis, Electrochim. Acta, 2001, 46, 3573–3578 CrossRef CAS
.
- J. Wei, X. Zhu and J. Ni, Electrochim. Acta, 2011, 56, 5310–5315 CrossRef CAS PubMed
.
- M. A. Rodrigo, P. A. Michaud, I. Duo, M. Panizza, G. Cerisola and C. Comninellis, J. Electrochem. Soc., 2001, 148, D60–D64 CrossRef CAS PubMed
.
- F. Xu, W. Yu, R. Gao, Q. Zhou, Q. Zhang and W. Wang, Environ. Sci. Technol., 2010, 44, 6745–6751 CrossRef CAS PubMed
.
- Y. Ito, Y. Tonogai, H. Suzuki, S. Ogawa, T. Yokoyama, T. Hashizume, H. Santo, K. I. Tanaka, K. Nishigaki and M. Iwaida, J. Assoc. Off. Anal. Chem., 1981, 64, 1448–1452 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4an02374a |
| This journal is © The Royal Society of Chemistry 2015 |