Solution structure: defining polymer film morphology and optoelectronic device performance†
Pascal
Wolfer
,
Ardalan
Armin
,
Almantas
Pivrikas
,
Marappan
Velusamy
,
Paul L.
Burn
* and
Paul
Meredith
*
Centre for Organic Photonics & Electronics, School of Chemistry and Molecular Biosciences and School of Mathematics and Physics, The University of Queensland, Brisbane QLD 4072, Australia. E-mail: p.burn2@uq.edu.au; meredith@physics.uq.edu.au
First published on 31st October 2013
Abstract
Film structure plays a critical role in defining the performance of all organic optoelectronic devices, with the importance clearly illustrated in the development of organic acceptor–donor bulk heterojunction (BHJ) photovoltaic (OPV) devices where solvent and/or thermal annealing of the deposited active layer affect solar cell output. Herein we report that the polymer–polymer interactions in solution, which are dependent on the thermal history of the solution, are a first order parameter in controlling the properties of the final active layer and hence device performance. We illustrate the key role played by organic semiconductor interactions in solution with the high efficiency donor–acceptor co-polymer, poly[N-9′′-heptadecanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)] (PCDTBT), and its blends with [6,6]-phenyl C71-butyric acid methyl ester (PC71BM). Differences in the cooling rate of the casting solution after dissolution can lead to a 20% variation in the ultimate efficiency of cells with identical active layer thicknesses with slow-cooled solutions giving rise to poorer devices. The oft-ignored intermolecular (polymer–polymer) interactions that occur in solution are manifest by dramatic differences in viscosity and are a function of concentration and molecular weight. Hence solution thermal history represents a critical new dimension in the processing landscape for organic polymer semiconductors.
Introduction
The performance of organic solar cells (OSCs) has improved dramatically in recent years, with advances due to a combination of new device architectures,1–6 processing protocols7–9 and materials developments.10–12 In particular, molecular engineering has led to fine-tuning of the optoelectronic properties of both solution processed and evaporable materials. Solution processed co-polymers with backbones comprised of donor and acceptor units have delivered OSCs with laboratory-scale device performances approaching those of inorganic thin-film cells.13–16 The relatively narrow optical gap of these polymers allows more complete harvesting of solar radiation, and charge separation and extraction in active blends with such polymers are often less reliant on film microstructure and phase separation as is the case for traditional systems based on poly(3-n-hexylthiophene-2,5-diyl) (P3HT).17,18 One promising class of donor–acceptor co-polymers are compounds containing carbazole and benzothiadiazole moieties,19,20 an example being the now-archetypical material, poly[N-9′′-heptadecanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)] (PCDTBT).21 This polymer delivers solution processed devices with efficiencies >7% when used in combination with the fullerene electron acceptor PC71BM ([6,6]-phenyl C71-butyric acid methyl ester) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by weight (PCDTBT
4 by weight (PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM). However, the reported efficiencies of devices based upon this now popular combination have varied significantly22,23 in spite of apparently simple solution processing protocols. In principle, co-dissolution of the two components in solvents such as 1,2-dichlorobenzene (o-DCB), allows facile fabrication of a bulk heterojunction (BHJ) blend structure – a bi-continuous network of the PCDTBT and PC71BM – with post-fabrication treatments such as solvent or thermal annealing not improving the photovoltaic performance.22,24,25 Addition of small amounts of dipolar solvents to o-DCB has been shown to improve device performance, which has been attributed to ‘improved’ blend nano- and microstructure.23
PC71BM). However, the reported efficiencies of devices based upon this now popular combination have varied significantly22,23 in spite of apparently simple solution processing protocols. In principle, co-dissolution of the two components in solvents such as 1,2-dichlorobenzene (o-DCB), allows facile fabrication of a bulk heterojunction (BHJ) blend structure – a bi-continuous network of the PCDTBT and PC71BM – with post-fabrication treatments such as solvent or thermal annealing not improving the photovoltaic performance.22,24,25 Addition of small amounts of dipolar solvents to o-DCB has been shown to improve device performance, which has been attributed to ‘improved’ blend nano- and microstructure.23
While the performance of polymer-based OSCs can strongly depend on the molecular weight of the semiconducting polymer, we have now found that the thermal history of solutions used for casting of the active layer can also have a dramatic effect on the ultimate device efficiency. In particular, to dissolve PCDTBT (and other high molecular weight semiconducting polymers) it is often necessary to heat the solution, although how this and the subsequent cooling of the solutions before film casting (often by spin or dip coating) is rarely, if ever, reported. Previous indications of the importance of solution processing temperature for PCDTBT come from size exclusion chromatography (SEC) measurements (showing different molecular weights and polydispersities) undertaken at different temperatures, and UV-visible (UV-vis) measurements whereby a bathochromic shift is observed between solutions at 135 and 25 °C.19,21 We have found that the rate at which the organic semiconductor solutions cool is critical, giving rise to devices with efficiencies that vary by up to 50%.
In this article we report the effect of polymer molecular weight, solution concentration, and solution thermal history on viscosity, and hence on intermolecular interactions, i.e., entanglements or aggregation between individual polymer chains prior to and during casting. We show that this thermal history has a significant effect on the ultimate device efficiency and thus should be considered a key processing parameter in optimizing an organic solar cell.
Results and discussion
The first stage in the study was to use solution viscosities to investigate how the level of intermolecular interactions of the PCDTBT chains in solution was related to polymer concentration and molecular weight. However, before discussing the results in detail it is necessary to note a few theoretical principles with regard to polymer solution viscosities. First, at low polymer concentrations, i.e., in the so-called dilute regime, individual polymer chains are isolated and only interact during brief encounters.26 In this concentration regime, the viscosity is dominated by hydrodynamic interactions between the polymer chains and solvent molecules, resulting from the relatively large differences in the size of the two components.27,28 When the polymer concentration is increased the chains start to overlap resulting in physical entanglements, and the point at which this occurs is called the overlap limit c*. The viscosity of these semi-dilute solutions is controlled by the entanglements, as the contribution of the entanglements to the solution viscosity is much more significant than effects resulting from the hydrodynamic interactions.29 In addition to solution concentration, the polymer molecular weight must also be considered when discussing polymer solution viscosities. In general by increasing the molecular weight of the polymer, the hydrodynamic volume occupied by a single macromolecular chain also increases and thus the hydrodynamic interactions between solvent and polymer chains get larger meaning that the viscosity for a given concentration is higher.28 As might be expected, the critical overlap concentration c* will decrease due to the larger hydrodynamic volume of the polymer chains, which are able to overlap at lower concentrations. Given the complexity of polymer solution viscosity theory,26–29 it is common practice to use a comparative approach to understanding the intermolecular interactions. In this case we have referenced the PCDTBT solution viscosities versus the “Drosophila” of organic solar cell materials, namely poly(3-n-hexylthiophene-2,5-diyl) (P3HT) of similar molecular weights (see Table 1).![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w, and polydispersities, PDI, of the PCDTBTs and reference P3HTs employed
w, and polydispersities, PDI, of the PCDTBTs and reference P3HTs employed
| PCDTBT |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w g mol−1
w g mol−1 |
PDI | P3HT |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w g mol−1
w g mol−1 |
PDI |
|---|---|---|---|---|---|
| 1 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.8 | R1 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.0 |
| 2 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.6 | R2 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.7 |
| 3 | 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
5.4 | R3 | 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.3 |
To this end, PCDTBT of three different molecular weights [![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w(PCDTBT-1) = 12
w(PCDTBT-1) = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1,
100 g mol−1, ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w(PCDTBT-2) = 47
w(PCDTBT-2) = 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1,
400 g mol−1, ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w(PCDTBT-3) = 122
w(PCDTBT-3) = 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1; see Table 1] were dissolved in o-DCB at 140 °C before being allowed to cool to room temperature with an average cooling rate of around 1.5 °C min−1 (the P3HT solutions were treated in the same manner). Once the solutions had reached room temperature their kinematic viscosities were measured at 20 °C in an Ostwald Micro Viscometer. To eliminate solvent related effects, the measured viscosities were normalized to the solvent viscosity, determined under the same conditions, and hence the viscosities are reported as the ‘relative kinematic viscosities’ (Fig. 1a). We found that the viscosities of the PCDTBT and P3HT solutions increased with increasing concentration and molecular weight, which is in line with theory. In addition, the influence of polymer molecular weight on solution viscosities is more pronounced at high polymer concentrations, cPolymer, i.e., in the semi-dilute regime above the overlap limit c*, where intermolecular interactions between the polymer chains dominate solution viscosities. While increases in viscosity for the three reference P3HTs (R1–R3) at a given concentration are relatively modest, those determined for PCDTBT batches 1–3 show a significantly larger change with concentration and molecular weight. At the low molecular weight end, the solution viscosity of PCDTBT-1 is similar to the P3HT reference, R1. However, the solutions of the PCDTBTs with medium and high molecular weights, i.e., PCDTBT-2 and PCDTBT-3, feature significantly higher viscosities than determined for the corresponding reference P3HTs, R2 and R3 (Fig. 1a). For example, a 25 mg ml−1 P3HT-R2 solution had a relative viscosity of 2.7, whilst that for PCDTBT-2 was 13.2 at the same concentration. For the highest molecular weight polymers P3HT-R3 and PCDTBT-3 the effect is even more pronounced: at a solution concentration as low as 5 mg ml−1, the former exhibits a relative solution viscosity of 1.3 whereas for the latter a value of 7.1 was determined. These results show polymer–polymer interactions, i.e., entanglements or aggregation between individual polymer chains, start to dominate solution viscosities of PCDTBT-2 and PCDTBT-3 at relatively low concentrations, and the nature of the polymer structure results in significantly different behavior from P3HT.
200 g mol−1; see Table 1] were dissolved in o-DCB at 140 °C before being allowed to cool to room temperature with an average cooling rate of around 1.5 °C min−1 (the P3HT solutions were treated in the same manner). Once the solutions had reached room temperature their kinematic viscosities were measured at 20 °C in an Ostwald Micro Viscometer. To eliminate solvent related effects, the measured viscosities were normalized to the solvent viscosity, determined under the same conditions, and hence the viscosities are reported as the ‘relative kinematic viscosities’ (Fig. 1a). We found that the viscosities of the PCDTBT and P3HT solutions increased with increasing concentration and molecular weight, which is in line with theory. In addition, the influence of polymer molecular weight on solution viscosities is more pronounced at high polymer concentrations, cPolymer, i.e., in the semi-dilute regime above the overlap limit c*, where intermolecular interactions between the polymer chains dominate solution viscosities. While increases in viscosity for the three reference P3HTs (R1–R3) at a given concentration are relatively modest, those determined for PCDTBT batches 1–3 show a significantly larger change with concentration and molecular weight. At the low molecular weight end, the solution viscosity of PCDTBT-1 is similar to the P3HT reference, R1. However, the solutions of the PCDTBTs with medium and high molecular weights, i.e., PCDTBT-2 and PCDTBT-3, feature significantly higher viscosities than determined for the corresponding reference P3HTs, R2 and R3 (Fig. 1a). For example, a 25 mg ml−1 P3HT-R2 solution had a relative viscosity of 2.7, whilst that for PCDTBT-2 was 13.2 at the same concentration. For the highest molecular weight polymers P3HT-R3 and PCDTBT-3 the effect is even more pronounced: at a solution concentration as low as 5 mg ml−1, the former exhibits a relative solution viscosity of 1.3 whereas for the latter a value of 7.1 was determined. These results show polymer–polymer interactions, i.e., entanglements or aggregation between individual polymer chains, start to dominate solution viscosities of PCDTBT-2 and PCDTBT-3 at relatively low concentrations, and the nature of the polymer structure results in significantly different behavior from P3HT.
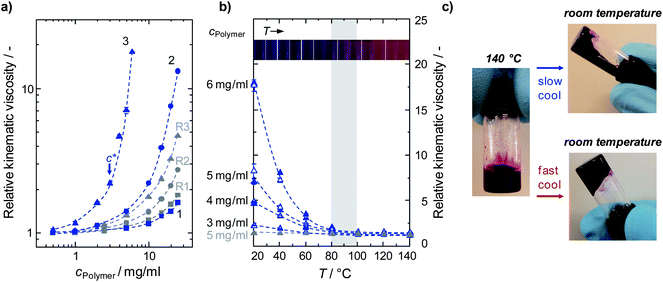 | ||
Fig. 1 (a) Solution concentration dependent relative kinematic viscosities of a series of PCDTBT (1 to 3, blue symbols) and reference P3HT (R1 to R3, grey symbols) molecular weights (cf. Table 1). For both polymers, the viscosity was found to increase with concentration and molecular weight. The high solution viscosities found for PCDTBT-2 and -3 can be attributed to the formation of intermolecular interactions already at relatively low concentrations. For PCDTBT-3, the critical crossover concentration (c*) from the dilute to the intermolecular interaction dominated solution viscosity regime is indicated with an arrow. Note that due to the complex solution behavior of the systems explored, c* could not be theoretically derived and had to be determined graphically instead. (b) Thermal evolution of relative viscosities for a range of PCDTBT-3 (full symbols) and PCDTBT-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM 1 PC71BM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w blend (open symbols) solutions. Relative viscosities of P3HT-R3 at 5 mg ml−1 (grey symbols) are presented for comparison. The inset shows the visual appearance of a PCDTBT-3 solution over a range of temperatures. Pictures were taken after each 10 °C step during a heating cycle. Between 80 and 100 °C, i.e., the temperature range where intermolecular interactions disappear, the solution color changes from dark-purple to pink. (c) Photographs of a PCDTBT-3 solution that was subject to a fast and slow cooling rate. The pictures illustrate the difference in viscosity found for solutions prepared with the two cooling rates in which higher viscosity is observed for the rapidly cooled solution. Both pictures were taken ∼10 s after turning the vial upside down. 4 w/w blend (open symbols) solutions. Relative viscosities of P3HT-R3 at 5 mg ml−1 (grey symbols) are presented for comparison. The inset shows the visual appearance of a PCDTBT-3 solution over a range of temperatures. Pictures were taken after each 10 °C step during a heating cycle. Between 80 and 100 °C, i.e., the temperature range where intermolecular interactions disappear, the solution color changes from dark-purple to pink. (c) Photographs of a PCDTBT-3 solution that was subject to a fast and slow cooling rate. The pictures illustrate the difference in viscosity found for solutions prepared with the two cooling rates in which higher viscosity is observed for the rapidly cooled solution. Both pictures were taken ∼10 s after turning the vial upside down. | ||
Having established the critical role of intermolecular interactions on solution viscosities of PCDTBTs of medium and high molecular weights, we next turn to the thermal stability of those interactions in a series of variable temperature viscosity experiments. To this end we focused on PCDTBT-3, as effects related to intermolecular interactions were most pronounced in this highest molecular weight polymer. Solution viscosities of PCDTBT-3 were found to possess strong temperature dependence at temperatures T < 80 °C (Fig. 1b). Between 80 and 100 °C the polymer solutions undergo a transition in which the relative viscosities of the PCDTBT-3 solution become similar to those determined for reference P3HT-R3. At temperatures T > 100 °C the measured relative viscosities are virtually temperature invariant. Thus the contribution of strong entanglements or aggregation towards the solution viscosities of PCDTBT-3 is large <80 °C, and negligible at higher temperatures. The transition between the two regimes can also be clearly seen in the optical appearance of the PCDTBT solutions (Fig. 1b inset). PCDTBT-3 was dissolved in o-DCB and then heated in 10 °C steps to 140 °C. A distinctive color change from dark-purple to pink was observed between 80 and 100 °C, i.e., exactly in the range where the influence of the intermolecular interactions diminishes. It is interesting to note that there is no hysteresis in the kinematic viscosities during a heating and slow cooling (1.5 °C min−1) cycle for the polymer only and polymer/fullerene solutions (see ESI Fig. S1†).
However, the active layers of BHJ solar cells contain not only an electron donor polymer (such as P3HT or PCDTBT) but also a high electron affinity component (an electron acceptor such as PC71BM) with the active layer formed by casting a solution containing these two components. It might be thought that addition of the acceptor component could disrupt the polymer–polymer interactions in solution and hence reduce the viscosity. We therefore undertook variable temperature viscosity measurements on PCDTBT-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM 1
PC71BM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w blends (the ratio delivering the highest device efficiencies).22 PC71BM was not expected to contribute to an increase in solution viscosities over and above that of the co-solvent (o-DCB). This was confirmed by measuring the viscosity of PC71BM over a wide concentration range (Fig. S2†). Importantly, as can be seen in Fig. 1b, the relative kinematic viscosities for the polymer and for the respective blend (1
4 w/w blends (the ratio delivering the highest device efficiencies).22 PC71BM was not expected to contribute to an increase in solution viscosities over and above that of the co-solvent (o-DCB). This was confirmed by measuring the viscosity of PC71BM over a wide concentration range (Fig. S2†). Importantly, as can be seen in Fig. 1b, the relative kinematic viscosities for the polymer and for the respective blend (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) are essentially the same across the whole temperature range showing that the PC71BM does not substantially disrupt the polymer network formed in solution.
4 w/w) are essentially the same across the whole temperature range showing that the PC71BM does not substantially disrupt the polymer network formed in solution.
To summarize our key finding so far: for PCDTBT, the influence of polymer–polymer interactions in solution varies with concentration, molecular weight, and temperature. Importantly, the major effects are seen for T < 80 °C, which is the temperature window in which BHJ active layers are typically cast. However, it is well known that during crystallization of polymers, the rate of cooling can affect the size of the crystal domain. Hence, one needs to address the question as to whether the cooling rate of the organic semiconductor blend prior to film casting affects the formation of intermolecular entanglements or aggregation of polymer chains in the solution state. To examine this question, the PCDTBT-3 solutions were allowed to cool slowly to room temperature by leaving them on a hot-plate that was switched off at 140 °C (a cooling rate of around 1.5 °C min−1), or fast-cooled by taking the same solutions reheated to 140 °C and then placing them in a temperature controlled water bath held at 20 °C. Critically, we observed significantly different viscosities for the two cooling regimes – with higher viscosities induced by a faster cooling rate (Fig. 1c and Table 2). This effect was more pronounced for higher concentrations and molecular weights (cf. Tables S1 and S2†). For example, a PCDTBT-3 solution of 3 mg ml−1 was found not to show a significant cooling rate dependency, whereas an increase in relative viscosity of more than 170% was observed for a fast-cooled 6 mg ml−1 solution when compared to its slow-cooled counterpart (Table 2). A cooling rate dependency was only observed for concentrations above the graphically determined crossover from the dilute to the semi-dilute regime, i.e., at cPCDTBT > 3 mg ml−1, where the polymer–polymer interactions dominate the solution viscosity (cf.Fig. 1a and Table 2). Most importantly, a similar trend was observed for blend solutions of PCDTBT-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM, although the absolute values differ slightly from those of neat PCDTBT solutions, especially at higher concentrations (Table 2). No comparable effect was observed for reference P3HT-R3 in the respective concentration range, demonstrating that the donor–acceptor structure of PCDTBT results in a significantly different response to the solution thermal history than observed for P3HT.
PC71BM, although the absolute values differ slightly from those of neat PCDTBT solutions, especially at higher concentrations (Table 2). No comparable effect was observed for reference P3HT-R3 in the respective concentration range, demonstrating that the donor–acceptor structure of PCDTBT results in a significantly different response to the solution thermal history than observed for P3HT.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM 1
PC71BM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w solution concentrations prepared with slow and fast cooling rates. For comparison, relative viscosities of P3HT-R3 are provided
4 w/w solution concentrations prepared with slow and fast cooling rates. For comparison, relative viscosities of P3HT-R3 are provided
| C Polymer mg ml−1 | Relative viscosity | Change % | ||
|---|---|---|---|---|
| Slow cooling | Fast cooling | |||
| 3 | PCDTBT | 2.21 ± 0.04 | 2.11 ± 0.05 | −5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 blend 4 blend |
2.25 ± 0.04 | 2.18 ± 0.03 | −3 | |
| 4 | PCDTBT | 4.65 ± 0.11 | 5.46 ± 0.13 | +17 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 blend 4 blend |
4.90 ± 0.07 | 5.70 ± 0.13 | +16 | |
| 5 | PCDTBT | 7.05 ± 0.31 | 14.55 ± 0.53 | +106 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 blend 4 blend |
8.25 ± 0.81 | 13.63 ± 0.75 | +65 | |
| 6 | PCDTBT | 17.86 ± 0.23 | 48.84 ± 2.67 | +173 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 blend 4 blend |
17.62 ± 0.67 | 74.83 ± 6.95 | +325 | |
| 5 | P3HT-R3 | 1.41 ± 0.06 | 1.40 ± 0.04 | −1 |
While these results are clearly important in understanding the basic properties and solution behavior of the semiconducting polymers in question – do these viscosity effects have any relevance to or bearing on the resultant BHJ organic solar cells? To answer this question we prepared a solution of PCDTBT-3 and PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w ratio) in o-DCB, which was then heated and either slow- or fast-cooled. The thickness of the PCDTBT
4 w/w ratio) in o-DCB, which was then heated and either slow- or fast-cooled. The thickness of the PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
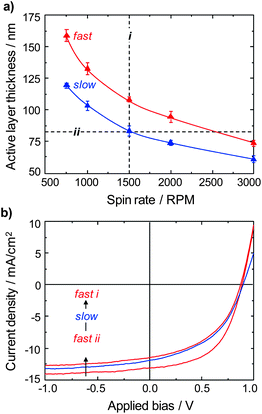
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM active layer is a critical parameter for optimizing device efficiency30,31 and as can be seen in Fig. 2a, significantly different spin-coating speeds are required with different thermal histories to achieve the desired target thickness of 85 ± 5 nm (the thickness dependence of the device performance (short circuit photocurrent) is illustrated in Fig. S3† and an active layer thickness of 85 nm was assumed to be optimal for all fabrication conditions explored). For the slow-cooled solutions a spin speed of 1500 rpm yielded an active layer thickness of 83 ± 5 nm (processing pathway i, Fig. 2a), with the resultant solar cells having an average power conversion efficiency (PCE) of 5.0% (Table 3; values were averaged over a minimum of 12 devices on three different substrates). If the same fabrication conditions [a spin speed of 1500 rpm (processing pathway i)] were applied to the fast-cooled solution the higher viscosity led to a 25 nm thicker active layer and the devices only had a PCE of 4.0%. As evident from Table 3 and from the current density–voltage (J–V) characteristics of typical devices in Fig. 2b (note the dark J–V curves are shown in Fig. S4†), the 20% decrease in efficiency mainly stems from a reduction of the open-circuit voltage (Voc) and fill factor (FF). When the correct thickness calibration was applied to the fast-cooled solution (processing pathway ii, Fig. 2a), active layers of the optimized thickness of 83 ± 5 nm could be deposited with a spin speed of 2500 rpm. The subsequent solar cells had an average PCE of 6.1%, i.e., a 20% increase over the equivalent device formed from a slow-cooled solution. The improvement can be seen to be mainly attributed to an increase in the fill factor from FFslow = 0.46 to FFfast = 0.53 (Table 3).
PC71BM active layer is a critical parameter for optimizing device efficiency30,31 and as can be seen in Fig. 2a, significantly different spin-coating speeds are required with different thermal histories to achieve the desired target thickness of 85 ± 5 nm (the thickness dependence of the device performance (short circuit photocurrent) is illustrated in Fig. S3† and an active layer thickness of 85 nm was assumed to be optimal for all fabrication conditions explored). For the slow-cooled solutions a spin speed of 1500 rpm yielded an active layer thickness of 83 ± 5 nm (processing pathway i, Fig. 2a), with the resultant solar cells having an average power conversion efficiency (PCE) of 5.0% (Table 3; values were averaged over a minimum of 12 devices on three different substrates). If the same fabrication conditions [a spin speed of 1500 rpm (processing pathway i)] were applied to the fast-cooled solution the higher viscosity led to a 25 nm thicker active layer and the devices only had a PCE of 4.0%. As evident from Table 3 and from the current density–voltage (J–V) characteristics of typical devices in Fig. 2b (note the dark J–V curves are shown in Fig. S4†), the 20% decrease in efficiency mainly stems from a reduction of the open-circuit voltage (Voc) and fill factor (FF). When the correct thickness calibration was applied to the fast-cooled solution (processing pathway ii, Fig. 2a), active layers of the optimized thickness of 83 ± 5 nm could be deposited with a spin speed of 2500 rpm. The subsequent solar cells had an average PCE of 6.1%, i.e., a 20% increase over the equivalent device formed from a slow-cooled solution. The improvement can be seen to be mainly attributed to an increase in the fill factor from FFslow = 0.46 to FFfast = 0.53 (Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM 1
PC71BM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w based organic solar cells prepared with three different processing protocols. Values and errors indicated represent the average and corresponding standard deviation over a minimum of 12 devices on three different substrates
4 w/w based organic solar cells prepared with three different processing protocols. Values and errors indicated represent the average and corresponding standard deviation over a minimum of 12 devices on three different substrates
PCDTBT-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM PC71BM |
Thickness calibration | Thickness nm | J sc mA cm−2 | V oc V | FF | PCE % |
|---|---|---|---|---|---|---|
| Slow cooled | Yes | 83 ± 5 | 12.2 ± 0.5 | 0.89 ± 0.01 | 0.46 ± 0.01 | 5.0 ± 0.2 |
| Fast cooled | No | 108 ± 5 | 12.0 ± 0.3 | 0.82 ± 0.01 | 0.41 ± 0.01 | 4.0 ± 0.3 |
| Fast cooled | Yes | 83 ± 5 | 12.9 ± 0.3 | 0.89 ± 0.01 | 0.53 ± 0.02 | 6.1 ± 0.3 |
Our results demonstrate quite clearly that the simple and oft-ignored processing parameter of solution thermal history can have a profound effect upon the ultimate solar cell performance of donor–acceptor co-polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene blends such as PCDTBT
fullerene blends such as PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM. It is generally accepted that different PCDTBT molecular weights and polydispersities will deliver different device efficiencies – however, that is an oversimplification of the real situation. Polymer–polymer interactions, i.e., the formation of an interconnected macromolecular network in solution, dictate viscosities even at relatively low concentrations. These interactions are controlled by multiple factors (including concentration and molecular weight), but the solution cooling rate also appears to be first order in governing device performance. Solar cells prepared with the same active layer thicknesses but cast from solutions with different thermal histories showed variations in PCE of up to 20%. So what is the origin of these differences: film structure and/or charge generation and/or charge extraction/trapping?
PC71BM. It is generally accepted that different PCDTBT molecular weights and polydispersities will deliver different device efficiencies – however, that is an oversimplification of the real situation. Polymer–polymer interactions, i.e., the formation of an interconnected macromolecular network in solution, dictate viscosities even at relatively low concentrations. These interactions are controlled by multiple factors (including concentration and molecular weight), but the solution cooling rate also appears to be first order in governing device performance. Solar cells prepared with the same active layer thicknesses but cast from solutions with different thermal histories showed variations in PCE of up to 20%. So what is the origin of these differences: film structure and/or charge generation and/or charge extraction/trapping?
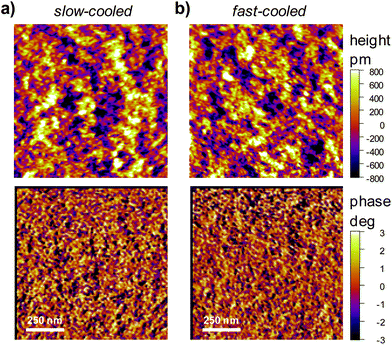
Although Atomic Force Microscopy (AFM) only gives information about the film surface structure, gross changes due to phase separation and/or crystallization of components can be inferred from this surface information. We therefore analyzed the surface topography of the PCDTBT-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM films produced from slow- and fast-cooled solutions. Both, height- and phase-images, show very similar features for films fabricated with solutions prepared with the two different cooling rates (Fig. 3). Both films were very smooth [root mean square (RMS) roughness Rq = 0.39 nm] and hence the differences in solar cell performance are not likely to be due to large differences in the nano- and microstructure of the blend.
PC71BM films produced from slow- and fast-cooled solutions. Both, height- and phase-images, show very similar features for films fabricated with solutions prepared with the two different cooling rates (Fig. 3). Both films were very smooth [root mean square (RMS) roughness Rq = 0.39 nm] and hence the differences in solar cell performance are not likely to be due to large differences in the nano- and microstructure of the blend.
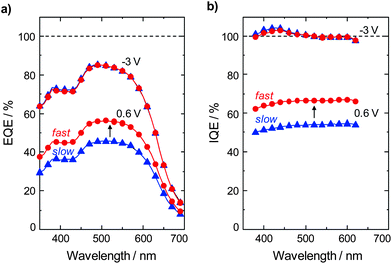
To understand whether the solution thermal history affects the active layer optical absorption, or charge generation and collection efficiencies we analyzed the External Quantum Efficiency (EQE) of solar cells made from the different solutions. When the devices were biased at −3 V (reverse bias) the EQE were identical (Fig. 4a). In addition, near normal incidence reflection and Internal Quantum Efficiency (IQE) measurements were obtained. The reflection measurements showed that the light absorption within the active layers was identical (see Fig. S5†) in both cases, and high IQEs (≈100% at −3 V) were also observed (Fig. 4b). These results indicate that identical charge generation and collection efficiencies occur under reverse bias. However, when the EQEs and IQEs were measured at a voltage closer to the maximum power point (Vmax power = 0.6 V) the devices made from the fast-cooled solutions were superior by ∼10%. Hence, one may conclude that the increased level of polymer entanglement or aggregation in solution delivered improved charge generation in and/or charge collection from the resultant active layer versus devices made from the slow-cooled PCDTBT-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM solutions.
PC71BM solutions.
Conclusions
In conclusion, our results clearly demonstrate that the thermal history of the casting solution used to form the active layer in PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM BHJ organic solar cells is an important parameter in defining the ultimate device efficiency. This part of the process in organic solar cell fabrication is often ignored and hence poorly controlled. Thus efficiency variations reported in the literature may not be simply due to polymer molecular weight or polydispersity differences but also dependent on how the solution has been treated. The origin of the phenomenon can be assigned to the strong effect of polymer–polymer interactions, i.e., the formation of a dense polymer network in rapidly cooled solutions, leading to improved carrier generation and extraction from the resultant active layer. One may speculate as to whether this is a generic property of high molecular weight semiconducting acceptor–donor polymers which characterize the now dominant narrow optical gap systems in high efficiency solution processed organic solar cells, and more generally high molecular weight semiconducting polymers used in organic optoelectronics. So, in optimizing such systems, we must consider the polymer concentration and molecular weight, as well as reporting as to whether the organic semiconductor casting solutions were heated to achieve dissolution and subsequently cooled, and whether the concentration was in the semi-dilute regime above the critical crossover limit c*.
PC71BM BHJ organic solar cells is an important parameter in defining the ultimate device efficiency. This part of the process in organic solar cell fabrication is often ignored and hence poorly controlled. Thus efficiency variations reported in the literature may not be simply due to polymer molecular weight or polydispersity differences but also dependent on how the solution has been treated. The origin of the phenomenon can be assigned to the strong effect of polymer–polymer interactions, i.e., the formation of a dense polymer network in rapidly cooled solutions, leading to improved carrier generation and extraction from the resultant active layer. One may speculate as to whether this is a generic property of high molecular weight semiconducting acceptor–donor polymers which characterize the now dominant narrow optical gap systems in high efficiency solution processed organic solar cells, and more generally high molecular weight semiconducting polymers used in organic optoelectronics. So, in optimizing such systems, we must consider the polymer concentration and molecular weight, as well as reporting as to whether the organic semiconductor casting solutions were heated to achieve dissolution and subsequently cooled, and whether the concentration was in the semi-dilute regime above the critical crossover limit c*.
Experimental
Materials
Poly[N-9′-heptadecanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)] (PCDTBT) batches PCDTBT-1 (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 12
w = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, PDI = 2.8) and PCDTBT-2 (
100 g mol−1, PDI = 2.8) and PCDTBT-2 (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 47
w = 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1, PDI = 2.6) were synthesized and purified in-house following the Suzuki cross-coupling protocols previously described.19 PCDTBT-3 (
300 g mol−1, PDI = 2.6) were synthesized and purified in-house following the Suzuki cross-coupling protocols previously described.19 PCDTBT-3 (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 122
w = 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, PDI = 5.4) was purchased from SJPC, Canada. Molecular weights of the different PCDTBT batches were determined with Gel Permeation Chromatography (GPC) in 1,2,4-trichlorobenzene at 135 °C by SJPC, Canada. Poly(3-n-hexylthiophene-2,5-diyl) (P3HT) batches P3HT-R1 (
200 g mol−1, PDI = 5.4) was purchased from SJPC, Canada. Molecular weights of the different PCDTBT batches were determined with Gel Permeation Chromatography (GPC) in 1,2,4-trichlorobenzene at 135 °C by SJPC, Canada. Poly(3-n-hexylthiophene-2,5-diyl) (P3HT) batches P3HT-R1 (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 22
w = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, PDI = 2.0, regio-regularity ∼94%), P3HT-R2 (
500 g mol−1, PDI = 2.0, regio-regularity ∼94%), P3HT-R2 (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 51
w = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, PDI = 1.7, regio-regularity ∼96%) and P3HT-R3 (
500 g mol−1, PDI = 1.7, regio-regularity ∼96%) and P3HT-R3 (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 126
w = 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1, PDI = 2.3, regio-regularity ∼97%) were purchased from Merck Chemicals, United Kingdom. Molecular weights of the different P3HT batches were determined with GPC in tetrahydrofuran (THF) at ambient conditions and regio-regularities were provided by the supplier. [6,6]-Phenyl C71-butyric acid methyl ester (PC71BM) was purchased from American Dye Source, Inc., Canada.
700 g mol−1, PDI = 2.3, regio-regularity ∼97%) were purchased from Merck Chemicals, United Kingdom. Molecular weights of the different P3HT batches were determined with GPC in tetrahydrofuran (THF) at ambient conditions and regio-regularities were provided by the supplier. [6,6]-Phenyl C71-butyric acid methyl ester (PC71BM) was purchased from American Dye Source, Inc., Canada.
Sample preparation
Solutions for kinematic viscosity measurements were prepared by dissolving the polymer or the blend components in 1,2-dichlorobenzene at 140 °C. After complete dissolution, the hot-plate was switched off and the solutions were allowed to cool to room temperature with a resulting average cooling rate of about 1.5 °C min−1. Subsequently, 0.7 ml of solution were transferred into an Ostwald Micro Viscometer for analysis. For viscosity measurements of fast-cooled solutions, the loaded viscometer was directly transferred from a 140 °C warm oil bath to a water bath at 20 °C.Pre-etched indium tin oxide (ITO) substrates for bulk heterojunction device fabrication and Atomic Force Microscopy (AFM) were purchased from Kintec and cleaned in a 90 °C warm Alconox solution. Subsequently, the substrates were scrubbed with a soft cloth followed by sequential ultrasonication in Alconox, de-ionized water, acetone, and 2-propanol. After drying the substrates under a nitrogen flow, a poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) (Baytron P VP Al 4083) film was spin-coated at 5000 rpm. The resulting 20 nm thick layer was baked at 170 °C for 10 min in air. Solutions of PCDTBT (7.5 mg ml−1, 2 ml) and PC71BM (60 mg ml−1, 1 ml) were prepared in 1,2-dichlorobenzene and combined at 90 °C to obtain a PCDTBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM ratio in solution of 1
PC71BM ratio in solution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w and a total material concentration of 25 mg ml−1. After stirring at the same temperature for about 10 min, the solution was split into two equal fractions. The first fraction was immediately removed from the hot-plate and placed on a metal surface at room temperature for the fast cooled sample. Subsequently, the hot-plate was switched off and allowed to slowly cool to room temperature with the second fraction still on the plate, resulting in an average cooling rate of about 1.5 °C min−1. Next, blend films were spin-coated on test substrates at different spin rates to calibrate the active layer thicknesses (cf. Fig. 2a). For this purpose, BHJ layer thicknesses were measured with a Veeco Dektak 150 profilometer. Subsequently, active layers produced with slow-cooled and fast-cooled solutions were spin coated for 100 s at 1500 and 2500 rpm, respectively, resulting in 83 ± 5 nm thick PCDTBT-3
4 w/w and a total material concentration of 25 mg ml−1. After stirring at the same temperature for about 10 min, the solution was split into two equal fractions. The first fraction was immediately removed from the hot-plate and placed on a metal surface at room temperature for the fast cooled sample. Subsequently, the hot-plate was switched off and allowed to slowly cool to room temperature with the second fraction still on the plate, resulting in an average cooling rate of about 1.5 °C min−1. Next, blend films were spin-coated on test substrates at different spin rates to calibrate the active layer thicknesses (cf. Fig. 2a). For this purpose, BHJ layer thicknesses were measured with a Veeco Dektak 150 profilometer. Subsequently, active layers produced with slow-cooled and fast-cooled solutions were spin coated for 100 s at 1500 and 2500 rpm, respectively, resulting in 83 ± 5 nm thick PCDTBT-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM films. For BHJ devices, 1 nm samarium/100 nm aluminum electrodes were thermally deposited under a vacuum of ∼10−6 mbar. The resulting device area was 0.2 cm2 with 6 devices per substrate.
PC71BM films. For BHJ devices, 1 nm samarium/100 nm aluminum electrodes were thermally deposited under a vacuum of ∼10−6 mbar. The resulting device area was 0.2 cm2 with 6 devices per substrate.
Viscosity measurements
Kinematic viscosities were determined using an Ostwald Micro Viscometer with a viscometer constant of 0.075 at 20 °C. For viscosity measurements performed at elevated temperatures, the viscometer constant was calibrated with 1,2-ethanediol. For measurements conducted at 20 °C, the viscometer was immersed in a temperature controlled water bath. For experiments performed in the temperature range between 40 and 140 °C, the viscometer was placed in a temperature controlled and thermally insulated oil bath. Solutions were allowed to equilibrate at the preset temperature for 15 min prior to analysis and viscosity values were averaged over 5 runs.Atomic Force Microscopy (AFM)
Atomic Force Microscopy was performed with an Asylum Research AFM MFP 3D, operating the device in the tapping mode with a cantilever resonant frequency of 140 kHz.Photovoltaic device characterization
Current density–voltage (J–V) characteristics were acquired in a N2 atmosphere using a Keithley 2400 Source Measure Unit. The simulated Air Mass 1.5 Global (AM 1.5 G) illumination was provided by an Abet Sun 2000 Solar Simulator. The intensity used throughout was ∼1000 W m−2 (the exact number being used for efficiency calculations) as determined by an NREL-calibrated silicon reference cell.External/Internal Quantum Efficiencies (EQE/IQE) analysis
External Quantum Efficiencies were measured with a PV Measurements Inc. QEX7 setup, using an integrated sphere and calibrated photodiode. The total reflection spectra of the solar cells were determined under the incident light angle of <10°. The parasitic absorption by the non-active layers was simulated using a computer code developed by van de Lagemaat et al. from the National Renewable Energy Laboratory (NREL) based on the transfer matrix method presented by Pettersson et al.32 The net light absorption within the active layer was obtained by subtracting the simulated parasitic absorption from measured total absorption within the device. The Internal Quantum Efficiency was determined by dividing the External Quantum Efficiency by the net absorption within the active layer.Acknowledgements
PW would like to thank the Swiss National Science Foundation (SNSF) for an Advanced Researcher Fellowship (PA00P2 145395). AA is funded by an University of Queensland International scholarship. AP is the recipient of an Australian Research Council Discovery Early Career Researcher Award (DE120102271), and UQ ECR59-2011002311 and UQ NSRSF-2011002734 funding. PLB and PM are supported by University of Queensland Vice Chancellor's Senior Research Fellowships. We acknowledge support from The University of Queensland (Strategic Initiative – Centre for Organic Photonics & Electronics). This work was performed in part at the Australian National Fabrication Facility Queensland Node (ANFF-Q) – a company established under the National Collaborative Research Infrastructure Strategy to provide nano- and microfabrication facilities for Australia's researchers. The authors kindly thank SJPC, Canada, for providing sample PCDTBT material and for performing the high-temperature GPC analyses.References
- C. W. Tang, Appl. Phys. Lett., 1986, 48, 183 CrossRef CAS.
- N. S. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474 CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145 CrossRef.
- J. G. Xue, S. Uchida, B. P. Rand and S. R. Forrest, Appl. Phys. Lett., 2004, 85, 5757 CrossRef CAS.
- J. Kim, K. Lee, N. E. Coates, D. Moses, T.-Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222 CrossRef CAS PubMed.
- F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2009, 93, 394 CrossRef CAS PubMed.
- J. Peet, M. L. Senatore, A. J. Heeger and G. C. Bazan, Adv. Mater., 2009, 21, 1521 CrossRef CAS.
- F. C. Krebs, T. Tromholt and M. Jorgensen, Nanoscale, 2010, 2, 873 RSC.
- R. Kroon, M. Lenes, J. C. Hummelen, P. W. M. Blom and B. de Boer, Polym. Rev., 2008, 48, 531 CrossRef CAS.
- J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. Zhan, Adv. Mater., 2010, 22, 3876 CrossRef CAS PubMed.
- A. Facchetti, Chem. Mater., 2011, 23, 733 CrossRef CAS.
- D. Muhlbacher, M. Scharber, M. Morana, Z. Zhu, D. Waller, R. Gaudiana and C. Brabec, Adv. Mater., 2006, 18, 2884 CrossRef.
- E. Wang, L. Wang, L. Lan, C. Luo, W. Zhuang, J. Peng and Y. Cao, Appl. Phys. Lett., 2008, 92, 033307 CrossRef.
- M. M. Wienk, M. Turbiez, J. Gilot and R. A. J. Janssen, Adv. Mater., 2008, 20, 2556 CrossRef CAS.
- L. Weiwei, K. H. Hendriks, W. S. C. Roelofs, Y. Kim, M. M. Wienk and R. A. J. Janssen, Adv. Mater., 2013, 25, 3182 CrossRef PubMed.
- Y. Kim, S. Cook, S. M. Tuladhar, S. A. Choulis, J. Nelson, J. R. Durrant, D. D. C. Bradley, M. Giles, I. McCulloch, C.-S. Ha and M. Ree, Nat. Mater., 2006, 5, 197 CrossRef CAS.
- G. Li, Y. Yao, H. Yang, V. Shrotriya, G. Yang and Y. Yang, Adv. Funct. Mater., 2007, 17, 1636 CrossRef CAS.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletete, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS PubMed.
- P.-L. T. Boudreault, S. Beaupre and M. Leclerc, Polym. Chem., 2010, 1, 127 RSC.
- N. Blouin, A. Michaud and M. Leclerc, Adv. Mater., 2007, 19, 2295 CrossRef CAS.
- S. H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297 CrossRef CAS.
- T.-Y. Chu, S. Alem, S.-W. Tsang, S.-C. Tse, S. Wakim, J. Lu, G. Dennler, D. Waller, R. Gaudiana and Y. Tao, Appl. Phys. Lett., 2011, 98, 253301 CrossRef.
- S. Wakim, S. Beaupre, N. Blouin, B.-R. Aich, S. Rodman, R. Gaudiana, Y. Tao and M. Leclerc, J. Mater. Chem., 2009, 19, 5351 RSC.
- T. Wang, A. J. Pearson, A. D. F. Dunbar, P. A. Staniec, D. C. Watters, H. Yi, A. J. Ryan, R. A. L. Jones, A. Iraqi and D. G. Lidzey, Adv. Funct. Mater., 2012, 22, 1399 CrossRef CAS.
- P. J. Flory and W. R. Krigbaum, J. Chem. Phys., 1950, 18, 1086 CrossRef CAS.
- W. R. Krigbaum and P. J. Flory, J. Polym. Sci., 1953, 11, 37 CrossRef CAS.
- A. Z. Akcasu and C. C. Han, Macromolecules, 1979, 12, 276 CrossRef CAS.
- G. C. Berry and T. G. Fox, Adv. Polym. Sci., 1968, 5, 261 CrossRef.
- T.-Y. Chu, S. Alem, P. G. Verly, S. Wakim, J. Lu, Y. Tao, S. Beaupre, M. Leclerc, F. Belanger, D. Desilets, S. Rodman, D. Waller and R. Gaudiana, Appl. Phys. Lett., 2009, 95, 063304 CrossRef.
- A. Armin, Y. Zhang, P. L. Burn, P. Meredith and A. Pivrikas, Nat. Mater., 2013, 12, 593 CrossRef CAS PubMed.
- L. A. A. Pettersson, L. S. Roman and O. Inganas, J. Appl. Phys., 1999, 86, 487 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3tc31812e |
| This journal is © The Royal Society of Chemistry 2014 |