Characterization of bimetallic core–shell nanorings synthesized via ascorbic acid-controlled galvanic displacement followed by epitaxial growth
Abstract
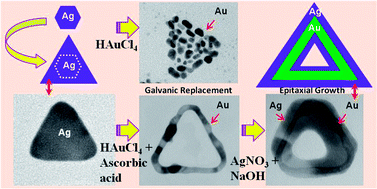
This paper describes the role of ascorbic acid in synthesizing bimetallic core–shell nanorings at room temperature. Using two-dimensional (2D) triangular silver nanoparticles as templates, we first synthesized 2D triangular gold (Au) nanorings via the galvanic replacement reaction and then overgrown Ag on Au nanorings via an epitaxial growth process. Transmission Electron Microscopy (TEM) and associated techniques were used for in-depth characterization. The TEM study revealed that single crystalline Ag nanoparticles led to the formation of continuous Au nanorings with single crystalline walls, and the void spaces which corroborate with the template shapes only when ascorbic acid was added to the growth solution. Both the silver nanoplates and gold nanorings have (111) planes as the basal planes. Subsequently we synthesized Au-core–Ag-shell nanorings using the previously synthesized Au nanorings as templates. Energy dispersive X-ray (EDX) line profile spectra and imaging, along with high-angle annular dark field scanning/transmission electron microscopy (STEM-HAADF), were used extensively for compositional studies in addition to energy filtered TEM (EFTEM) imaging.

 Please wait while we load your content...
Please wait while we load your content...