A study of a fluorine substituted phenyl based complex as a 3 V electrolyte for Mg batteries†
Abstract
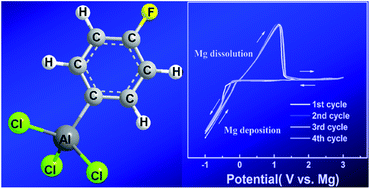
An electrolyte with a wide electrochemically stable window and high efficiency for reversible Mg deposition/dissolution is a key component of Mg battery systems. In the present study, functional-group-substituted phenyl-based Mg battery electrolytes have been prepared by direct reactions between the Lewis acid AlCl3 and various fluorine substituted Lewis bases. The substitution effects of these functional groups on the anodic stability of the electrolyte and the efficiency for Mg deposition/dissolution have been studied by electrochemical analysis and first-principles density functional theory calculations. This study indicates that the para-substituted fluorine complex (4-F-PhMgBr)2-AlCl3/THF is an excellent 3 V electrolyte for Mg batteries with respect to the electrochemical stability and efficiency of reversible Mg deposition/dissolution. Both the experimental results and theoretical calculations are consistent and indicate that electron-withdrawing groups with small steric effects on phenyl rings improve the electrolyte stability and reversibility by decreasing the HOMO and increasing the LUMO energy levels of the complex component, while electron-donating groups have profound detrimental influences. This investigation provides further understanding of the electrolyte chemistry of Mg batteries and advances the design and optimization of new electrolytes.

 Please wait while we load your content...
Please wait while we load your content...