A single source-precursor route for the one-pot synthesis of highly luminescent CdS quantum dots as ultra-sensitive and selective photoluminescence sensor for Co2+ and Ni2+ ions†
Abstract
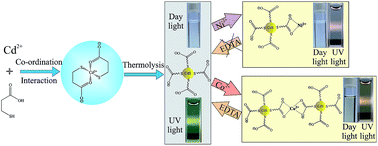
In this study, we have demonstrated a facile, simple one-pot and low cost method for the synthesis of 3-mercaptopropionic acid (MPA)-capped, water-soluble CdS quantum dots (QDs) with highly tunable optical properties. Initially Cd2+ coordinates with MPA at about pH 5, and the CdS QDs were then formed at a higher pH (7–12) under refluxing conditions through the disruption of coordination interaction with the release of sulfur. Here MPA played a dual role, as both, a source of sulfur and as a stabilizer. The particle size and the optical properties of the as-prepared CdS QDs were found to be dependent on the refluxing time for a given concentration ratio of the reactants and pH of the initial mixture. The broadness and large Stokes shift of emission of MPA–CdS QDs are due to the surface-trap state photoluminescence (PL). The PL peak around 510 nm–650 nm is due to the recombination of shallow trapped electrons in sulfur vacancy defect states with holes in the valence band, and a ∼665 nm peak (shoulder) arises from deep-trap states. The origin of the longer lifetime is presumed to be due to the involvement of surface-trap states and their environment. Use of MPA as a capping agent eventually enhances the water solubility as well as the stability of CdS QDs, which makes them useful for the ultra-sensitive detection of Co2+ and Ni2+. The selective coordination interaction of Co2+ and Ni2+ with MPA–CdS QDs through the carboxyl group of MPA provides a turn-off photoluminescence-based assay for sensitive detection of these metal ions without any interference of other commonly coexisting metal ions. The limit of detection (LOD) is 10 nM for Co2+ ions and 50 nM for Ni2+ ions. Co2+-induced color (from colorless to yellow) and UV-vis spectral change of MPA–CdS QDs is the simple way to distinguish Co2+ from Ni2+ in a higher concentration range (more than 5 µM). On the other hand the lower stability of the Co(II)–MPA complex than the Ni(II)–MPA complex provides a disodium salt of ethylenediaminetetraacetic acid (EDTA)-induced, time dependent turn-on photoluminescence-based technique to distinguish Co2+ from Ni2+ in the entire range of concentrations. EDTA-induced time dependent PL recovery of MPA–CdS QDs occurs via rapid dissociation of Co2+ ions from the surface of QDs than that of Ni2+. Thus our synthesized MPA–CdS QDs offer a very simple, rapid, cost-effective, turn-off–on photoluminescence-based technique for ultra-sensitive and selective detection of either Co2+ or Ni2+ in aqueous solution without interference of other common metal ions.

 Please wait while we load your content...
Please wait while we load your content...